SleepNon24VLiDACMel - VLiDACMel therapy for entrainment of treatment-resistant sighted non24

Man with too long a day. A metaphor of the non-24 circadian rhythm sleep-wake disorder, by Alec Gray. Reproduced with permission.
Foreword
This is an experimental protocol for 24h entrainment of treatment-resistant sighted non24.
This is a work-in-progress documentation of the author's self-experiment. Hence, it will continue to evolve over time. Check out later this document for updates.
This work takes an evidence-based approach based on a mostly clinical literature review when possible and self-experimental using a combination of sleep diary, manual data logging and automatic vitals monitoring where the data is lacking in the literature (sighted non24 is pretty rare after all). The goal being to design and assess the effectiveness of therapies to manage sighted non-24.
As of July 2020, the protocol is considered mature, as it reproducibly allows for a stable (but not constant) entrainment of the author's circadian rhythm to a 24h cycle. Furthermore, all the observed effects could be elucidated by previous studies, which provides a framework to predict how this therapy works in various scenarios. This experiment is also following the new approach of radical open science, where the experiments progress is publicly accessible at nearly all stages. What remains to be explored are the following points:
- Milestone 1 (done July 2020): Complete this document to fully describe the therapy and the theoretical physiological pathways underlying it, as well as the practical details to adjust it on an individual basis.
- Milestone 1.5 (done August-October 2020): Reproduce the shorter than 24h circadian period with very long bright blue light therapy. This would allow to adjust backwards the circadian rhythm (ie, sleep and wake up earlier) without having to freerun forward.
- Milestone 2 (done October 2020): Assess the necessity of each step by elimination (ie, try to keep all steps but remove one at a time, if no effect then can permanently be removed). After this milestone, the protocol will be including only the minimal set of steps necessary for entrainment of the author's circadian rhythm.
- Milestone 2.5 (done November 2020): Update document with critical findings from side notebook. All major aspects of the therapy (such a very long light therapy being more effective than brighter light therapy) were found to be strongly supported by previous (but unpopularized) research, and adequate references were added. Added a simplified protocol (set of rules, 2 pages). The protocol is now considered mature.
- Milestone 2.6: Update document with more findings from side notebook. (done during 2021-2022)
- Milestone 3: Systematization of the therapy by circadian rhythm monitoring using wearable devices. Just like diabetes became medically manageable when glucose and insulin monitoring devices could be made, there needs to be a device to monitor the circadian rhythm in order to properly time the therapies on a daily basis and monitor their effects as well as chaotic biological fluctuations. (done during 2021-2022)
- Milestone 3.5: Reproduce the shorter than 24h circadian period with very long bright blue light therapy, continuously for several weeks, while monitoring vital signs and body temperature, in order to objectively assess the phase advance produced by very long bright blue light therapy. (Failed as of 2023)
- Milestone 4 (done 2022 - database is complete and publishable): Publication of the database of vital signs and sleep logs for this self-experiment to allow for third-party review and analyses. Database may be published in a peer-reviewed journal. See the Wearadian project on GitHub for more details on the acquisition system and access to the database.
- Milestone 5 (partially done as of 2023 - protocol rewritten, references not rewritten but can be automatically done with custom tool): rewrite this protocol more concisely and with references in academic style instead of hyperlinks (using Zettlr) for publication in a peer-reviewed journal. — Idea: convert to a MyST Markdown or AsciiDoc document (using Pandoc?), then simply auto-extract links and move them to the end of the subsentence it was highlighting (should also take into account the "(see also here, here and here)", but likely will require some manual cleanup - but the heavy lifting would be automatically done).
- Milestone 6 (done in March 2024): achieve one year of continuous entrainment at a stable phase. Being entrained for one year logically means that it is possible to stay forever entrained if the conditions are repeated. This is hence the ultimate n-of-1 efficacy milestone.
In addition to the therapy's protocol, there is also a TROUBLESHOOTING section towards the end of this document, which aims to answer the most common questions about the various therapies for non24 and clarify how they work and how to optimize them according to the current scientific knowledge. This section is much longer than the therapy outline, and hence it is written for the curious reader to further their knowledge and/or answer their questions about or around circadian rhythm disorders. Reading the Troubleshooting section is not mandatory, rather the reader is invited to search there in case of a specific question that is not answered in the therapy outline.
This therapy was designed to treat sighted non24. Since the tools influencing the circadian rhythm are the same for all humans (and actually most research was done on typical sleepers but are applicable for people with circadian rhythm disorders), most parts are also be applicable to DSPD with some slight changes (mostly that the goal of DSPD is to phase advance gradually, whereas non24 aims to freeze the circadian rhythm in place with a treatment-induced daily phase advance that counteracts the natural intrinsic daily phase delay). For ASPD, it should be possible to use the same tools too but timed at the opposite, under the phase delay part of the zeitgebers' PRCs (eg, being exposed to light therapy in the evening instead of at wake-up, as did Czeisler et al in a case study). Findings for individuals with a circadian rhythm disorder can also provide generalizable insights for typical sleepers as previous studies have done.
Since this protocol was written, the Circadiaware collective was founded by several developers to make open-source tools to further help with the analysis of circadian rhythm phenomena and the management of circadian rhythm disorders. You may find interesting tools there such as WebActogram, a tool to instantly screen circadian rhythm disorders, and Wearadian, a protocol design to automatically monitor 24/7 the sleep and circadian rhythm patterns out of lab.
DISCLAIMER: this protocol is not scientifically peer-reviewed and not clinically validated. It was tested on a sample size of only 2 individuals with non24 since birth and under a controlled home environment. Hence this protocol cannot be formally recommended, it should still be considered experimental and maybe risky. If you do try, it would be at your own risk (please at least ask a physician to accompany you!).
IMPORTANT HEALTH NOTE: this therapy cannot be used by individuals with epilepsy or macular degeneration or other retinal diseases or malformations (eg, aphakic people born without crystalline lens and pseudophakic who received intraocular lens implants), as these populations are at risk when using light therapy. It is also risky for people with motor disorders such as restless legs syndrome or periodic limb movement disorder as both bright light therapy and melatonin can independently increase melatonin levels which can trigger motor symptoms, however these symptoms should disappear following discontinuation of the therapy.
This document was first written and self published by Stephen Karl Larroque in June 2020, from material collected since August 2019, with substantial iterative updates over the years.
Last update: February 2025.
ORCID: https://orcid.org/0000-0002-6248-0957
Contact me on GitHub by opening an issue.
To print the document, select the text (click on the first paragraph, then SHIFT+left click at the end of the page) and right-click on the selection, then select print (ensure the option "print selected text only" is checked), in order to remove the top navigation bar that can hide text on some pages.
How to read and navigate this document
There is a button "Table of contents" at the top left corner that will show you a navigation bar with where you are in the document and allowing you to quickly jump to any section.
Each section in the document can be linked to, but there is a bug that makes the first link copy not show the correct anchor, so try to just copy the link twice and the second one should include the correct HTML anchor.
For people with sighted non-24 or DSPD who just discovered they have this disorder, I recommend first starting in the Diagnosis and sleep diary section in Troubleshooting, where you will find all the informations you need to start documenting your sleep disorder and find a sleep medicine doctor experienced with circadian rhythm disorders who can help you with getting a formal diagnosis and accommodations that are necessary for school and work, given these are extremely debilitating disorders.
Afterwards or if you have a circadian rhythm disorder but you already are diagnosed or experienced with the clinical process and are looking for a therapy protocol to entrain the circadian rhythm period or phase advance the phase, you can proceed to read the therapy's protocol. I recommend to first read the Quick protocol, to get a nice fast introduction with the big ideas clearly laid out, then if you like what you are reading and want more details, you can proceed to read the Simplified protocol or even the Full protocol.
For people who do not have a circadian rhythm disorder but are suspecting they know someone with such a disorder, for caregiver and for clinicians, you may be mo interesting in reading the Introduction to the non24 disorder (which also covers other circadian rhythm disorders, notably the effects on health). Then you can continue reading on with the section on Zeitgebers to learn more about circadian rhythm science. For clinicians or the curious minded ones, the next sections afterwards review the scientific and medical literature on sleep to explain the various choices that led to the design of this therapeutic protocol, and some insights into what may help design future circadian rhythm therapies.
Preface
My name is Stephen Karl Larroque. I am a researcher in the neuroscience of consciousness. I was born with the non-24 circadian rhythm disorder, got diagnosed the first time in my twenties, and it started to become impossible to ignore in my thirties. Facing the sparsity of knowledge and effective treatments for this disorder, this prompted the start working on my own to find evidence-based approaches to improve the management of this disorder, which ultimately led to the VLiDACMel protocol presented below. The non-24 disorder affects my lineage over at least 2 generations of direct ancestors (so I am the 3rd), which strongly suggests that it is of genetic cause, and hence will likely affect my future children.
Although I was not trained to work on this specific field of circadian rhythm science, and hence claim no authority, I found myself in the exceptional circumstances of being trained in the scientific method and specifically in biomedical science, and being afflicted by a disorder I could study with this method and by building on my predecessor's works.
This protocol as is presented in this document is publihed with no guarantee of any kind of medical use nor of safety, please regard it simply as informational content. I publish it in the hopes that such a protocol with a review of the previous evidence in the theory of circadian rhythm and circadian rhythm disorders combined with the preliminary results from my self experiment, with a clear set of rules that optimized the therapy's efficacy during this self-experiment, may help in the design of future experiments by other researchers and lead to a faster investigation and finding of new therapeutic avenues for circadian rhythm disorders.
The current document has multiple levels of reading depending on how much you want to invest time in reading its content:
- a Simplified Protocol of VLiDACMel is presented as a set of rules for legibility. It contains the most crucial information, but lacks the subtleties of some parameters that can reduce the efficacy of the therapy.
- a Quick 2 minutes VLiDACMel protocol which is even more concise, for those who just want a quickstart to the core elements necessary for entrainment.
- For a more complete understanding of the protocol, the Full Protocol section outlines the entire protocol with links to the most important academic works that underlies it, as well as explain the various adjustment factors to optimize its efficacy.
- Then, the Troubleshooting section presents an in-depth review of the science of circadian rhythm and circadian rhythm disorders, with all the links to the academic sources, this section and its subsections are primarily addressed to scientists or the very curious reader as it gets much more technical and requires the use of jargon, although the author tried to summarize in layman terms the key points in the opening paragraph of each subsection, and keeping the jargon at the minimum required for accuracy.
Although this document is primarily aimed at sighted individuals with a non-24 sleep-wake circadian rhythm disorder, it is also mostly applicable for other circadian rhythm disorders such as DSPD and night shift work disorder given the same biological pathways are involved and hence the same therapies are likely applicable, see the sections "Adaptations of this protocol for other circadian rhythm disorders (DSPD, nightshift workers)" for more specific instructions for each disorder. The information contained herein may also be partially or fully applicable to insomnia, given the strong links with circadian rhythm disorders.
Introduction
This document describes a protocol for the entrainment of sighted non-24, which was designed using an evidence-based approach from a scrupulous examination of previous research, and self-experimentation to determine the factors influencing therapy's efficacy or circadian rhythm (dis)entrainment.
This section describes the starting point of this experiment and the methodology followed to derive the therapy's protocol and its results over the years. If you are interested in the reasons for its inception and some observations of its results on a single case, read on, otherwise if you just want to get started, skip to the next section.
Here are some sleep graphs of the early results from using this therapy in February to April 2020:
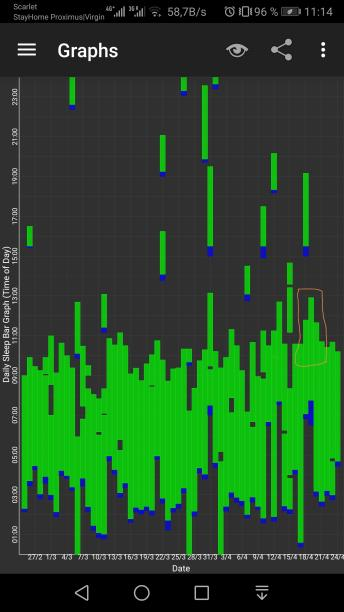
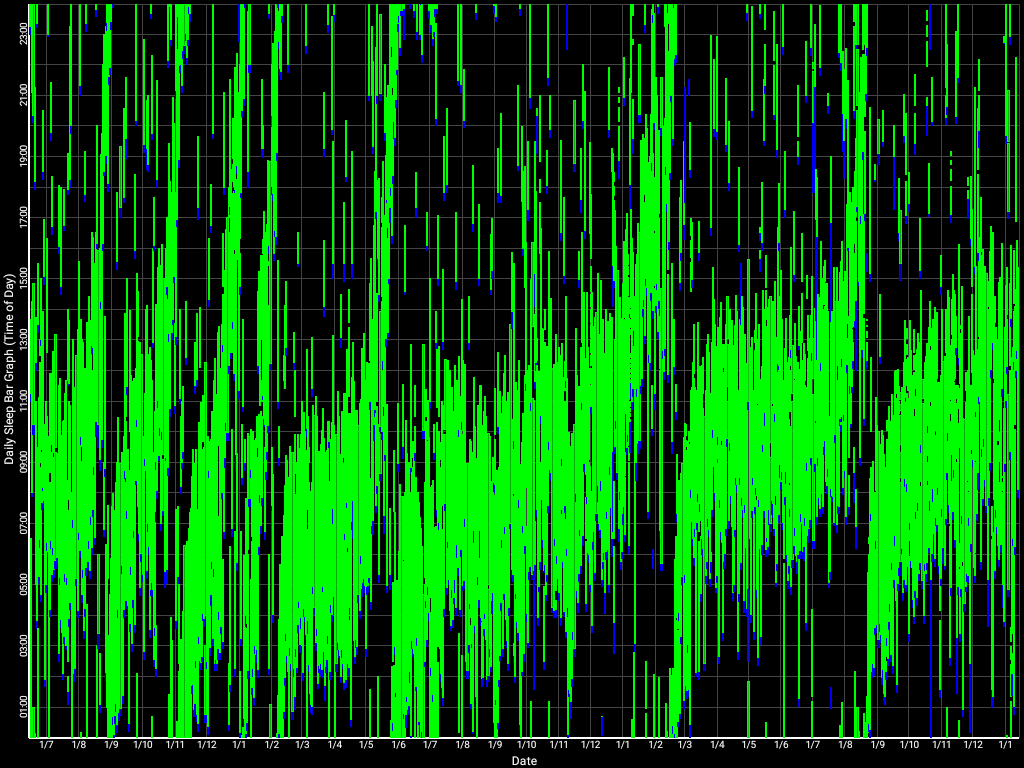
Zoomed out, here is my full sleep diary over 1 year, with the working therapy at the end:
The graph above shows a relatively stable entrainment over 2.5 months. As of December 2020, the author was entrained for 6 months, which is significantly much more than any published therapy protocol before. In comparison, all the author's previous attempts, most using published protocols, failed after 2 weeks to 1 month. The entrained (right part) of the graph was through the use of 1-2h of daily light therapy.
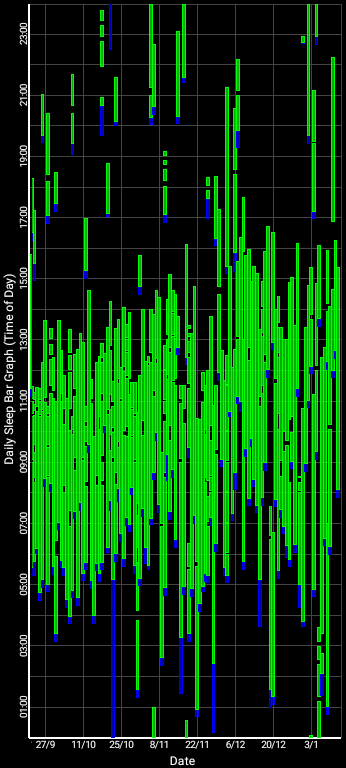
Here is the result with very long light therapy of more than 5h everyday for 10 days. This result is especially interesting as it was never observed before, with an inverse freerunning pattern: waking up 30 min earlier every day and up to 1h30 (one full ultradian cycle) earlier on the last day (which prompted the premature stopping of the self-experiment because this became uncontrollably too early):
This first experiment with very long light therapy (on the far right) was started on about the 3rd of June, after 1 more month of freerunning as can be seen during May. The very long light therapy resulted in a staggering reduction of circadian period tau under 24h at 23.5h on average and 22.5h the last day! Everything else was held constant (same melatonin intake time, same meal eating time, same daytime activities and environment), only light therapy duration was extended to reduce the circadian period under 24h. This very long light therapy experiment had to be stopped because of waking up way too early. See the Backward Cycling Therapy protocol below for more information.
Before this working therapy, the author tried: 1- melatonin only, 2- light therapy lamps + melatonin, 3- strict ketogenic diet only with timed meals (time-restricted feeding), 4- intermittent fasting (or even complete fasting for a few days), 5-carbs-only diet, and of course strict sleep hygiene, 6- chronotherapy, 7- chronotherapy with light therapy (ie, advancing light therapy 1h earlier than last target wake up time every 3 days). None of those therapies worked.
The latest working therapy protocol designed by the author, which worked for 2.5 months and reproduced for 4 months (still ongoing) at the time of this writing, is named VLiDACMel, which stands for:
- Very long Light therapy at wake-up (after minimal core body temperature), the most important tool of this therapy,
- Dark therapy in the evening,
- Avoid eating Carbohydrates when Melatonin is high in the blood,
- Take exogenous instant-release Melatonin timed before DLMO (measured via core body temperature or approximated via 3 days average of wake-up times).
- And always curate a sleep diary to assess changes in the circadian rhythm phase and properly adapt the treatments and to assess the conditions to optimally sleep restoratively.
Concisely, this therapy is founded on the following 3 points:
- Light exposure control: light therapy glasses Luminette at wake-up (or another light source of 500lux with optimized light angle to stimulate ipRGCs in the nasal retinal hemiregion) to phase advance and hence reduce circadian period (biological day duration). The exposure must be "very long", so use for 2-5h from wake up using relatively low intensity bright light of 500 lux. Exposure duration to light therapy can be modulated to fine-tune the wake-up time (ie, with longer exposure, the participant will wake up earlier and earlier), and this modulation is the primary way this therapy allows for flexible readjustment of the sleep schedule on a daily basis without having to freerun a full cycle again. Light therapy must always be combined with dark therapy in the evening (ie, avoidance of light exposure to avoid unwanted phase delay), by using blue light filters and dimming the brightness of any light emitting device/lamps (or use blue blocker sunglasses if environmental light sources cannot be controlled).
- Sleep induction and consolidation by melatonin: use melatonin instant release pills, taken at a time calculated relatively to the individual's DLMO (not the bedtime), and avoidance of wakefulness inducing drugs such as caffeine. This both consolidates sleep (ie, ensures you sleep your full night and not wake up too early or in the middle of your night causing unwanted sleep deprivation) and phase advance (allow to sleep and wake up earlier). The effect of melatonin is additive with light therapy. This step can be temporary, as melatonin can be dropped later on if the user feels too drowsy during days after melatonin, but it's good to do at least for a few weeks at first to magnify the sleepiness feeling so that the user re-learns to recognize it.
- Food timing and diet composition control: never eat after melatonin intake and reduce/minimize carbohydrates intake. In the experiment above, I was half the time under a strict ketogenic diet, and half under a balanced diet including carbs. The ketogenic diet is not necessary, but it can help at first before phasing it out.
Update on current findings of efficacy for this therapy:
The first threshold to consider any treatment potentially effective was set to 1 month of entrainment, entrainment being defined as an average wake-up time under 1 ultradian cycle (a time window of 1.5-2h), such as wake up between 9am and 11am. A secondary threshold to consider a treatment really effective is set to at least 6 months of entrainment, as evidenced by circadian rhythm measures (eg, core body temperature, not necessarily the sleep-wake patterns). A third threshold to consider a treatment effective and robust is set to at least 1 year of continuous entrainment, as to ensure the treatment allows robust entrainment despite seasonal variations in sunlight exposure and ambient temperature (ie, robustness against environmental variability).
As of April 2021, very long (5h-9h) bright light therapy plus dark therapy achieved threshold 1, and is investigated for the 2nd and 3rd thresholds. Melatonin alone passed the 1st threshold but not the 2nd.
As of August 2021, since late February 2021 the very long (5-9h) bright light therapy plus dark therapy protocol (ie, updated VLiDACMel protocol, with melatonin being optional and not used during this period) achieved thresholds 1 and 2 but failed the 3rd threshold. Indeed, the entrainment (ie, stabilized/frozen circadian phase) worked for ~7 months, whereas with no therapy entrainment (to sunlight) lasts 1 month maximum for the author. This is significantly better than no therapy, but still the entrainment is lost once or twice a year. It should be noted that the entrainment is not lost at once, but rather there is a slight remaining daily shift that accumulates over time and so the wake up time changed from 8am in late February 2021 to 4pm in late July 2021, so that the circadian phase was now akin to a DSPD pattern. The author then decided to freerun to become again more quickly in phase with the day-night cycle. This is still a failure since the entrainment couldn't be maintained fully stable. A potential improvement to this therapy may be by adjuncting a hyper photosensitizing drug, this will be explored in the future (there is a section about these drugs below in the Troubleshooting section).
In summary, the current state (as of August 2021) of efficacy of the VLiDACMel therapy is a maintenance of entrainment for 6-7 months for the author (compared to 1 month without). Anecdotal reports from other users suggest that some have obtained (much) better results, while others have reported worse (in general individuals with comorbidities that prevent the continuous use of light therapy or melatonin, such as RLS and PLMD). Elders seem to be more responsive (often, melatonin alone is sufficient for their entrainment) than younger individuals.
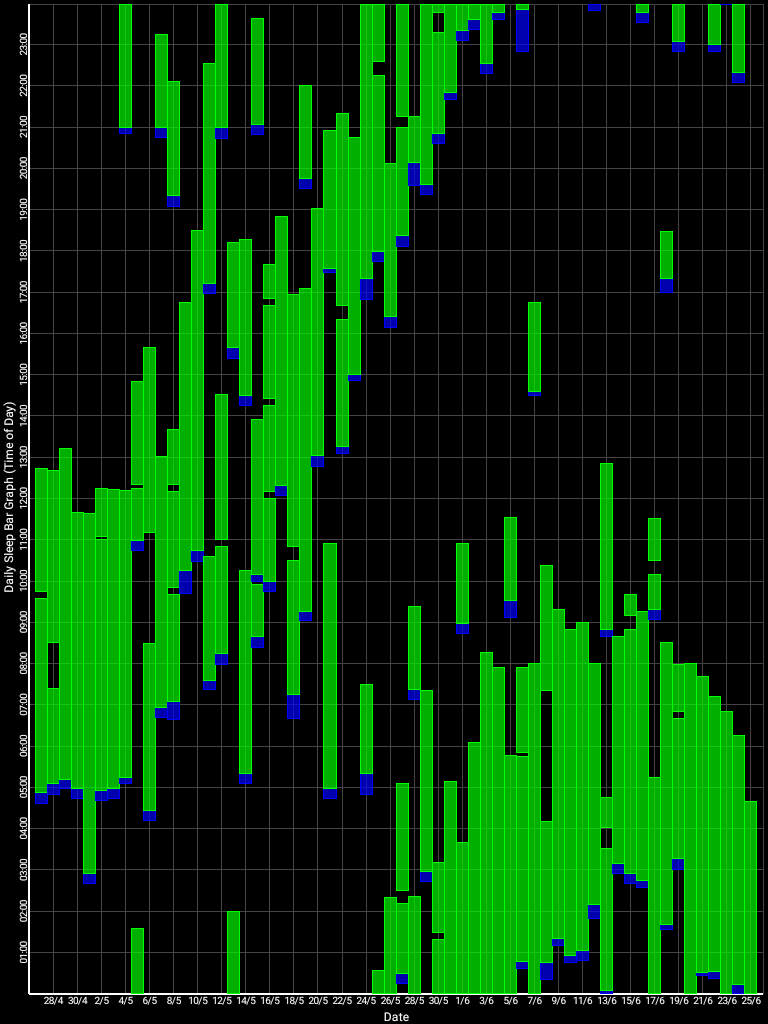
As of January 2022, despite more than a month (during mid October to November) without any therapy (because the light therapy glasses had to be sent back to the producer for exchange for an upgraded model), 2-4h of daily therapy allowed to drastically reduce the freerunning speed, but did not allow for a full freeze, although it is very close. Indeed, between end of September 2021 and mid-January 2022, the wake up time shifted from 1pm to 4pm, which is only a 3h phase delay under 3.5 months, in other words a (3*60)/(30*3.5) = 1.71min/day of daily freerunning delay. Compared to the author's natural daily freerunning delay of 20 to 30min/day, this is a 91% to 94% reduction in freerunning speed! And it is worth accounting for the fact that most of this phase delay happened during the period without any therapy, so this average daily freerunning delay is misleading as it was in reality mostly frozen before and after this period without therapy, as shown in the sleep diary below:
This represents the first successful significant slowdown, almost frozen, freerunning of the author's circadian rhythm during winter. Although this was still partially unsatisfactory due to the less than ideal absolute timing of the circadian night, as it was slowed down in an already delayed phase, it is still a significant improvement over having no control when there is no light therapy. This period of winter 2021 represents the first time the author could avoid a full freerunning loop. Worth noting is that this was achieved without being exposed to sunlight the vast majority of the time, with the author waking up past sunset on most days, under which conditions it is a considerable achievement to mainain a relatively entrained circadian rhythm and positive mood. Furthermore, this allowed the author to experience the mood modulation effects of light therapy (or the lack thereof), which prompted the author to modify this document to emphasize the mood modulation effect as being as important as the circadian phase shifting effect.
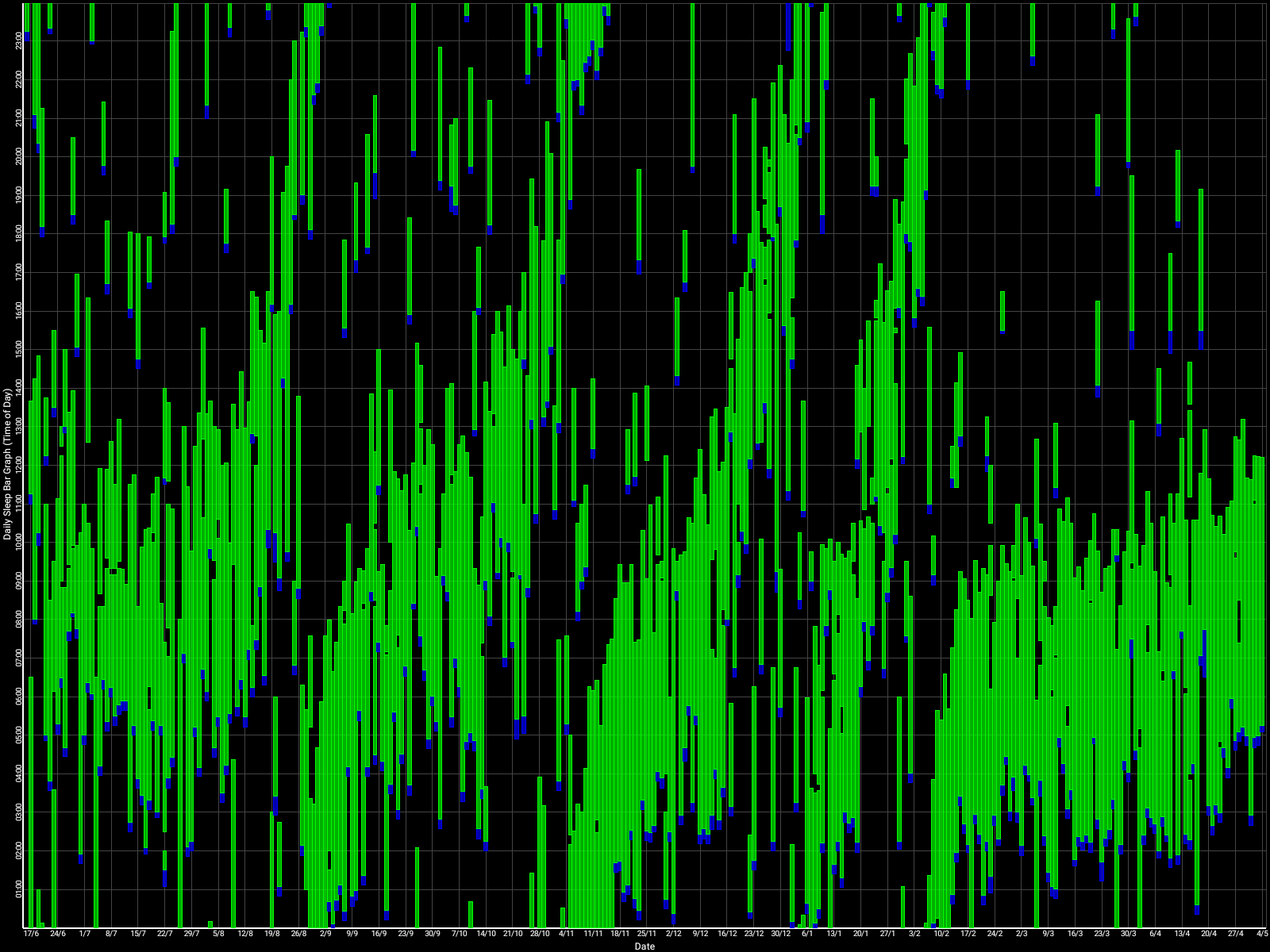
Here is a wide picture including all of the 2021 year, which includes a forced freerunning in August 2021 to realign the circadian night with a more favorable timing in preparation for an important personal event (and this method succeeded):
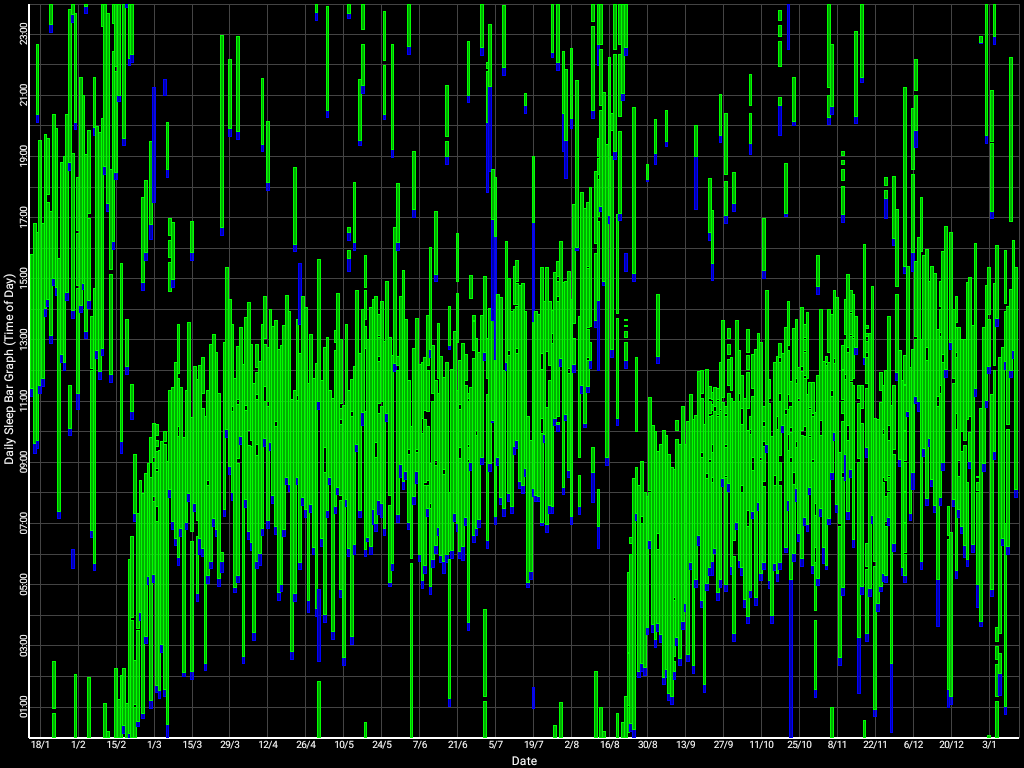
Here is the whole sleep diary over several years since the start of this whole experiment, spanning from July 2019 to January 2022, starting with no therapy at the far left, then attempting melatonin therapy with limited success, to finally end up with very long light therapy for the last three cycles (starting from 1/6/2020, at the middle of the chart):
Note that during 2021, most of the chaotic sleep that can be observed were caused by unavoidable appointments or sleep disruptors (eg, environmental noises). The effect of the latter were greatly disrupted since starting using the Hibermate earmuffs and sleep mask in December 2021, which greatly improved sleep quality and reduced sleep fragmentations.
As of August 2022, light therapy had to be mostly stopped since January 2022, being used about once every 2 weeks (or more precisely 3 weeks every 3 other weeks) due to duties and appointments. This allowed to observe that my circadian rhythm reverted under just a month or so to its original state, with a freerunning period of about 24.3h (20-30min delay per day). This allows us to infer two things:
- Despite use of light therapy daily over a year, effects aren't sustained over the long term: the beneficial effects of light therapy (and the other items in the VLiDACMel protocol) are only sustained as long as the therapy is used, hence, it is a maintenance therapy, not a curative therapy;
- VLiDACMel and light therapy are arguably safe to experiment with, as even using circadian waveform shaping (an extreme manipulation protocol) did not result in any long-term effects, discontinuation of the therapy is sufficient to recover the original circadian rhythm state after a short time (a few weeks).
(TODO: add sleep diary figure)
Hence, after 2.5 years of (self-)experimentation, we can deduce the benefits and limitations of the VLiDACMel protocol: it is likely a safe protocol, which can reduce by more than 90% the daily freerunning delay, but it cannot freeze/eliminate fully the delay, and the effects are sustained only as long as the protocol is used (although keep in mind that missing one or two days is not an issue since light therapy has an inertia period where effects are still sustained despite discontinuation, but not over weeks). Further research should test this protocol over a bigger sample to confirm these findings, as this would indeed be the first known effective maintenance therapy for the sighted non-24 disorder with the potential to significantly improve the quality of life and social integration of this population, although unfortunately not a curative treatment and not eliminating fully the impairments and handicaps caused by this disorder.
Two-minutes quickstart version of the VLiDACMel therapy protocol
A quickstart for those who don't have the time to read or don't need the details and references. Other versions with more precisions are available below.
Target populations: individuals with a circadian rhythm disorder, especially sighted non-24 for whom this protocol is optimized for, with undamaged ipRGC retinal cells as evidenced by preserved pupillary light reflex (PLR), since the ipRGC cells regulate both the PLR and circadian manipulation and the PLR was found to be a reliable discriminator to detect DSPD and especially sighted non-24, strongly suggesting that for at least some people with sighted non-24 or DSPD, one root cause is that they are less sensitive to bright light. Thus, it is arguably likely that this therapy should work for any individual with a preserved pupillary light reflex. Hence, this protocol should work for individuals with a sighted non-24 disorder, some blind non-24 (those sensitive to relative coordination to sunlight), and those with a delayed sleep-phase disorder (DSPD) with some slight modifications as indicated in the "Adaptations for DSPD" section. With further adaptations, as indicated in the relative sections, the protocol should also work for other circadian rhythm disorders (night shift work disorder, ASPD, etc). If you don't know what these disorders are or if you are unsure if you are affected, please read in the "Diagnosis" section (inside the Troubleshooting part) the instructions to monitor your sleep-wake patterns using a sleep diary or a core body temperature sensor at home, or via salivary melatonin sampling in a hospital.
Improvements you can expect: if you have sighted non-24 or blind non-24 with preserved pupillary light reflex, you can expect a drastic slowdown of your freerunning period proportional to the duration of bright light therapy. Those with a shorter circadian period, ie, closer to 24h, should see most benefits, as they can become quasi entrained to 24h, whereas it is expected to be more difficult to entrain for individuals with longer circadian periods, with those with a >26h circadian period obtaining little benefits (they will still get a slowdown, but it may not be a clinical improvement in practice for them). For DSPD, you can expect to consistently sleep and wake up a few hours earlier, proportionally to the duration of bright ligth exposure. For ASPD, you can expect to consistently sleep and wake up later. There can be minor side effects such as additional fatigue or dizziness, but no severe adverse effect, and all effects should disappear after discontinuing therapy.
Who should NOT use the therapy (contra-indications): If you have an ocular illness, ask your doctor before if you can use light therapy, otherwise melatonin can still be used. If after starting the therapy, motor dysfunctions appear or are worsened (eg, restless legs) then stop the whole therapy (both light therapy and melatonin) right now, and talk to your doctor about getting tested for a motor disoder (PLMD, RLS) or ADHD. If on the other hand you can sustain being under the sunlight, you will probably be fine. If you don't have a contra-indication to sunlight or light therapy exposure, then apriori you can safely use certified light therapy devices such as Luminette as they are medically screened and validated against FDA or european health safety norms, and the effect of light therapy on the circadian rhythm is always reversible under a few weeks of discontinuation (there is unfortunately no circadian plasticity contrary to what was presumed in the past).
Therapy's main steps, in chronological order of when to do them in the day:
- Find your circadian night. It's when you sleep more than 6h with little to no wake ups in the middle of the night (no fragmentation). If you have non-24, freerun until your circadian night happens close to the time you want to freeze your sleep-wake schedule. Naps are allowed. Write a sleep diary all the time to monitor your sleep patterns, and bring it to a circadian rhythm disorder specialist to get diagnosed and accommodations. For diagnosis, 2-4 weeks of sleep diary data is enough, but sleep diaries are the best tool currently available to self-manage circadian rhythm disorders, hence you should continue writing them all the time. Using an electronic sleep diary can be easier over the long term (Sleepmeter Free on Android, and its widget, or a digital spreadsheet).
- On natural (unrestricted) wake up, use light therapy glasses such as Luminette v3 for 2-8h using the lowest or medium light intensity setting. No alarm clocks, just wake up naturally. Don't use light therapy lamps, only glasses. For the first light therapy session of the day, keep your eyes closed for 30s while turning the lights on, to reduce the likelihood of headaches or dizziness due to sudden cortisol secretion caused by sudden bright light, then slowly open your eyes. Light therapy is by far the most important element of this therapy. Light therapy has two major effects: it advances earlier your circadian rhythm phase (circadian shifting effect), making you wake up earlier, and it improves your energy levels, mood and productivity (antidepressant effect). The antidepressant effect is as crucial as the phase advance effect, as it allows to remain motivated and enjoy activities, whether or not the circadian phase advance effect is sufficient for the individual to fully freeze the phase, the antidepressant effect is well worth by itself. Very long outdoors sunlight exposure is even more effective than any artificial light therapy device, but it's difficult to administer properly and constantly: on unfavorable weather (rain, snow) it is highly uncomfortable and difficult to time as sunlight must be blocked (even indoors) before your minimal core body temperature point, and sunlight cannot be used past sunset, which can be a big issue during winter in some regions of the world with particularly shorter days.
- Side-note: Light therapy is most effective when started in the circadian morning. The above step assumes that even if you don't know when your circadian morning is, by constantly using light therapy at wake up, at some point one of your wake ups will happen in your circadian morning, and then light therapy will "freeze" your wake up time in place. This strategy has more chances to work because the wake up time (sleep offset) is much more tightly coupled with the circadian rhythm than the fall asleep time (sleep onset) (for references, see the section Seasonal variations in zeitgebers or why Bedtime and wake up time are independent (dual-oscillator model)). But you can also choose to detect your circadian morning beforehand to increase the odds the therapy works and minimize the risks of unwanted effects (ie, faster delay when exposed in the circadian evening/night). To know when is your circadian morning, try to use a sleep diary such as Sleepmeter Free, and infer approximately using the following algorithm: wait for a long sleep session, and then start light therapy at the wake up from this long sleep session just after and the following days. How to know what is a long sleep session for you? For the vast majority of adults, the average night sleep needs per 24h is 7-9h of sleep, although some adults need more (10h or more) or less (very rare, usually people who think they are short sleepers are in fact sleep deprived), but this duration can only be achieved when sleeping in phase with the circadian night or under extreme sleep pression built from severe sleep deprivation. Any sleep duration less than that should be considered an out of phase sleep, and hence not to be counted. Remember sleep works by discrete increments, called ultradian cycles, that last ~2h, hence short sleep sessions can last at most the maximum sleep you can get for your age minus one or two ultradian cycle (eg, for an adult having average sleep needs of 7-9h, they can sleep out of phase a maximum of 4-6h, these short sleep sessions should be discarded). Once you experience a sleep session long enough to fully fill your sleep needs (eg, 7-9h), start the therapy on wake up, and redo therapy at the same time the following days. You should notice your wake up time should not move or at least not as fast as before after a few days of therapy, if the timing was correct. If not, wait for another long sleep session to retry, as sometimes a long sleep session may be caused by extreme sleep pressure instead of circadian alignment, and hence be a false positive. Keep in mind that the disadvantage of the inference method is that you can wait for weeks before a long sleep session happens depending on your circumstances (especially if you restrict your sleep because of duties), whereas using a core body temperature sensor allows to know the circadian profile under just 48h. Skin temperature sensors do not work for this purpose, unless MAYBE wrist skin temperature on the radial artery with an adequately totally enclosing cotton wrist band.
- WARNING: do NOT use bright light therapy while driving, as you need your full vision for your safety!
- Do not use cheap light therapy lamps, such as Beurer TL30: they don't emit enough blue light and they require you to be way too close (nose on the lamp), so they are in practice ineffective. Check their manual, it should specify the distance to get the promised quantity of lux, and you will find you need to be literally the nose on the lamp for it to be effective, which is impractical and unrealistic given how long the therapy needs to be done to be effective. Expensive light therapy lamps can be effective but they are more cumbersome and much more expensive than light therapy glasses of similar melanopic intensity (ie, intensity of bright light at your eyes level), but some users with non24 report even greater efficacy with them, or better, in combination with light therapy glasses.
- Also, bright light therapy is unnecessary when exposed to outdoors sunlight, even if cloudy or behind a glass (eg, driving), it counts as additional bright light therapy (ie, UVs filtering does NOT matter, only the quantity of Lux at eye level does). But as a rule of thumb, consider artificial bright light therapy using light therapy glasses is always necessary indoors (this is not 100% accurate as some indoors conditions can be sufficient, but it is highly variable with just head orientation, hence this rule of thumb - use a lux sensor app on your smartphone to see how much lux varies indoors), and optional or unnecessary outdoors when there is sunlight exposure (even if indirect or very cloudy).
- If you are short on money and don't want to spend it on light therapy glasses unless you know it's going to work for you: you can try sunlight therapy instead for a few weeks, which consists in going outside (don't stay in a building, you need to get outdoors) to get exposed to sunlight at natural wake up, and stay outdoors for at least 1-2h, every single day. This is free, and you will get similar benefits to light therapy glasses (sunlight is the brightest lamp!), but it's very cumbersome: you need to do that every single day even when the weather is rainy or snowy, and even when it's cold. Hence, to make it easier, try this preferably during the warmer months, such as summer in northern countries. If your circadian rhythm stabilizes (freerunning slows down for non24 or phase advance = waking up earlier naturally for DSPD), then consider buying light therapy glasses, now that you know it works for you. This is not just for comfort, but for efficacy: during winter months, sunlight cannot be used, since it rises up too late (eg, if you want to wake up at 7-8am but dawn happens only at 9-10am then this won't work, you need artificial light therapy exposure before 7-8am to lock in your circadian phase).
- How to assess if light therapy is safe for you? The general rule is: if you have an eye condition that makes you limit your exposure to sunlight, then you should also avoid artificial light therapy. But if you can get exposed to sunlight without any particular limit, there is no reason to expect artificial light therapy to be any more dangerous, as it is magnitudes less intense. But I'm not an ophtalmologist, so please check with a professional.
- Tip: Keep the light therapy device on your bed table, so that you can easily reach for it first thing in the morning while still in bed.
- During winter, should you use artificial light therapy longer than sunlight exposure (eg, if sunset is at 4pm, should you continue past that)? It depends on how long the exposure you need to be entrained. If you need 5h of exposure minimum, then this is achievable before 4pm by starting at the latest at 11am. So indeed you may use artificial light therapy longer during summer than during winter, but during winter you may have to use artificial light therapy past sunset if you need longer exposure.
- 3h-5h before your natural fall asleep time (this should equal 12-15h before your last wake up time) is usually the start of the circadian evening, start dark therapy: dim to the minimum or switch off lamps and screens, and filter blue light using blue light filter apps and prefer red light lamps such as Yeelight 1S. Use a lux meter app on a smartphone to confirm, you should see 0 lux at best, or at least less than 10 lux. If you can't modify your environmental lights, wear orange or red tinted blue blocker glasses (use UVEX or red-tinted laser safety glasses that filter blue and green lights). Avoid eating or consuming carbohydrated meals or drinks past this time. Never consume alcohol, at any time, as it majorly disrupts the circadian rhythm. Avoid caffeine in the circadian evening, and also in the day if you are not an avid user, as effects can carry over 48h.
- Pro tip: you are doing dark therapy right if your eyes stay accommodated to darkness (ie, dilated pupils letting more light rays entering in) all the time during your circadian evening.
- Pro tip2: even a short burst of artificial bright light during your circadian evening is enough to modify your circadian phase durably, so equip your environment with smart lighting such as RGB LED lamps programmable with an app (such as Yeelight), or dim portable led lamps with automatic movement detection, so that you can go to the bathroom, kitchen, toilets without getting exposed to bright lights.
- During the night, eliminate sources of noise and sleep interruptions. Tools such as black silk eye masks, earplugs, and very-low-profile earmuffs such as the Hibermate headset, white/brown noise machines or apps such as Noice can allow to greatly reduce the impact of environmental noise on your sleep. The Hibermate combined with in-ear plugs is a highly recommended aid to reduce sleep fragmentations due to sleep disruptions such as noise and unwanted light exposure. Bright light during sleep directly damages cardiometabolic health.
- The 3h-5h rule is not really precise, it should work for most people but the timing of the start of the circadian evening, but there is actually a wide window of variability between different people, so if this does not work, feel free to start dark therapy and melatonin earlier or later until you find the sweet spot, effect should happen the same night or the night after at worst, so feedback is near instantaneous and makes this an easy to iterate process.
- If you cannot fall asleep:
- If you tend to watch TV or a computer screen as half of americans do in 2023, prefer instead to use a smartphone: with its smaller screen, and settings set to minimum brightness and a reddish blue light filter app, such properly configured smartphones screens will have minimal impact on the circadian rhythm (you can check with another smartphone with a lux meter app that your eyes get 0-1 lux from the smartphone screen).
- If it takes really too long (40min), you can wake up and do interruptible activities so that you don't lose all your time, but prioritize going back to bed at anytime at the slightest feeling of tiredness.
- Avoid any bright light exposure whatever you do until you fall asleep. Bright light during the circadian evening or night will inhibit melatonin and phase delay your circadian phase.
- Optional: You can take melatonin at the same time you start dark therapy, dosage 0.3mg up to 3mg (or sometimes more for some people - melatonin has been tested safe for all ages including toddlers with dosages up to 10mg for infants and 6600mg for adults), instant release and in blister packs. For some people, taking melatonin 12h before the natural wake up time is a good starting point, the effects should work the very same night, and it's possible to then modify the timing (earlier or later) each day by increments of 30min until the sweet spot of maximal effect is found. If feeling drowsy during days after melatonin intake, you can reduce melatonin dosage or stop it once entrainment to light therapy works and only if you do not consume any stimulant (eg, caffeine in coffee or tea or energy drinks, ADHD medications, etc).
- Note: Melatonin and bright light therapy work on distinct (so-called "EM") circadian oscillators: melatonin affects directly the sleep onset time, whereas bright light affects the wake up time. To simplify, melatonin kickstarts the circadian night, whereas bright light kickstart the circadian day. Bright light also affects the sleep onset time after a few days of "catching up" with the new wake up time, whereas melatonin has almost no effect on the wake up time. Hence, melatonin is only optional for circadian entrainment, and mainly serves to accelerate phase advancing of sleep onset or amplify sleep drowsiness, whereas bright light is mandatory.
- Melatonin and other compounds will degrade with exposure to light, humidity or hot temperature. To slow down degradation, place the melatonin contener inside a resealable plastic bags (or tie an air-tight knot with the plastic bag opening), and place the bag in an obscure place at ambient, normal temperature. Avoid liquid melatonin, as it degrades under a few days after opening, prefer solid form tablets. Prefer pure melatonin products, with no other compounds (eg, valeriane) as they will have higher quality control standards (ie, dosage will more likely be as labelled).
- Maintenance: Once entrainment is achieved, the treatment must be continued as-is with the parameters (ie, light therapy timing and duration, melatonin timing and dosage, dark therapy setup, etc) you found effective for maintenance of benefits. Indeed, the therapy is effective only as long as it is used, otherwise effects are lost under a few weeks, but the therapy can always be restarted later. Of course some adjustments can be done on a day-to-day basis: if waking up later, you can do light therapy longer the next day ; if waking up too early, you can start light therapy later at the usual time you found effective for entrainment to avoid waking up earlier and earlier ; melatonin and dark therapy should usually be started always at the same time but can be advanced to ensure you do not miss the window, etc. Do not expect a totally immutable circadian phase, this is not a mechanical clock: even when fully entrained, sleep remains a biological process and hence naturally noisy, so it is likely perfectly normal to observe over time natural variations in the fall asleep time and wake up time under a window of one sleep cycle (ie, 1h30-2h00 for adults, 1h-1h30 for children). Note also that between seasons it is normal to sleep and wake up later or earlier depending on the varying sunrise and sunset times.
- Previously, it was here noted that "the therapy could not be 100% effective and freeze totally in place the circadian rhythm due to uncontrollable environmental factors", but the author achieved in 2023-2024 a highly robust circadian phase and set at a time that cannot be caused by environment (eg, consistently waking up 2h earlier than sunrise during winter, the same absolute time defined in autumn and even before DST change) and only varying under one sleep cycle window, and most often under a window less than 30min. However, this requires extreme mastery of the protocol and of exposure to environmental factors such as sunlight, hence for less experienced users, expect to get misaligned from time to time: for individuals with non-24, simply discontinue therapy temporarily to freerun a few weeks until the ideal time is reached again; for DSPD, increase the exposure duration, or discontinue therapy temporarily to find where your circadian phase currently is, to re-time light therapy optimally after the minimum core body temperature, or use a core body temperature sensor to find it right away.
Most helpful tools, used on a daily basis by the document's author:
- Sleep diary (Sleepmeter Free on Android, and its widget for an electronic diary, or the AASM template for a paper diary, 2021 updated version here, or there is a digital spreadsheet , or the plees-tracker open-source app on Android)
- On Android 14+, SleepMeter cannot normally be installed, but it is possible to force installation (thanks to eatnerdsgetshredded for the tip!): enable USB debugging on your phone (you need to enable Developer Mode, no root needed), then connect your phone to a PC with USB debugging, and then you can use the following ADB command: `adb install --bypass-low-target-sdk-block app.apk`
- Luminette v3 (for light therapy)
- Hibermate (includes eyemask, to eliminate sleep disruptions)
- Earplugs (to eliminate sleep disruptions)
- Core body temperature or melatonin sampling (for circadian phase monitoring)
- Some labs offer direct circadian rhythm estimation through core body temperature (usually via a rectal probe, sometimes with a 3M SpotOn or another zero-heat-flux thermometer) or via melatonin sampling. This has the great advantage of allowing to observe the circadian rhythm despite masking effects, such as social obligations (eg, having to set up an alarm clock to go to work or school, then the sleep diary won't allow to robustly estimate the circadian rhythm profile and parameters, but the core body temperature or melatonin sampling can).
- Don't try to use skin temperature sensors to derive your circadian profile. They may be much cheaper, but skin temperature does not reflect the circadian rhythm since humans are homeothermic animals, so only core (internal) body temperature is self-regulated, whereas skin temperature is affected by ambient temperature. There is an exception for wrist skin temperature but this requires a very specific kind of setup and analysis.
- Yeelight 1S RGB light bulb (automatic dark therapy)
- Blue blocker / red tinted laser safety glasses (for portable dark therapy)
- Noice white noise machine app on Android, open-source and free. Here is a preset the current document's author made for newborns but which also works for adults: InfantWombSim for Noice v1.3.3, WombSimulator for Noice v2. A non-free equivalent software for iOS is Dark Noise, but the source-code is available.
- The book Sleep Misfits: The reality of Delayed Sleep Phase Syndrome & Non-24 by Sally Cat is highly recommended, being the only book currently written compiling the experience specific to patients living with non-24 and DSPD handicaps. Reviews note that this book can help in validating one's own experience, and help with acceptance and coping with the handicap.
- Optional, and only when freerunning and NOT using bright light therapy: Long release caffeine tablets (Lucovitaal 200mg per tablet). Just tike one tablet (200mg) during the circadian morning, usually at natural wake-up. The long-release form ensures a constant inhibition of adhenosine and hence of sleep pressure, and the tablet form avoids the diuretic effect of hot liquid coffee. The long release tablet form makes caffeine a pharmacological compound with more stable pharmacodynamic properties, more reproducible and dosable. However, the same usual issue with caffeine are also present here: caffeine tends to delay the circadian rhythm, and for slow caffeine metabolizers (this is defined genetically), then it can stay in the bloodstream too long and carry over into the night and next day. In practice, this should only be used while freerunning (when phase delays are not an issue). When using both light therapy and caffeine, caffeine can often cause a weird insomnia the night just after intake, with a wide sleep fragmentation gap during the circadian night sleep session, that resorbs after discontinuation of either light therapy (but hence causing freerunning resuming), or long-release caffeine. Nevertheless, it can be a great tool to ensure being able to perform for a whole day, even under sleep deprivation, and reduce the likelihood of random wakefulness drowsiness bouts that is intrinsic to non-24, but it can only be used as a "wildcard" for a single day, with a necessary rest day the next day, it certainly cannot be used on a daily basis (except if freerunning). In that, it can be seen as a non-prescription alternative to modafinil, another wakefulness-promoter that was found to produce effects of similar magnitude to caffeine.
Variants of the protocol:
- Phase-delay bright light therapy, which is to get exposed to bright light such as Luminette in the circadian evening and night (ie, start 3-5h before circadian night and up to 4h after the start of the circadian night but no later than 4h before the circadian wake up time) can be used only for individuals with non-24 (not DSPD, there is a theoretical risk to turn into non-24!) to speed up the circadian freerunning by 2x up to 3x. This can be used as a complementary therapy to VLiDACMel, when the circadian phase slipped out too late due to the residual uncorrectable freerunning, to realign one's circadian phase with the day-night cycle, and then restart the VLiDACMel therapy.
- If you need to restrict your sleep (eg, for work or other circumstances), consider adopting a biphasic sleep pattern, which is to sleep twice under your circadian period: once during the latter half of your circadian night, and once during the circadian siesta which is about 12-15h after the start of the circadian night. For example, if your circadian night is currently at 2am-10am, then you can try to have a nap between 2pm-5pm, and then sleep the latter half of your circadian night between 6am-10am (you won't be able to sleep the first half because of a lack of sleep pressure due to the nap). You will usually sleep less during the nap than during your circadian night. This works because for most purposes (health, cognitive performance), what matters most is the total sum of sleep durations over one circadian period. This strategy is commonly used by successfully adapted shift workers. It however does not prevent freerunning, but it can be combined with VLiDACMel to slow it down.
Simplified protocol
This is a simplified version of the full protocol presented as a straigh-to-the-point set of rules, without the rationale nor the explanations, which may be easier to present the therapy to patients. See the Full Protocol and the Troubleshooting sections below for more detailed explanations and references.
Jargon
- phase shift: earlier or later shift in the timing of the circadian rhythm and hence of the natural wake up and bedtime, with phase advance being earlier and phase delay later
- biological or circadian night/day: relative day or night in phase with the individual's circadian rhythm (respectively low period and high period - core body temperature reflects the same trends). The biological/circadian night is the ideal time for the individual to get a long and reparative sleep, and inversely it will be very difficult to sleep during the biological/circadian day.
Who can use this therapy?
- Target populations: individuals with a circadian rhythm disorder, especially sighted non-24 for whom this protocol is optimized for, with undamaged ipRGC retinal cells as evidenced by preserved pupillary light reflex (PLR), since the ipRGC cells regulate both the PLR and circadian manipulation and the PLR was found to be a reliable discriminator to detect DSPD. Thus, it is arguably likely that this therapy should work for any individual with a preserved pupillary light reflex. Hence, this protocol should work for individuals with a sighted non-24 disorder, some blind non-24 (those sensitive to relative coordination to sunlight), and those with a delayed sleep-phase disorder (DSPD) with some slight modifications as indicated in the "Adaptations for DSPD" section. With further adaptations, as indicated in the relative sections, the protocol should also work for other circadian rhythm disorders (night shift work disorder, ASPD, etc). If you don't know what these disorders are or if you are unsure if you are affected, please read in the "Diagnosis" section (inside the Troubleshooting part) the instructions to monitor your sleep-wake patterns using a sleep diary or a core body temperature sensor at home, or via salivary melatonin sampling in a hospital.
- Contra-indications: If you have an ocular illness, ask your doctor before if you can use light therapy, otherwise melatonin can still be used. If after starting the therapy, motor dysfunctions appear or are worsened (eg, restless legs) then stop the whole therapy (both light therapy and melatonin) right now, and talk to your doctor about getting tested for a motor disoder (PLMD, RLS) or ADHD. If on the other hand you can sustain being under the sunlight, you will probably be fine. If you don't have a contra-indication to sunlight or light therapy exposure, then apriori you can safely use certified light therapy devices such as Luminette as they are medically screened and validated against FDA or european health safety norms, and the effect of light therapy on the circadian rhythm is always reversible under a few weeks of discontinuation (there is unfortunately no circadian plasticity contrary to what was presumed in the past).
What results can be expected
- Freerunning should stop or get slower for those with a long circadian period (ie, >26h). This means there won't be "night-walking" phases anymore, where you sleep during the day and are awake the whole night.
- However, this therapy does not guarantee a constant sleep-wake schedule, which will likely remain highly variable from day-to-day in a 6h timeframe: one day you can wake up at 1pm, the next at 11am, the next at 2pm, the next at 9am (and feel sleepy at according times).
- Longer sleep on average than without the therapy, and with a higher quality and less to no interruptions.
- However, biphasic sleep (ie, "weird insomnia") and irresistible naps will still occur somewhat regularly, mostly at random.
- Improved overall health, especially cognitive performance and mood.
- However, mood swings and cognitive drops (ie, "zombie-like" states) will still happen somewhat regularly, mainly dependent on how long you slept earlier.
Preparations
- Start to write a sleep diary (template) with the fall asleep time and wake up time, everyday, including naps. Continue to curate this sleep diary all the time, this is the most essential tool to self-monitor the circadian rhythm and better manage the disorder. Digital sleep diary such as Sleepmeter Free on Android are recommended as they also generate sleep charts, which are easier to monitor and diagnose by doctors.
- Try to find where is your circadian night (ie, when you sleep a long session of a duration of about the optimal amount of sleep you need ± one ultradian cycle, eg, on average for humans this would be 6-9h of sleep).
- If you want to plan future schedules based on your past sleep patterns, prefer to use the wake up time as a reference, as it is a reliable estimator of the circadian rhythm, whereas bedtime is not.
- Before starting the therapy: Freerun (ie, sleep when naturally tired and wake up without an alarm clock) until you wake up close to your ideal wake up time. The therapy will then freeze in place the circadian rhythm and sleep-wake schedule. Nap as much as needed to reduce as much sleep deprivation as possible, this improves the therapy's efficacy. This applies only for non-24, other circadian rhythm disorders such as DSPD should not freerun.
Start the VLiDACMel therapy
- At natural wake-up (biological morning):
- Avoid alarm clocks, allow yourself to wake up naturally.
- Waking up naturally is necessary to observe your natural sleep-wake patterns and also to time correctly light therapy, as it needs to be used after the minimum core body temperature point (CBTmin), otherwise it can cause the opposite (delaying) effect, and the natural wake up time is strongly coupled with your circadian rhythm (but not the fall asleep time).
- Avoid pulling all-nighters, they do not change the circadian rhythm and only cause more tiredness, prefer naps instead.
- Use light therapy glasses (Luminette, Re-Timer) at wake-up every day for several hours (2-8h) with the minimal intensity setting (500lux).
- Start with eyes closed for the first minute or so to help the pupil gently accomodate and avoid dizziness due to sudden bright light exposure, which may otherwise cause headaches and migraines due to the sudden pump in cortisol secretion due to sudden bright light exposure.
- Light therapy is the strongest tool for circadian rhythm entrainment, and is the foundation of this protocol: it modulates both the wake up time, the minimal core body temperature (and hence circadian rhythm) and the stop of endogenous melatonin secretion (DLMOff).
- Light therapy has two major effects: 1) it advances earlier your circadian rhythm phase (circadian shifting effect), making you wake up earlier, and 2) it improves your mood, energy levels and productivity (antidepressant effect). The antidepressant effect is as crucial as the phase advance effect, as it allows to enjoy activities even when the phase advance effect may not be sufficient for some individuals to fully stabilize their sleep schedule.
- Note that light therapy only has an indirect effect on sleep onset time, so it won't make you fall asleep earlier nor even feel sleepy earlier, because sleep onset and wake up times are regulated by distinct circadian oscillators ("EM" oscillators). You either need to wait several days for the sleep onset to "catch up" with the new wake up time, or you can try to use melatonin as indicated below to force a phase advance in the sleep onset, since melatonin directly affects the sleep onset oscillator.
- At first, light therapy will slow down the daily phase delay in the wake up time (wake up earlier), but not the bed time, so it's possible to experience a reduced sleep duration at first. Both times gets synchronized after several days.
- After 10 days you should see the full effect of the light therapy, with a reduction of your daily phase delay (ie, you'll sleep less later every day, or hopefully be entrained). This delay is due to photic history.
- If after 10 days that's not enough to stay entrained, increase the duration, not light intensity. Indeed, increasing the duration is more effective than increasing the intensity of light therapy. There is no limit to how much phase shift can be gained from light therapy since there is no PRC dead zone, which can allow to achieve 8h of phase advance in 5 days by using 5-8h/day of bright light therapy.
- If you cannot wake up naturally without an alarm clock due to obligations, then do not use light therapy and postpone this therapy, as it's crucial to use it after the minimal core body temperature point, which happens slightly before the natural wake up time, and not before, as confirmed by the AASM CRSWD 2015 Guidelines. Once you know when your natural wake up time is, then you can use light therapy even if you need to use an alarm clock, based on your approximative prediction of when is your circadian morning and day (tools such as Circalog can help - but are still in development).
- Do not restrict your sleep and nap as much as needed to feel rested (so don't use alarm clocks), as sleep deprivation and sleep restriction reduce the effectiveness of light therapy due to adenosine buildup.
- About consistency and room for error: what happens if you cannot use light therapy at the same time and for the same duration every days?
- In practice, this is a very common occurrence, nobody can comply 100% all the time with such a long and constraining protocol. What matters is that you can start light therapy at about the same time around natural wake up on most day (more or less 1h), and that you can use about the same duration (more or less 2h). With experience, you will learn what is your sweet spot in terms of duration to achieve your goal (entrainment for non24, phase advance for DSPD), memorize this duration as it is very important.
- On other days, when you cannot use light therapy as you optimally would need to, then you can always use light therapy partially, this will maintain some if not most of light therapy effects thanks to photic history inertia (as long as the partial light therapy days are less frequent than the full light therapy days).
- What if you wake up earlier than usual? If it's 1-2h earlier, you can start using light therapy if you want to progressively wake up earlier. On the other hand, if you are satisfied with your current circadian phase and just want to keep it, then wait later to start light therapy at around a similar time you naturally wake up usually. If you wake up more than 2h earlier, there is a risk that exposure to bright light can fall before the minimal core body temperature point and hence cause a phase delay, so in this case you should avoid all bright light exposure (not just light therapy!) until later around your usual natural wake up time, then start light therapy and you can get exposed to other bright light sources such as sunlight.
- What if you wake up later than usual? Start light therapy at wake up, and then use light therapy longer to compensate with more duration to increase efficacy, BUT always stop before the circadian evening. Eg, if usually you do light therapy at 10am and stop at 2pm, but today you wake up at noon, then you start light therapy asap and stop at 4pm. However, if you do light therapy at 10am and usually stop at 5pm, and today you wake up at 2pm, then you should do light therapy from 2pm to 5pm or max 6pm, then stop because sun sets and your circadian evening is starting.
- Why this works: Since there is no PRC dead zone, it's ok to start light therapy later if waking up later than usual or if unable to start light therapy at wake up, even hours after natural wake up and it will still be effective, as long as it can be finished before the biological evening and night (just like for SAD therapy). Starting later than in the circadian morning (ie, hours later than natural wake up) is less effective, but this can be compensated by increasing duration.
- What if you have to stop light therapy in the middle of the session? No problem, you can restart later. Studies shown that intermittent light therapy (15min of light therapy per hour) is as effective as continuous light therapy to shift the circadian phase. The same principle applies if you need to take a nap in the middle of your light therapy session: you can do light therapy before and after, as long as you stop before your circadian evening. Note however that blue light therapy tends to reduce the ability to nap and drowsiness according to studies.
- What if you stop light therapy during several days?
- Missing one or two days of light therapy is not catastrophic thanks to the inertia induced by photic history, but it should be resumed as soon as possible.
- If missing a longer time, do not worry: the circadian rhythm takes time to return to its original state after treatment discontinuation, about as long as it takes for the therapy to reach maximum efficacy (ie, maximum phase advance). Just try to resume therapy, to resume progress to maximum efficacy. Nobody can follow any therapy systematically all the time, this therapy was designed with practicity in mind, and is field tested daily by its author as well as hundreds others under freeliving conditions.
- In the worst case scenario, if light therapy is discontinued for a week or two, most effects will be gone, but no worries: if you are responsive to light therapy, that's all that matters, you can achieve again the same results you got before by just restarting the protocol from 0.
- Some people claim that light therapy only works when done with an extreme rigor, as missing one day is enough to lose all progress. This is false, just like having to skip lunch because it's past noon would be inane. Just like any biological process, there is some margin we can use.
- Needs some trial-and-error to find the sweet spot for how long and when to use the light therapy, some people are light hypersensitive while others are hyposensitive. Timing is taken care of with this protocol, since you just need to start at natural wake up.
- During winter or in latitudes where days are shorter and sunlight dimmer, longer light therapy sessions are needed. For the author, up to 9h/day is necessary during winter.
- Very long bright light therapy alone should be sufficient to entrain you. After 10 days, you should start to feel the sleepiness sensation appearing every day at the same time (although it can be feeble and fleeting), hinting that you are entrained and the time you can fall asleep even if you still feel slightly energized. If this sensation does not appear, check if there is any hidden caffeine in the food or beverages you consume such as 0% coke, as caffeine's effects carry over up to 48h including phase delay. It's likely good idea to also avoid any wakefulness inducing drug such as tea and modafinil and nootropics.
- The phase advance obtained is proportional to the duration of exposure to bright light, eg, 5-8h of daily bright light exposure can produce a phase advance of 8h over 5 days.
- Some drugs can increase the entrainment to bright light therapy such as hyper photosensitizing drugs (antidepressants, dopaminergic stimulants such as ADHD medication, histaminics), and be decreased with others (eg, antihistaminics, alcohol, caffeine).
- Experimental: low doses of aripiprazole may be used as a complement to increase entrainment to bright light therapy, due to aripiprazole's agonism of histaminergic H1 receptors. Supplementing in vitamin B12 may serve a similar purpose (see also here). Vitamin A is necessary to synthesize all opsins in the eyes, including the melanopsin pigment necessary for ipRGC cells and entrainment to bright light to work, hence it is necessary to ensure adequate levels of vitamin A, via supplementation if needed. These complementing drugs may be useful for treatment-resistant cases or periods of very diminished sunlight exposure such as winter in occidental countries, but they can cause addiction, tolerance and side effects such as akathisia.
- Naps are allowed. Naps are the main tool to manage sleep pressure, just like bright light therapy is the main tool to regulate the circadian rhythm. Reducing the sleep pressure via naps likely improves the efficacy of bright light therapy.
- WARNING: do NOT use bright light therapy while driving, as you need your full vision for your safety!
- Also, bright light therapy is unnecessary when exposed to outdoors sunlight, even if cloudy or behind a glass (eg, driving), it counts as additional bright light therapy (ie, UVs filtering does NOT matter). But as a rule of thumb, artificial bright light therapy using Luminettes is always necessary indoors (this is not 100% accurate as some indoors conditions can be sufficient, but it is highly variable with just head orientation, hence this rule of thumb - use a lux sensor app on your smartphone to see how much lux varies indoors).
- Light therapy effect does not dissipate over time (ie, no tolerance buildup, no desensitization), its efficacy only depends on the duration and intensity and timing you use it, and a few device related factors that are taken care of by certified devices such as Luminette.
- Avoid alarm clocks, allow yourself to wake up naturally.
- In the biological evening (3-5h before naturally falling asleep or 12-15h before the last wake up time):
- Start dark therapy 2-3h before expected fall asleep time, which means avoiding bright and blue-green lights, but dimmed red light is ok.
- Dark therapy is a necessary complement to light therapy: if you do light therapy, you need to do dark therapy.
- Dim or switch off all ambient lights and screens to the minimum brightness.
- Avoid blue, green and white room lights, replace them by red lamps such as RGB LED bulbs like Yeelight 1S.
- No exception: even a short exposure to bright light can suppress melatonin and phase delay the circadian rhythm! Even with eyes closed! So also change bathroom lights, small push button portative lights with a red plastic filter taped on top can be helpful as secondary lights for evenings.
- To filter blue lights of screens, apps can be used such as Twilight on Android and LightBulb on Windows.
- Blue blockers glasses, including red lens laser safety glasses, can be used instead of dimming environmental lights and screens, especially useful outdoors or at offices where it's not possible to control the environmental lighting.
- Do not compare with others, as photosensitivity is highly variable: some people are 50x more sensitive than others and with lights as low as 6lux, and light hypersensivity is very common in people with circadian rhythm disorders including non24. Having wider pupils increases light hypersensitivity, and pupillary light reflex is faster for those who are hypersensitive and indicates a circadian rhythm disorder.
- To check if you're doing dark therapy right, check if your pupils are dilated (ie, you can see in the dark). This works because the ipRGC cells control both the circadian rhythm shifting effect and pupillary light reflex from bright light exposure. Alternatively, use a lux meter app on any modern smartphone with a light sensor, the reading should be below 10 lux and ideally below 1 lux (= a candle flame's light) - make sure to orient the sensor where your eyes are looking, as orientation matters to get accurate lux readings. A higher end alternative is to wear a LYS melanopic lux (mLux) light sensor necklace (only for iPhone owners).
- Optional: Use melatonin pills in the biological evening several hours before bedtime (not just 1h before), as melatonin needs to be taken before the body start producing melatonin (DLMO point).
- Prefer instant-release, sublingual, pure melatonin tablets as they are more effective, are generally of higher quality in over-the-counter products and degrade more slowly (especially in in blister packs). Over-the-counter melatonin (without prescription) fitting these criteria can be as good as medical-grade melatonin, but for inexperienced beginners, it's highly recommended to ask a doctor for a prescription for medical melatonin, to ensure to test with high quality melatonin. Otherwise, low quality melatonin may have no effect, or have effect at first and then quickly dissipate after just a few days due to degradation to light or humidity.
- Dosage should be between 0.5-3mg for first timers. The optimal dosage can vary a lot between individuals and by age, so it's possible to use higher dosage, such as 10mg especially for children who naturally have higher endogenous melatonin levels. There is no risk of overdosage in practice (humans have consumed up to 6600mg/day without any serious side effect). A good starting point for adults is to try 2-3mg, and after 2 weeks if you feel drowsy during the days after melatonin intakes, try to lower the dose below 0.5mg.
- Trial-and-error is required to find the sweet spot for optimal timing and dosage to maximize effect on the circadian rhythm while minimizing next-morning drowsiness. The effect should be felt the very same night, so if the effect is mild or negligible, you can try to change the timing of melatonin intake by increments of 30min each day under the window of 12h-15h before the natural wake up time, until you find the sweet spot of maximal effect.
- If melatonin is used in combination with bright light therapy, then timing does not matter much and it can be taken just 1h before the expected fall asleep time, because then melatonin mostly serves to induce sleep (by stimulating melatonin type 1 receptors) and maintain the circadian rhythm in place (ie, prevents delays), whereas bright light therapy serves to advance/shorten the circadian rhythm.
- Melatonin is not necessary for entrainment if very long bright light therapy and dark therapy are used and no stimulant (eg, caffeine) is consumed. But it is still recommended to use melatonin at first, to consolidate the circadian rhythm faster and magnify the sleepiness feeling so that you can better recognize when your body can sleep. However, it can cause drowsiness up to 48h, hence after a few weeks, either lower dosage or melatonin can be discontinued.
- Melatonin has a direct effect on the sleep onset timing, but not on the wake up time, since both are regulated by distinct circadian oscillators ("EM" oscillators). Hence, if you only use melatonin, you will still continue to wake up later than you want. Thus, melatonin necessarily needs to be combined with another therapy that primarily work on the wake up time circadian oscillator, such as bright light. Indeed, we can view bright light as the tool to activate the circadian day/light oscillator, and melatonin the one to activate the circadian darkness/night oscillator.
- Avoid eating and caloric drinks when melatonin is high in the blood(R1, R2) + avoid alcohol: no meals, especially carbohydrates, in the biological evening and night and also after taking melatonin pills(R) and not too early in the biological morning (ie, skip breakfast if waking with an alarm clock or if waking up before sunrise in winter), because melatonin impairs insulin and glucose processing (including in typical sleepers).
- 0% drinks are allowed all the time. But avoid zero sugar coke as it usually contains caffeine, except when "no caffeine" is specifically mentioned (Coca-Cola Zero Sugar Zero Caffeine). Prefer 0% limonade or other non-caffeinated sodas.
- Low-GI (Low Glycemic Index) food such as pasta, coffee and food allergies such as lactose should be avoided even at lunch as they impair sleep quality and can impair the circadian rhythm well into the next day (24h from ingestion).
- Ketogenic diets, although unnecessary for entrainment, may ease it by forcefully desynchronizing the digestive (intestines and liver) system's clocks through the body temperature modulation and stabilization by increased lipids (triglycerides) input. Ketogenic diets can also reduce sleep duration of about 1 ultradian cycle without loss of sleep quality and may improve digestive issues.
- Do not underestimate the importance of food on the circadian rhythm. Whereas the brain is the central clock guided by light input through the eyes' ipRGC cells, the digestive system is the peripheral (body) clock guided by food intake. The intestines produce >400x more melatonin than the brain. If no extra care is given about the meals timing, it's possible for the central and peripheral clocks to become desynchronized which can lead to metabolic/circadian syndromes.
- Alcohol use, even when occasional, is strongly associated with circadian rhythm and melatonin disruptions, with more alcohol causing more disruptions. If you are already dependent on alcohol, improving your circadian rhythm with melatonin may help in reducing alcohol dependence.
- Avoid any stimulants and wakefulness inducing drug including caffeine, such as tea and modafinil and nootropics. Avoiding stimulants can reduce the need for melatonin use, as tiredness will naturally build up. Stimulants such as caffeine both reduces the homeostatic sleep pressure over up to 48h but also slightly phase delays the circadian rhythm each day, which can build up to a lot of phase delay over time. There is also some evidence that caffeine, including in tea, can cause muscle cramps including nocturnal legs cramps (NLC) which is a major sleep disruptor.
- Prepare a good sleeping environment:
- Sleep with a black silk eye mask or use thick curtains to reduce unwanted exposure to sunlight during your sleep (inhibits your melatonin levels and increases sleep fragmentation). Cut a straight line in the middle of a standard eye mask to make a nose hole which will greatly improve the fit and hence light obstruction.
- Reduce environmental noise, sleep with ear plugs and a very-low-profile sleep earmuffs such as the Hibermate if necessary. Try various kinds of ear plugs, some will be more comfortable than others depending on your ears. Very-low-profile sleep earmuffs such as the Hibermate can be used alone or in combination with in-earplugs, providing excellent portable isolation to noisy environments. This combination is highly recommended.
- If you can't sleep under 30min of going to bed, wake up and do something else, come back about 1h30-2h (one ultradian cycle) later when you feel some hints of tiredness.
- Talk with your co-living relatives to let them know you need to sleep, potentially at odd hours, without interruptions.
- Avoid sleeping pills (hypnotics drugs) such as benzodiazepines and non-benzodiazepines, they are inadequate to treat circadian rhythm disorders and insomnia.
- If you snore regularly, this means your airways are obstructed and sleep quality is impaired. Consider getting a sleep study for sleep apnea, although snoring is not a reliable sign of sleep apnea. Meanwhile, you can try to sleep more on your sides by removing your pillows (as we are more likely to snore on our backs), and use nasal sprays to clean the airways before sleep.
- If you have other health issues that disturb your sleep (such as sleep apnea, digestive issues, fungal infections, restless legs syndrome or any kind of inflammation), treat them too. Comorbid physical diseases often cause or worsen sleep issues and can hence jeopardize anything you try to improve your circadian rhythm disorder, potentially both by decreasing sleep quality (ie, being a sleep disturbance) and by directly affecting your circadian rhythm in some cases. This is especially the case for comorbid disorders that have a circadian pattern (ie, they trigger more often during the circadian night, such as RLS and PLMD and night legs cramps).
- The VLiDACMel therapy will stop your freerunning, but your sleep quality depends on more than that. If you still feel tired under the VLiDACMel therapy, look for those other health issues that may be the cause of sleep disturbances and hence decreasing drastically your sleep quality and make you feeling tired the whole day after, without guilt or shame for taking care of this essential and universal need.
- Sleep apnea is a relatively common cause of insomnia and circadian rhythm disorders. If you snore loudly and regularly and have impaired sleep quality, call sleep clinics until you find one that provides an at-home sleep study kit, so that you can get diagnosed of sleep apnea at home. Meanwhile, you can screen yourself by recording your snoring at night, there are free apps such as Do I Snore Or Grind app on Android, or a simple audio recorder will do, then look at the waveform to find the most loud events. However, note that snoring is not a reliable sign of sleep apnea.
- Grinding teeth during sleep, formally called bruxism, can be a sign of another sleep disorder that requires treatment.
- Although comorbid physical disorders need proper treatment to reduce their impact on sleep, the sleep issues always need to also be treated in their own rights with treatments targeted at sleep, even when there are co-morbid physiological diseases or psychological disorders.
- Check StuffThatWorks and other online medical resources for potential non prescription treatments or management strategies for your other afflictions. StuffThatWorks is a database collecting feedbacks from patients themselves about their symptoms and what treatments worked best for them. For instance, FODMAP elimination diet was reported to be the most effective to manage Irritable Bowel Syndrome (IBS). For night legs cramps (NLC), supplementation in potassium and magnesium can eliminate the issue, especially if following a restrictive diet such as the ketogenic diet or FODMAP elimination diet. Anecdotally, both of these management strategies worked for the author of the present document.
- Disregard your mental states, cognitive activity and stress, they can not affect your circadian rhythm, as by design, core body temperature cannot be altered by psychological processes since its maintenance is crucial for human's survival (homeothermic endothermic animals). When the entrainment therapy works, you should feel tired at the end of your circadian day, no matter what cognitive activity you are engaged in. Mental states may however affect your sleep quality, but not your circadian rhythm.
- Maintenance: Once entrainment is achieved, the treatment must be continued as-is with the parameters (ie, light therapy timing and duration, melatonin timing and dosage, dark therapy setup, etc) you found effective for maintenance of benefits. Indeed, the therapy is effective only as long as it is used, otherwise effects are lost under a few weeks, but the therapy can always be restarted later. Note however that the therapy is not 100% effective, it cannot totally freeze the circadian rhythm in place (due to disturbances in daily life that prevent following consistently the therapy, and varying uncontrollable external factors such as sunlight intensity and duration exposure), hence expect to get misaligned from time to time: for individuals with non-24, simply discontinue therapy temporarily to freerun a few weeks until the ideal time is reached again; for DSPD, increase the exposure duration, or discontinue therapy temporarily to find where your circadian phase is, to re-time properly light therapy.
- Start dark therapy 2-3h before expected fall asleep time, which means avoiding bright and blue-green lights, but dimmed red light is ok.
Other advices
- Always put one's sleep first.
- Disregard sleep hygiene. Your sleep is not "dirty", you have a disease, that needs special accommodations and tools to manage. Evidence shows sleep hygiene does not work. Avoid behavioral chronotherapy too.
- Some sleep is always better than no sleep.
- Plan how to handle sleepless nights:
- Sleepless nights and premature wake ups will always continue to randomly happen due to non-24, as there is unfortunately an uncurable insomnia component.
- It's important to plan what to do during these sleepless nights. Trying to sleep for hours, alone, in the dark, will only cause a loss of time, running thoughts and depressive feelings of powerlessness. Realizing that doing activities during sleepless nights is acceptable and even advisable is definitely the most important realization of people with non-24, as this reduces time spent in a depressive setting while allowing more time for activities.
- A good strategy is to strike a deal with oneself to always put one's sleep first, but to allow to get up and do activities if unable to sleep for more than 30min. If you can't sleep and do not feel tired after 30min of trying, get up and do something. But whenever you feel tired, try to go back to sleep/nap as your top priority. If again it does not work after 30min, you can get back up and do activities. This is similar to the core tenets of sleep hygiene, do not stay in bed for too long if you cannot sleep.
- Make sure to avoid getting exposed to bright light (ie, use dark therapy) during sleepless nights. Hence, screens are allowed, but only dimmed to the minimum and with a blue light filtering software.
- Learn how to detect and handle sleep deprivation and circadian misalignment:
- Learn to detect the signs of sleep deprivation by reading this excellent medical review and also this other excellent systematic review. In summary, the symptoms are those of jetlag, or alcohol hang over since sleep deprivation is similar to alcohol intoxication, which include but is not limited to the following symptoms: attentional issues, mood dysregulations (easier to get angry, even at neutral events), motor coordination issues (letting objects fall more often), tiredness (but this symptom is not necessarily present or not throughout the day), cardiac anomalies, gastric upset.
- Handle sleep deprivation just like alcohol intoxication, hence avoid similarly risky situations (eg, driving, taking important decisions, ...). Sleep loss is the major cause of accidents by far, not alcohol nor other drugs. Also keep in mind that just like people often underestimate the effect of alcohol intoxication on them or their level of intoxication, there is a tendency to underestimate the amount of sleep deprivation and its effects on our decisions and cognitive abilities (and other physiological and psychological health aspects).
- Indeed, sleep deprivation causes major cognitive and mood disturbances to a similar extent to being drunk, including alternating euphoria and anhedonia.
- Just like alcohol causes a hangover that takes days to clear up, expect a sleep deprivation episode to require days or even weeks of good sleep to fully recover, and headaches and mood and other cognitive impairments are not surprising meanwhile.
- Are there other effective therapies? Maybe, but there are a lot that are not working or even detrimental. Check if these interventions are known to modify the core body temperature (search on google scholar or pubmed), if not they are likely ineffective to shift the circadian rhythm.
- Some therapies may work better for some than others, but effective therapies have an objectively measurable effect on everybody. And of course there are therapies that have no effect at all for everybody.
- If you hear someone claim they were treated with a miracle therapy, ask for how long and a proof such as a sleep diary. If they can't produce a sleep diary over at least 1 month post-treatment of a stable sleep pattern, consider the therapy ineffective unless more follow-up data is provided. Transient improvements are common due to how elastic sleep can be, but it does not last more than a few weeks if the therapy is ineffective.
- Avoid benzodiazepines and non-benzodiazepines (Z drug) sleeping pills. They work for some weeks, then they stop working because of tolerance, and then if you stop you'll have an even worse insomnia, so that you'll be compelled to continue using them just to sleep as bad as you were before starting the sleeping pills. That's why current medical guidelines recommend the use of sleeping pills to be limited to 4 weeks maximum, to then be phased out for other alternatives such as melatonin. This applies to all sleep disorders, including insomnia. For circadian rhythm disorders, sleeping pills are never recommended and even disadvised.
- Avoid studies using "self-reported sleep measures", as they are the worst and most inaccurate kind of measure. This usually refers to periodically asking the subject to say how much they think they sleep on average. This kind of self-reported, subjective measure is known to have very poor accuracy due to poor recall. Prefer studies using objective measures of sleep such as actigraphy and core body temperature, or at least a sleep diary.
- As we grow older, we typically need more light therapy (due to lens darkening) and smaller dosage of melatonin (due to lower endogenous levels) to get the same effects.
- Work on accepting the disorder. This will likely involve going through the grieving process of foregoing your previous life or comparison with the social expectations. But that doesn't mean passively suffering from the disorder, but rather to actively improve your management of it and organizing your life in a sustainable manner around it, putting your sleep needs first. For additional infos on the steps of the acceptance process, see the dedicated section below.
- If you need to explain what it feels like living with a chronic illness, read the Spoon Theory by Christine Miserandino.
- There are variant therapies such as phase-delay bright light therapy and sleep schedules or tricks such as adopting a biphasic sleep that can be used in practice to complement the VLiDACMel protocol, for example if you want a faster phase resetting, phase-delay bright light therapy can be helpful. Most of these therapies and tricks are described elsewhere in this document.
WIP: self-monitoring: core body temperature modulation is the core signalling way to propagate circadian rhythm changes throughout all body's cells. Can allow to monitor both the circadian rhythm, and optimally time melatonin and other therapies by observing their direct effect on the core body temperature.
This protocol should result after about 10 days in at least a significantly reduced daily phase delay, or even entrainment.
Following this protocol should not be exhausting, on the contrary, it requires that the participant is fully rested before starting and during the therapy, as sleep deprivation reduces the therapy's efficacy, hence alarm clocks should be avoided, as they not only reduce the therapy's efficacy but also mask the sleep-wake patterns and hence make individualized assessment and monitoring of sleep-wake patterns and therapeutic adjustments difficult.
Belief, strictness and self-discipline are not required. Only compliance to use the devices at the indicated time is necessary for the therapy to be effective. The goal is to get educated on how sleep works and what (external) factors can influence it, not wish it into working how we want. By controlling these factors, this allows to get some control over the sleep-wake patterns.
It's also crucial for the clinical practitioner to explain the complexity of this therapy and instruct the patient how to adapt it to his needs.
Full protocol
This therapy aims to allow for the entrainment of the circadian rhythm to a 24h cycle (ie, entrainment is the stabilization of the sleep schedule) for individuals with a non-24 circadian rhythm sleep-wake disorder (ie, a circadian period longer than 24h). The therapy works by first waiting for the circadian rhythm to naturally and progressively shift towards the ideal wake up time, at which point the therapy should be started to "freeze"/entrain the circadian rhythm in its current state. In practice, this works by using tools that will phase advance (ie, reduce the circadian period), their combination being additive. Since the individual's sleeping schedule does not necessarily follows the circadian rhythm, we will use the terms of "biological day" and "biological night" to refer to the day and night as defined by the circadian rhythm and hence the ideal sleeping schedule, not by the individual's current sleeping schedule.
A previous study found that a combination of melatonin and light therapy could entrain all 6 individuals with non-24, but with limited long term success. The protocol below attempts to address the long term issues by identifying the key parameters for successful entrainment and clarifying how to adjust the therapy on an individual basis to get the optimal results for long term entrainment and for the necessary day-to-day adjustments (eg, spectral composition and duration of light therapy, timing of melatonin, etc.), as well as adding new tools that were not explored before (such as food control).
The therapy was self-experimented by the author (34 years-old, formally diagnosed thrice over 10 years).
The target populations for this therapy includes individuals with a circadian rhythm disorder, especially sighted non-24 for whom this protocol is optimized for, with undamaged ipRGC retinal cells as evidenced by preserved pupillary light reflex (PLR), since the ipRGC cells regulate both the PLR and circadian manipulation and the PLR was found to be a reliable discriminator to detect DSPD. Thus, it is arguably likely that this therapy should work for any individual with a preserved pupillary light reflex. Hence, this protocol should work for individuals with a sighted non-24 disorder, some blind non-24 (those sensitive to relative coordination to sunlight), and those with a delayed sleep-phase disorder (DSPD) with some slight modifications as indicated in the "Adaptations for DSPD" section. With further adaptations, as indicated in the relative sections, the protocol should also work for other circadian rhythm disorders (night shift work disorder, ASPD, etc). If you don't know what these disorders are or if you are unsure if you are affected, please read in the "Diagnosis" section (inside the Troubleshooting part) the instructions to monitor your sleep-wake patterns using a sleep diary or a core body temperature sensor at home, or via salivary melatonin sampling in a hospital.
Disclaimer: The author thoroughly designed and self-tested this protocol after several failed variations. The author does not guarantee that this protocol will work for anyone else, or that all steps are necessary, but all steps laid down below were tested under many variations (by elimination and by changing parameters), and this is the only combination that was found to consistently work so far. Please keep in mind that if the protocol is only done partially (eg, skipping some steps), this may reduce the effectiveness (or not work at all). But even when all steps are followed, this may not work for some individuals. This protocol is shared in the hope it can be helpful for future research or to other individuals with non24.
Preparation phase
Two weeks before doing the therapy: sleep without alarms to fulfill your sleeping needs, and wait for your circadian rhythm to shift naturally until it's close to the target sleep schedule (particularly the wake up time):
- Sleep according to your own natural rhythm for 2 weeks. It is crucial to be well rested before starting the therapy, as this is necessary to reduce fragmentation in your sleep schedule and circadian rhythm by eliminating sleep deprivation, which was also shown to reduce light therapy effectiveness. Indeed, sleep deprivation can cause chronic insomnia as shown by Randy Gardner's experiment. If necessary, buy an eye mask and ear plugs to prevent external factors from disrupting your sleep.
- Write down your wake-up time and falling asleep time every day in a journal (use Sleepmeter on Android). This will serve 2 purposes: you can get a formal diagnosis from a specialized sleep doctor with 2 weeks of sleep logs showing a non24 pattern, and it also allows you to better know what affect your sleep and better know your own sleep patterns. Indeed, it's not uncommon that we overestimate the duration of our sleep, and for non24 individuals the daily phase delay (ie, it's often shorter than you think).
- Take this opportunity to get to better listen to your body and recognize the signs of sleepiness tiredness signalling your body is ready to sleep. It takes at least 2-3 days of good sleep (good duration AND circadian alignment) for the body to recover and feel fully working. For individuals with non-24, this can be a genuinely new experience to NOT feel sleep deprived, since they only lived under sleep deprivation before. It is extremely helpful to know what it's like to not be sleep deprived, and to learn to differenciate when you are sleep deprived and when you are not, as it will help in knowing when to adjust the treatments timing and dosage for you.
- After the 2 weeks, calculate the average wake-up time over the last 3 days. Subtract the sleep duration you need to feel the most refreshed after sleep (usually 7-8h for adults) + 2 hours from this average wake-up time to calculate the DLMO (dim-light melatonin onset). Example: if the average wake-up time over 3 days was 6am, and you need 7h of sleep to feel refreshed, your DLMO is at 6-7-2 = 9pm. Subtract 2-4 hours from this DLMO time to get the ideal time window to take melatonin. Using the previous example, the melatonin intake window would be between 5-7pm.
- Now, wait for your sleep to cycle and come close to the ideal time you would like to freeze in-place. Indeed, there is no proven way to cycle backward (ie, wake up earlier and earlier, also called phase advance), but if you phase delay enough (ie, sleep later and later, which happens naturally for people with non-24 and is called "freerunning"), you'll eventually reach the wake-up time you would like. If you are too eager and start the therapies while sleeping out of phase with your circadian rhythm, this will not work, may worsen your phase delay and increase sleep deprivation temporarily, and hence ultimately discourage you. Hence, it's crucial to be patient to wait for your biological night to be in phase with the actual night. This is usually noticeable as when the circadian rhythm is in phase with the day-night cycle, your sleep will be on average more restorative and longer. Start the next steps below when your wake-up time is around 2-4h before the ideal time you would like to wake up. Meanwhile, continue to write a sleep log.
Note: Be careful to track the biological night's sleep and using this sleep session as a reference for all the calculations in this protocol, and not the siesta (nap time). Humans circadian rhythms naturally have a biphasic sleep with 2 sleep gates : one for the biological night sleep, and one for the siesta about 12h later, but then when sleeping during the siesta only a half night (3-5h) can be slept at most. Since both sleep gates are regulated by the circadian rhythm, knowing the timing of one allows to estimate the timing of the other: for example, if the siesta happens at 6-7pm, the biological night (the other sleep gate) is at 6-7am. See the Biphasic sleep section for more info. A very good indicator to differenciate both types of sleep in practice is the sleep duration, as the siesta can only lasts for half (3-5h) of the biological night sleep (7-9h on average for adult humans). Also, humans are more prone to do a siesta if chronically sleep deprivated (but do not avoid the siesta if you are chronically sleep deprived, as this will allow to reduce the sleep pressure and increase the likelihood you sleep during your biological night on the next days). Furthermore, the biological night sleep duration is dependent on sleep pressure, so that as a rule of thumb, if an individual sleeps a siesta, this amount will be subtracted from the biological night sleep: for example, if you sleep for 4h during the siesta, you can only sleep 4h during the biological night sleep ; if you sleep 2h during the siesta, you can sleep 6h during the biological night sleep. It's the bedtime that will be delayed, not the wake up time (eg, if your biological night sleep is 6am-2pm, and you take a 4h siesta at 6pm-10pm, then you'll be able to sleep your biological night sleep at 10am-2pm, not 6am-10pm, due to reduced sleep pressure so you'll need more time to build it before being able to fall asleep).
Reminder: it is crucial to wait for your circadian rhythm to be in phase with the ideal timing you wish before starting the therapy, as otherwise the treatments will be mistimed and hence will not work or even make your sleep temporarily worse, as for example light therapy can increase sleep fragmentation if mistimed.
For researchers, technically this preparatory phase is akin to a combination of a multiple nap protocol and a constant bed rest protocol, in that sleep and naps are permitted ad libitum in order to reduce or eliminate the effect of sleep pressure and avoid masking by alarm clocks or other factors. However, some factors such as light exposure, food timing and social events are not controlled since this is done in the wild. For these factors, asking the patient to log them such as by using the Pevlog app can allow to take them into account when assessing the circadian rhythm from sleep logs or actigraphy.
Entrainment therapy
After the 2 weeks of natural sleep, use now this combination of therapies everyday, laid out here in chronological order of use during the day, and the major steps emboldened:
- Continue to write down a sleep diary of your sleep and wake up times, optionally along with any other information you think pertinent for your sleep. This is the swiss army knife of non-24 management: the sleep diary not only helps with diagnosis, but it's also crucial to properly time the treatments relatively to the circadian rhythm and spot early signs of transient (dis)entrainment and other changes in your circadian rhythm once you get entrained, so that you can react fast enough to adapt your therapy to stay entrained (eg, by increasing or shortening the light therapy's duration or melatonin timing or dosage). Due to the ever changing circadian rhythm in this disorder, it's necessary for individuals with the non-24 disorder to always maintain a sleep diary.
- Very long light therapy: use 500 lux bright light therapy at wake-up for 2-8h with an angle towards your nose to "freeze" your circadian rhythm by constant phase advance or even reduce circadian period to less than 24h. Light therapy also inhibits melatonin secretion and increases vigilance and mood. This is the strongest tool (zeitgeber) to manipulate the circadian rhythm, for both for the central clock (suprachiasmatic nucleus in the brain) but also for all peripheral clocks of all the organs throughout the body. Light therapy has two major effects: it can shift the circadian rhythm phase earlier or later (circadian shifting effect), making the individual more likely to wake up earlier or later accordingly, and it improves energy levels, mood and productivity (antidepressant effect). The antidepressant effect is as crucial as the phase advance effect, as it allows to enjoy activities even when the phase advance effect may not be sufficient for some individuals to fully stabilize their sleep schedule. Light therapy is optimally delivered via light therapy glasses such as Luminette v3 or v2 for 2-8h directly as soon as you wake up. If you are in a dimly lit environment, start with the eyes closed for a few seconds to allow for the eyes pupils to contract, before opening your eyes for the rest of the session, this will reduce minor side effects of sudden bright light exposure such as dizziness and migraines, which may otherwise be caused by the sudden pump in cortisol secretion due to sudden bright light exposure. The longer the exposure, the proportionally more phase advance you will get (see also here). It's possible to increase the duration of light exposure to more than 5h (see also here), in which case you may wake up earlier and earlier (but be careful because the effect increases over time, being maximal at 10 days due to photic history, so you may end up waking up too early!). The lowest setting, 500 lux, is sufficient with the Luminette. In case of incomplete entrainment after 10 days, increasing the duration is more effective than increasing the intensity of light therapy, as the author of the present document self-experimentally arrived at the same conclusion before finding previously published validation, which means that this effect is so robust that it is noticeable and reproducible on an individual basis. This is likely because light intensity has a low saturation point (1000lux to 2000lux), whereas duration has none (no PRC dead zone). Longer exposure to bright light also eliminates biphasic sleep. In theory, longer exposure to light may be necessary depending on age, as the eyes lens (cristallin) are obscuring and acting as a blue-light filter with age (see also here), although in practice age does not affect the response to light therapy as only melatonin inhibition is impaired by age but not the circadian phase advance which remains the same, and with some studies showing that light therapy produce the same phase shifting effects regardless of age or sex.
- Do not restrict your sleep and nap as much as needed to feel rested, as sleep deprivation and sleep restriction reduce the effectiveness of light therapy, as adenosine buildup was shown to inhibit the effects of bright light on the circadian rhythm.
- At first, light therapy will slow down the daily phase delay in the wake up time (wake up earlier), but not the bed time, so it's possible to experience a reduced sleep duration at first. This is because light therapy instantly synchronizes the DLMOff (stop of endogenous melatonin secretion, which coupled with the wake up time and production of cortisol) but it takes several days to entrain the DLMOn (start of melatonin secretion).
- If you are unsure whether light therapy will work for you, the sleep diary may help: if over the course of one full freerunning revolution, a pattern of relative coordination can be observed (ie, faster freerunning when out of phase with the day-night cycle, and slower when in phase), this is due to sunlight, and hence is strongly suggestive of responsiveness to light therapy. See the extended section below about relative coordination.
- If you want a cheaper alternative to the Luminette, any bright light should be sufficient to get some phase advance, although it will be less effective and reliable than the Luminette since it's enriched with blue light and it's very ergonomic with a precise and constant distance to the eyes. A computer screen at max brightness can be sufficient (if it emits at least 100 lux), or simply changing the orientation of your work desk to get more sunlight can make for a "free" light therapy, as long as you get at least 500 lux. This can be tested with a lux meter app on most smartphone by using the light sensor that is usually included to automatically adjust the screen brightness depending on environmental light exposure. Several users reported success with various DIY devices, such as make-up mirror lights, strong neon lights or with a cheap $30 Beurer TL30 lamp. Just ensure the light is close enough to your eyes to get enough lux. The light source also needs to be oriented in the peripheral view as to target either the parafoveal or nasal region of the retina as these regions much more effectively suppress melatonin, not the inferior nor superior nor temporal regions of the retina. There is also the Lys Circadian app on iOS which can use the phone's camera to finetune the result using color with a proprietary algorithm, or their dedicated LYS button sensor that can be worn as a necklace and detects more accurately melanopic circadian lighting.
- When going outdoors during the day, and if you are living in a timezone with a wide range of hours of daylight (eg, NOT northern Norway, where there can be as few as 4h of indirect sunlight per 24h during winters), then you do not need to use light therapy glasses during the time you are exposed to direct outdoors sunlight, even if cloudy, since sunlight is the most intense bright light therapy that exists. Keep in mind the spectral composition of light is bluer in the mornings than in the afternoon and evening, hence if you only get exposed to outdoors sunlight late in the day, it may be worth to use light therapy glasses a hour to get the benefits of optimal spectral composition. Just count outdoors sunlight exposure as additional bright light therapy. Indoors sunlight exposure is more tricky and may be insufficient, see above for ways to measure with apps.
- Bright light therapy is even more important if you use more than 1mg of melatonin (supraphisiological levels), because there will be residually higher levels of melatonin next morning that will cause drowsiness, and bright light therapy can forcefully inhibit that. Suppressing melatonin by light in the morning can reduce the carry-over hangover when taking a supraphysiological dose because of the photic history (see also here) and because light eliminates melatonin faster than natural elimination.
- Light is likely the most powerful tool we have to control the circadian rhythm, as it was shown in animals that light can entrain even without the SCN pacemaker.
- Light also increases serotonin levels and hence vigilance, particularly at wake-up when sleep inertia is at its highest, since light impacts both the circadian rhythm C and the homeostatic (sleep pressure) process S (which makes sense since cordycepin, an adenosine analog and adenosine being the biological basis of the homeostatic process S, has a huge impact on the circadian rhythm C, showing that both processes are inter-dependent). Hence, the control of light exposure using a combination of light therapy and dark therapy, through the modulation of both light intensity and color, is an optimized therapy.
- Light therapy improves mood as much as antidepressants, and is even recommended by systematic reviews authors as a first-line treatement for seasonal and non-seasonal major depression.
- During the rest of the day, after the wake-up light therapy, it is advisable to continue to be exposed to bright light throughout the rest of your natural day, in order to optimize photic history, as it was shown that 6.5h of bright light exposure (whether continuous or intermittent) at wake-up shifts the circadian phase way more than only 1h of light exposure, and with continuous light suppressing melatonin more than pulsed light (hence continuous light is likely more efficient to increase vigilance). Another study shown that exposure to 5-8h of bright light daily phase advance the circadian rhythm of 14 typical sleepers by 8h over 5 days of treatments only, confirming the viability of very long bright light therapy to treat severe circadian rhythm misalignments. Hence, contrary to what previous research suggested, light PRC curve has no dead zone (see also here), hence it seems there is virtually no limit to how much phase advance can be obtained with longer exposure to bright light. This also means that bright light always affect the circadian rhythm, so that a thorough control of exposure to light is necessary (ie, dark therapy). See also this reddit comment.
- Although bright light exposure matters more in the morning for circadian rhythm entrainment, being exposed to bright light during the whole biological day allows for a more consolidated sleep, as prior light therapy during the circadian day increases endogenous melatonin levels at night. Hence, after the morning light therapy session is done, it's preferable to stay exposed to bright light of at least 500 lux for most the rest of the day. If this is not possible, either buy a bright neon light, or do a longer light therapy session in the morning to compensate for the lack of light exposure the rest of the day.
- During the first few days of light therapy, the sleep schedule may see some chaotic variations in the timing. This is normal and shows that the light therapy works, the sleep schedule should stabilize over the next days.
- Just like melatonin, the optimal light intensity and duration will need some trial-and-error, since there is a 50-fold difference in light sensitivity across individuals.
- It takes about 10 days for light therapy to be fully effective, because of photic history. This means you will notice a snowball effect where light therapy may produce more phase advance over time (ie, your wake up time will stabilize more and more or even be earlier and earlier depending on the light therapy duration).
- Why use low light intensity (500lux on Luminette and Re-Timer) and long duration instead?
- Low saturation point for intensity (<= 2000 lux, or ~1000 lux according to Rea et al) and 100 lux produces already half of the circadian shifting effects of a 10K lux, so 500lux produces at least more than half of the maximum that can be obtained with max intensity.
- Proportionally more phase advance with no limit (no PRC dead zone, see also here) with more duration contrary to intensity.
- Reduce eye hazard risk, since light phototoxicity is a factor of intensity, color and duration, so if we use light therapy for longer, then it's safer to reduce intensity (see also here and here).
- During winter or in latitudes where days are shorter and sunlight dimmer, longer light therapy sessions are needed. For the author, up to 9h/day is necessary during winter.
- If you plan on taking a nap, you can use light therapy after the nap, as long as you are waking up before your circadian night. If you use light therapy before, you may not be able to nap. If you are sleep deprived (sleep duration < 6h for most adults), then it's preferable to prioritize napping, as sleep deprivation reduces light therapy efficacy due to adenosine buildup.
- Very long bright light therapy + dark therapy alone should be sufficient to entrain you. After 10 days, you should start to feel the sleepiness sensation appearing every day at the same time (although it can be feeble and fleeting), hinting that you are entrained and the time you can fall asleep even if you still feel slightly energized. If this sensation does not appear, check if there is any hidden caffeine in the food or beverages you consume such as 0% coke, as caffeine's effects carry over up to 48h including phase delay since caffeine can modify core body temperature and is hence a zeitgeber. It's likely good idea to also avoid any wakefulness inducing drug such as tea and modafinil and nootropics.
- Most light therapy glasses including Luminette can be used with prescription glasses, however make sure they do not have a blue light filtering coating.
- Experimental: hyper photosensitizing drugs such as aripiprazole and antidepressants may be used as a complement to bright light therapy, due to the agonism of histaminergic H1 receptors which increases photosensitivity and entrainment to bright light. This can explain why low doses of aripiprazole was found to be effective to treat DSPD (see here and here). This may be useful for treatment-resistant cases or periods of very diminished sunlight exposure such as winter in occidental countries. But due to their fast tolerance build-up and side effects such as akathisia, it's not recommended for the sole treatment of circadian rhythm disorders, although their hyper photosensitizing effect can be leveraged if they are needed to treat a co-morbid disorder such as ADHD or depression.
- CONTRA-INDICATIONS: bright light therapy can NOT be used by individuals with epilepsy or macular degeneration or other retinal diseases or malformations (eg, aphakic people born without crystalline lens and pseudophakic who received intraocular lens implants), as these populations are at higher risk when using light therapy. Light hypersensitivity (photophobia), as is common in people with ADHD and can be caused by drugs such as methylphenidate, should be considered carefully. Here is a list of drugs potentially causing light hypersensitivity (photophobia). Ask your physician if light therapy is safe if you have light hypensensitivity, and restrict usage of Luminette to the lowest light intensity setting of 500lux, and always start by closing your eyes for 30 seconds when turning on the light therapy to allow the pupil to contract and avoid side-effects associated with sudden bright light exposure.
- WARNING: Do NOT use light therapy glasses while driving! You need your full vision unhampered for your own safety. Also they are unnecessary under outdoors conditions as you will be exposed to plenty of direct sunlight.
- Naps are allowed. Naps are the main tool to manage sleep pressure, just like bright light therapy is the main tool to regulate the circadian rhythm. Reducing the sleep pressure via naps likely improves the efficacy of bright light therapy.
- Given bright light therapy provides the most phase advance when done a hour or two before the natural wake-up time, why don't we use an alarm clock to increase the therapy's efficacy? Because it's at this point, a hour or two before the natural wake-up time, that the PRC curve flips from maximally negative to maximally positive. So if one get exposed to bright light a bit too early, the risk is to be exposed in the maximally phase delaying part instead of the maximally phase advancing part, and hence causing an unwanted phase delay that can totally negate the phase advance obtained in the later part of the PRC curve or even worsen the freerunning speed. Furthermore, this flipping point is very hard to estimate and varies a lot between individuals. Hence, by recommending to start light therapy only at the natural wake up time, we indeed miss some of the maximal phase advancing part, but we avoid all risks of getting (maximally) phase delayed. In other words, we aren't too greedy, we play it safe.
- Analogy: imagine that light therapy is like a pince that is stretching your circadian rhythm, a spring, to the left (earlier time). If you stop using light therapy, the spring naturally tends to contract more and more to the right (later time). But it doesn't happen at once. You can use light therapy again at any point in time to stretch the spring again. It may take a few days to go back to where you were depending on how long you missed light therapy (eg, if you missed one day, you can recover usually in one day, if you missed 2, you need 2 days of light therapy, etc).
- Optional: Timed big main meal, which is to take your main meal at the middle of your circadian rythm's day. This synchronizes your circadian rhythm thanks to your intestines regulation of the circadian clocks (it's the biggest producer of melatonin). You can eat a breakfast, but it should be relatively small, and there should be only one big meal during the day (eg, the sort of meal that you feel like you ate enough for the whole rest of the day - but be careful of not over-eating!). More than entraining your circadian rhythm, timing meals allows to avoid circadian misalignment, as eating food during your biological night or too close to it can impair several regulatory functions such as insulin and glucose. This is true not only for non24 and DSPD but also for typical sleepers as well, although the former may be even more at risk due to a mutation in melatonin type 2 receptor (MT2) which seems to be more prevalent in individuals with a circadian rhythm disorder.
- Important: Reducing the quantity of consumed carbohydrates is highly beneficial in any case, as each 1% reduction improves the metabolism and reduces risks of obesity and metabolic disorders, including sleep, according to a meta-analysis. It's also an advised treatment to deal with postprandial sleepiness and particularly reactive hypoglycemia.
- Timed big meals provide very little additional circadian stability apriori and it's disadvised for those who try to manage their weight, so it's not recommended to focus in this step, rather focus on light and dark therapy and melatonin, and simply avoid ingesting calories and especially carbohydrates during the circadian night.
- If you really need to eat in the evenings, prefer to consume tropical fruits such as bananas, which were shown to cause an uptick of melatonin in the blood 2h after consumption, although note that it is likely only the receptor-independent antioxydative activity that is promoted, it is unlikely to help with sleep, but this hypothesis was not tested so we don't know. But at least, fruits are a healthy kind of meal and stack if you need to eat, especially when late in the evenings.
- Optionally, it's possible to follow a strict ketogenic diet with timed big main meal.
- The ketogenic diet is not necessary for entrainment, but it allows to eliminate the effect of carbs (postprandial sleepiness, sugar crash) as well as disconnect the digestive clock with the brain circadian clock, hence it can ease entrainment. The effects will start only after you reach a ketosis state as indicated by the highest 2 levels on the ketostix (measurement bands of ketosis from urine). Following a strict ketogenic diet is kind of the extreme of the carbohydrate reduction treatment for postprandial sleepiness. A strict ketogenic diet, as defined for epilepsy treatment and diabetes management, is a diet with limited carbohydrates (<=50g of wet carbs (sugar+fibers), including <= 20g of sugar carbs per day), medium proteins and lots of lipids (fat). Proteins should be kept in limited amounts, as to not compensate for the lack of carbs by eating too much proteins, as proteins will get converted to carbs, preventing reaching the highest levels of ketosis as indicated by the last 2 colors on the ketostix.
- In practice, the author of the present document observed the following phenomena during self-experimentation, which fits with recent research findings:
- Desynchronization of the whole body circadian clock, which has two paradoxical effects in practice: 1- a faster daily phase delay during freerunning (1h of delay per day in the author's case, instead of 30min/day usually); 2- reduced daily phase delay (ie, shorter circadian period) during the entrainment phase. This may be explained by the preliminary evidence on mice showing that the ketogenic diet can modulates the body's circadian clock, so that under ketosis, food has an increased circadian rhythm resetting effect, by increasing the intestines time clock gene expression and switching off liver's time clock genes and melatonin secretion — in other words, the peripheral (ie, body) circadian clocks will rely more on the food timing, which is a lot easier to control than other zeitgebers, and with bigger meals having an increased resetting effect. Another study on mice also observed that the ketogenic diet induced a shorter circadian period and hence a phase advance.
- DEPRECATED: Reduction of the sleep duration (by one ultradian cycle, so about 1h30-2h shorter sleep) while improving sleep quality (so there is no loss in sleep even though the duration is smaller, which eases the maintenance of a stable sleep by compensating the too long awake period of individuals with non24 by a shorter sleep), as also observed on a study on epileptic children under the ketogenic diet.
- UPDATE 2021: although during the first ketogenic diet run (over 3 months) seemed to reduce sleep duration with no side effect, the second ketogenic diet trial over 3 more months 1 year later did not show similar benefits. The difference is that coke beverages (containing caffeine) were excluded in the second run. It seems the caffeine's effects remains well over one day and carry over to the next day, so that this is the likely cause of reduction of sleep duration in the first run. In the second run, when sleeping in circadian alignment, the author could sleep a full 8h night and a bit more. Hence, the ketogenic diet did not show any significant effect on sleep nor on dreams during the 2nd run, which means that the ketogenic diet does not appear to improve, nor impair, sleep. It can hence be used by individuals with the non-24 disorder for other purposes in parallel to an entrainment therapy (eg, the ketogenic diet may be part of a diabetes management therapy).
- Reduction of hunger (eases the avoidance of the detrimental melatonin/insulin/carbs interaction in the biological evening).
- If you choose to do a ketogenic diet, plan to start it ahead, at least 2 weeks before the rest of this protocol, as to have enough time for your sleep to adapt and stabilize with the new diet. Also make sure to use vitamins and minerals supplement, and salt a bit your homemade food, to avoid the risk of electrolytes insuffisance contributing to the dreaded keto flu. If the strict ketogenic diet shows efficacy to you for entrainment, you can later transition back to a healthy diet (such as the scientifically designed DASH diet as recommended by the NIH, and combine with the openfoodfacts.org search engine filtered by Nutri-Score and NOVA to find healthier food products in practice) with carbs in reduced quantities compared to your old diet, and you should also keep the benefits in insulin resistance reduction even after stopping the ketogenic diet (as long as you don't revert back to your old diet).
- The ketogenic diet may also improve sleep indirectly by:
- weight loss, as weight surplus is associated with obstructive sleep apnea and snoring, which may resolve with weight loss.
- reducing digestive issues for individuals with irritable bowel syndrome disorder as it reduces or eliminates the intake of FODMAP, since they are specific kinds of carbohydrates, which are avoided in the ketogenic diet. In other words, there are no FODMAPs in lipids nor proteins, so the ketogenic diet is a good option for those with FODMAP allergy.
- To learn more about the ketogenic diet both in theory and in practice, read this and this excellent reddit posts.
- Start dark therapy, which is the avoidance of bright blue-green lights, 2-3h before natural bedtime. Dimmed red lights are OK, either by changing room lights with RGB LED such as Yeelight 1S, or by using orange or red-tinted blue blocker sunglasses (or laser safety glasses) if you can't change the environment lights. Start dark therapy simultaneously to ingesting melatonin pills (if you use melatonin). Doing dark therapy allows to both preserve the natural secretion of melatonin, and avoid unwanted circadian phase shifting and detrimental health effects of night-time bright light exposure such as on the cardiometabolic system. Indeed, light can suppress both melatonin and shift the circadian rhythm (independently of whether melatonin is suppressed, see also here and here). Both the intensity and color of light (see also here and here) matter in circadian rhythm shifting and melatonin suppression by modulating the ipRGCs receptors (see here for intensity, and here and here for color), hence you need to avoid bright lights in your biological evening, both by dimming down all lights, and by filtering blue light. Dark therapy is necessary to keep a robust gain from the other therapies, by ensuring there is no unwanted shift in your circadian rhythm by uncontrolled factors such as uncontrolled light exposure.
- The author strongly recommends red-tinted laser safety glasses, which are the most filtering kind of glasses and will filter both blue and green parts of the spectrum as well as dim light (as they often include a dimming layer like sunglasses). For more infos to find these glasses, look in the dedicated section on Dark Therapy in Troubleshooting below. If not available, a good alternative but less filtering and with no dimming layer are the UVEX glasses with SCT-Orange coating as blue light filters, they are very inexpensive and highly effective according to several independent reviews (see the dedicated section on Dark Therapy below). Use the UVEX Skyper if you want to hang outside with it, or the UVEX S0360X Ultra Spec if you want to use prescription glasses under, as the Ultra-Spec are big enough to fit prescription glasses under, but not the Skyper. A dimming layer can be manually added to UVEX amber glasses, by using VLT shading films for cars windows.
- If you don't have access to such glasses, a more inconvenient and less reliable but working alternative is to modify the environment: dim down / switch off all lamps (including your computer screen intensity) and install blue light filters softwares on your computer (advised: LightBulb or f.lux) and smartphone (Twilight on Android). If you have a changing color LED lamp (eg, Living Colors), use it as a bed lamp by setting it to full red color (the blue LED should be switched off if you selected an appropriately full red color).
- Why start the dark therapy about 2-4h before target bedtime? Because melatonin takes 1-2h to produce drowsiness effects from its DLMO point, and it takes 1-2h for melatonin secretion to reach it's DLMO level from the moment it starts its production (or from when the light inhibition/exposure is stopped). Hence, the dark therapy should be started 2-4h before the natural bedtime. This is further supported by evidence from a study of home lighting, which found that increased melanopic illuminance 3h before bedtime was correlated with increased wakefulness for 90 more minutes past bedtime.
- Dark therapy is the natural complement to light therapy: whereas light therapy phase advances your circadian rhythm (ie, you wake up earlier), dark therapy prevents unwanted phase delays due to light exposure, which concretely makes you feel more fatigued at the wanted time.
- Optional: Kickstart your melatonin secretion with a melatonin pill and hence sleep and help its consolidation, several hours before bedtim, by taking melatonin at the same time you start dark therapy: take melatonin in instant release form, if possible sublingually dissolving tablets. The optimal efficacy of melatonin is dictated by two factors: 1. ingestion before DLMO, 2. dosage high enough or timing close enough to DLMO for exogenous melatonin in bloodstream to overlap with DLMO. The timing is crucial and requires some trial-and-error, as melatonin must be taken relatively to one's current circadian rhythm (ie, bedtime and wake up time), NOT the target bedtime contrary to what current regulations state. Indeed, it's necessary to take melatonin before the body starts producing it (called the DLMO point), and the body starts producing melatonin a few hours before you go to bed, as melatonin is one of the things that cause sleepiness feelings and allow to sleep a full night (sleep consolidation). The dosage does not change the magnitude of circadian phase shifting effect, so it can be as low as 0.1mg or up to 3mg, but only higher doses > 1mg (supraphysiological) can produce hypothermia (as also shown here), so that "nighttime increase in sleepiness was achieved only after administration of high doses" and doses such as 3mg are "more likely to produce a substantial phase shift" although this needs confirmation. However, dosage does matter for the timing of intake, as it's necessary for melatonin from pills to overlap with the natural endogenous melatonin secretion (DLMO), as to simulate an earlier dusk and trick the body into thinking it started producing melatonin earlier. Since higher dosages remain longer in the bloodstream (see also here), they provide more leeway in timing and produce effects even if taken very early, whereas lower dosages need to be taken much closer to DLMO (but never after - hence lower dosages require more accurate calculations of DLMO), hence higher melatonin doses (1-3mg) are likely easier to time for beginners. However, both the DLMO timing (60% have a DLMO outside the 2-3h before bedtime range) and the dosage (between 10-fold variability and 35-fold variability) required are highly variable between individuals. Although melatonin can shift the circadian rhythm via the type 2 receptors, its main purpose is to stabilize the circadian rhythm and consolidate sleep, hence to maintain the benefits from more efficient tools for phase advance such as light therapy. Melatonin is also a powerful antioxydant that reduces or eliminates the detrimental health effects of sleep loss, but this (receptor-independent extracellular) effect is only obtained with very high doses (8mg/kg/day for humans).
- Theoretical optimal timing for melatonin intake: 3h to 4h before the DLMO (dim-light melatonin onset, the time at which the body starts producing melatonin), which means up to 7h before the time you usually fall asleep. The DLMO can be assessed by salivary samples every 30 minutes in a clinic by spending a whole day and night in a dim-lit room (<50 lux) as to allow melatonin to build up, uninhibited by any light. This procedure is hence very cumbersome. Although this is certainly useful for other circadian rhythm disorders with a fixed circadian rhythm but shifted such as DSPD, the utility is greatly reduced for individuals with non-24 since their circadian rhythm and DLMO are shifting all the time, hence an assessment will become obsolete in a matter of days.
- Practical optimal timing for melatonin: Since daily DLMO assessment is impractical for individuals with non-24, the other way is to approximate it. There are mostly 3 ways:
- 3-5h before natural bedtime (not target bedtime), by assuming that on average DLMO happens 2-3h before the natural bedtime as in Lewy's PRC. However, it was shown that the DLMO-to-bedtime is highly variable between individuals, 60% have a DLMO bigger or smaller than 2-3h before bedtime (range: -0.3h to 5.8h). Hence, another study rather recommends 5-7h before natural bedtime (3-5h before DLMO). However, a study shown that bedtime is a poor predictor of the DLMO, hence it's easy to mistime melatonin administration when using bedtime as the basis of calculations.
- 9-11h before the midpoint of the sleep period. The midpoint of the sleep period(s) (see also here and here) was found to be the most reliable predictor of the DLMO and the circadian rhythm, and hence sleep midpoint should be the preferred method to calculate the adequate timing for melatonin administration.
- 13-15h from your wake up time, by translating the results from this study about the optimal timing relatively to the sleep midpoint. Indeed, the wake-up time is a reliable predictor of the DLMO and the circadian rhythm similarly to the sleep midpoint, and the author observed it can be used to monitor entrainment (see the dedicated section below). Some redditors with non24 reported that melatonin intake 12h before their last wake up time allowed them to robustly entrain.
- There is also an experimental "melatonin split-dosing" scheme, where a low dose of melatonin is taken before the DLMO (pill taken 3-7h before natural bedtime) to maximally shift the circadian rhythm, and a higher dose 1-2h before natural bedtime to maximally induce sleepiness (similarly to a sleeping pill). This approach is not yet validated and was only used for one published sighted non-24 case. The idea with split-dosed melatonin is to target both types of melatonin receptors with an optimally timed and dosed melatonin for each separately, instead of trying to target both with a single pill. In addition, this also doubles the likelihood of getting at least one timing right, and hence reduce the risk of disentrainment. However, given that exogenous melatonin needs to overlap with endogenous melatonin for maximal circadian rhythm shifting, it may be more effective to also use a high melatonin dose before DLMO (and hence high dose for both intakes). Thus, the author would recommend to test 1-3mg before DLMO (3-7h before bedtime), and 2-3mg 1-4h before bedtime. An individual with DSPD tried this scheme and reported great results, greater than with any other method they tried, although the dosage require some experimentation.
- Notes:
- these timings are given for a 25-45 years-old adult with an average ideal sleep duration of 7-8h. Younger individuals sleep more and elders sleep less, hence the timings should be recalculated accordingly.
- since there is so much variability between individuals, and also the optimal timing depends on the dosage, finding the optimal dosage for you will require some trial-and-error, so consider the timings above as starting points and feel free to experiment and change the timing of melatonin until you find the sweet spot that works best for you.
- if the wake up time changes after starting the therapy (eg, waking up earlier), then the melatonin intake time must be recalculated accordingly for optimal efficacy.
- the current DLMO point can be approximatively calculated more robustly given the last wake up time as follows (assuming as in Lewy's PRC that it's 2-3h before natural bedtime): DLMO point = (wake up time - optimal sleep duration (~8h for non-old adults) - 2) mod 24. However, the DLMO point is highly variable between individuals, so the number "2" (as in 2h before natural bedtime) in the previous calculation may be a different value for different individuals.
- Optimal dosage for melatonin: for adults, start with 2-3mg. For children, up to 10mg may be necessary. If the feeling of tiredness is not present 2h after intake, increase dosage. If you feel drowsy the next day after melatonin intake (ie, need to take naps), decrease dosage (can go as low as 0.1mg).
- Melatonin is not necessary for entrainment if very long bright light therapy is used and no stimulant (eg, caffeine) is consumed. But it is still recommended to use melatonin at first, to consolidate the circadian rhythm faster and magnify the sleepiness feeling so that you can better recognize when your body can sleep. However, a too high dose for the individual can cause drowsiness up to 48h after intake of melatonin (ie, carry over effect), hence after a few weeks, either lower dosage or melatonin can be discontinued. Indeed, it is counterproductive if the therapy maintains the individual in lowered vigilance state during the day, improving wakefulness is as crucial as improving sleepiness, and bright light therapy can do both, whereas melatonin only tackles the latter but can impair the former, hence melatonin should be dosed with care. Nevertheless, melatonin's effects will disappear after just a few days of discontinuation even after months of continuous use, since there is no addiction nor tolerance nor irreversible receptors desensitization, even at very high doses such as 6600mg/day for a month.
- Avoid any stimulants and wakefulness inducing drug including caffeine, such as tea and modafinil and nootropics. Avoiding stimulants can reduce the need for melatonin use, as tiredness will naturally build up. Stimulants such as caffeine both reduces the homeostatic sleep pressure over up to 48h but also slightly phase delays the circadian rhythm each day, which can build up to a lot of phase delay over time. There is also some evidence that caffeine, including in tea, can cause muscle cramps including nocturnal legs cramps (NLC) which is a major sleep disruptor.
- What also matters is that it dissolves in sublingual instantly, as instant-release melatonin is more effective - if you can't find an immediate/fast release melatonin, you can use long/prolonged release melatonin but crushed to avoid the coating (parent compound) from delaying the melatonin intake) during the ideal melatonin window time you calculated before.
- Melatonin not only helps with phase advance, but also consolidates the 2nd half of your night's sleep, so you are more likely to sleep your full night rather than be woken up earlier because of various disturbances.
- Ensure melatonin quality and preservation against environmental degradation:
- Prefer to get a medical prescription for melatonin if you are a beginner, this will ensure you get high quality melatonin to test, otherwise with low quality melatonin there may be no effect or the effect may dissipate after just a few days because of degradation to light or humidity (see below).
- Over-the-counter melatonin secretion quality is not regulated as a medical drug, hence the dosage can vary 10-fold from pill to pill. Prefer sublingual tablets containing only melatonin and no other "sleep-inducing" compound, as the only-melatonin tablets were found to be the most consistently qualitative with a dosage closer to what is claimed on the label. The same study found that chewable gummies are the worst in terms of variability of melatonin dosage. In the USA, prefer melatonin supplements with the USP seal, which means that the product was voluntarily tested and meets US Pharmacopeia Convention standards, or from a pharmacy.
- In some cases, some individuals may feel excited after taking melatonin. This may be due to serotonin residues which are present in ~26% of over-the-counter (low quality) melatonin products. It's then advised to change for another (higher quality) melatonin product.
- Since melatonin degrades quickly when in contact with air (but not temperature) in tablets from the 2nd day and attaining -41% of efficacy on the 6th day, and even faster in liquid form (see also here), the packaging is crucial. The best packaging is a sealed wrapping per dose, such as aluminium foils commonly called "blister packs", which are common in European countries but unfortunately rare in USA, so that each tablet is protected from environmental degradation until it's used. Avoid bottles, as all tablets will begin to degrade from the moment the bottle is opened to take the first tablet. It's very much like food conservation: after being opened, liquids spoil the fastest, bottles of tablets and gummies spoil on a medium rate of one or two weeks, and per-tablet wrapped aluminium packages do not spoil other than past the preservation date of the unopened package. If you want to extend the conservation time of bottles of tablets, you can try to place the bottle in a ziploc resealable plastic bag, and place in a closet in normal temperature, so as to isolate your medicinal products from air humidity, warm temperature and sunlight, these factors being the biggest ones for accelerated spoiling.
- If the effect of melatonin drops dramatically after a week or 2, or when you buy a new package, then an issue with quality control or environmental degradation is likely the reason, since there is no habituation to melatonin. Try to buy another brand of sublingual tablets of pure melatonin (no other compounds) in blister packs.
- Melatonin has 2 effects because there are 2 types of receptors: type 1 causes drowsiness/sleepiness, and type 2 causes a phase advance of the circadian rhythm. The effect of drowsiness is most obvious as it happens very fast, under an hour, after taking melatonin pills. This is why most users use melatonin close to bedtime, but this doesn't allow the benefits from the circadian rhythm shifting effects as the intake is later than the DLMO. If you want the circadian rhythm effects, take melatonin before your DLMO, as calculated with the methods outlined above.
- Higher doses of melatonin are at the expense of drowsiness just after and up to the next morning. If you experience brain fog in the morning after taking melatonin the evening before, you can try to reduce the dosage or use bright light therapy on wake up (choose blue light emitting devices as blue light is the most effective to reduce drowsiness and increase vigilance compared to other colors such as green light which has no effect on vigilance and can only temporarily melatonin suppression effect).
- Using a higher dosage of melatonin is not going to be harmful, it's just that you'll have too much melatonin in your bloodstream for what you need, so that there will be huge residual levels the next morning and you'll feel very drowsy and hard to wake up. But otherwise no detrimental health effect, and in fact high dosages of melatonin have more antioxydant effect as this is an extracellular effect (receptor-independent effect) and it needs a much higher concentration than what is needed to shift the circadian rhythm (receptor-dependent effect). But for circadian rhythm disorders, you want to avoid the next-morning drowsiness so it's best to use the lowest dose that mimics the dosage you would have in your bloodstream with natural endogenous melatonin.
- Hence, for first timers with melatonin, 1 to 2mg is likely the best dosage for adults (5-10mg is better for children), because it's big enough to ensure an effect, but not too big so that the pill can be split in half to get down to physiological levels of melatonin (ie, as much melatonin as you have naturally in your blood during sleep, hence no brain fog the next morning).
- Children and teenagers have naturally much higher levels of melatonin in the blood than adults, hence higher doses such as 5-10mg are necessary for them. A 1-year long-term clinical trial found melatonin to be effective with dosages between 2mg to 10mg per day in children.
- The opposite is also true: older adults need lower doses of melatonin to get the same effect than younger adults.
- Melatonin is contra-indicated for people with the Restless Legs Syndrome (RLS) and other dopaminergic related diseases, as melatonin interacts with dopamine and can make the symptoms worse (although it still improves sleep, particularly for people with a comorbid circadian rhythm disorder such as non-24). Some users reported that lower doses of melatonin produce less side-effects so using a <1mg melatonin dose may be more manageable. There are also anecdotal reports of bright light therapy also worsening RLS, likely by indirectly increasing melatonin levels through photic history.
- Do not fear overdose, melatonin has been used up to 6600mg/day for 35 days in humans without any serious adverse effect.
- Avoid eating and caloric drinks (especially carbs) when melatonin is high in the blood, to prevent insulin inhibition and hyperglycemia before and during the circadian night and early circadian morning + avoid alcohol, as Panda et al also recommend in a review. As a practical rule, apply the rule above when starting dark therapy, consider melatonin is rising and that you should avoid eating and caloric drinks. The pancreas has both insulin and melatonin receptors so that each one inhibits the other at the protein level. When melatonin is high, insulin is inhibited and if you eat, then glucose will remain high in your blood and cause a superficial diabetes throughout the night, as insulin is necessary to process glucose. Hence, melatonin impairs insulin production and glucose processing (even in typical sleepers), and insulin impairs melatonin processing. This may have detrimental effects on health as it's hypothesized to be one of the cause of chronic diabetes type 2 and obesity, and may also disturb the ability to sleep as the high blood glucose and hence available energy will cause the individual to feel more energetic past bedtime. Researchers suggest that this may be a biological safeguard mechanism to avoid hypoglycemia during the night since we spend a long time without eating while we sleep, and hypoglycemia can be very dangerous (diabetics often have this issue at night time), but this safeguard assumes that the individual do not eat when supposed to sleep by the circadian rhythm and melatonin rhythm.
- In practice, this means you need to avoid eating during your biological night, as well as avoid eating at least 2h or ideally 4h before your natural bedtime. Avoid eating too early in your biological morning, so if for example usually you sleep until noon but today you have an appointment at 8am, skip breakfast (too early breakfast was demonstrated to be detrimental to the health of people with DSPD). Also if you take melatonin pills, don't eat after, always eat before, and it doesn't matter if it's daytime or nighttime, eating after melatonin intake is always bad.
- Low to no-carbs 0% drinks (including water) are allowed at any time, and it's possible in theory that low-carbohydrates food such as ketogenic diet may have less of an impact on the circadian rhythm and health, although this needs confirmation. Be careful with zero sugar coke as it usually contains caffeine, except Coca-Cola Zero Sugar Zero Caffeine, although this should not prevent entrainment (the author of the present document unknowingly consumed zero sugar coke with caffeine for more than 6 months while still being entrained... But having more difficulties falling and staying asleep). After switching to 0% lemonade and discontinuing caffeine, it became much easier to feel sleepiness and fall asleep at a regular time, to the point that melatonin could be discontinued (but bright light therapy + dark therapy must be continued).
- Low-GI (Low Glycemic Index) food such as pasta, coffee and food allergies such as lactose should be avoided even at lunch as they impair sleep quality and can impair the circadian rhythm well into the next day (24h from ingestion).
- The glucose processing impairment by melatonin is worsened for those with a genetic mutation in the melatonin type 2 receptors (MTNR1B rs10830963 and rs10830962 and rs1387153). This mutation is associated not only with increased diabetes type 2 risk and other metabolic syndromes such as obesity, but also with circadian rhythm disorders such as DSPD (see also here) and non24. Because of these strong links between metabolic syndromes and circadian rhythm disorders, researchers now calls to rename them as circadian syndromes. Hence, in practice, it is advised to plan meals at times when melatonin levels are low (see also here). This means no meal past melatonin pills intake, during the biological night and not too early in the biological morning (ie, skip breakfast if waking with an alarm clock). The absolute time does not matter, what matters is when you eat outside of your biological night (eg, if you currently ideally sleep during the day, you can safely eat at night). Indeed, it was shown on individuals with DSPD that if they ate too early (particularly those with a constrained sleep schedule, ie, waking up earlier for work), their glucose tolerance was worse in the morning due to melatonin being still present in the body due to their delayed circadian rhythm.
- Figure 1 of this study on DSPD shows that there are two variants of the mutation: rs10830963C and rs10830963G, with those with two G alleles (GG) having a DLMOff even later than those with only one (CG). The author of the current document highly suspects than individuals with sighted non-24 may commonly have the GG mutation.
- This strategy is kind of, but not exactly like, a time-restricted feeding schedule, but instead of restricting by time, meal timing is restricted by when melatonin is expected to be present in the body. This may hence explain the anecdotal reports of sleep improvements after doing a time-restricted feeding schedule such as intermittent fasting. If this is the case, equal benefits in terms of sleep and circadian rhythm can be obtained by just avoiding to eat when melatonin is high in the blood.
- The author of the present protocol found in practice that it's necessary to avoid meals at least 6h before the sleep onset (fall asleep time) for the last meal to have no impact on the circadian rhythm.
- During winter, humans tend to have a longer melatonin secretion that stops later in the morning compared to summer due to later sunrise, hence it's necessary to eat later during winter than summer to adapt to the longer melatonin secretion profile (eg, skip breakfast if waking up earlier than sunrise).
- Alcohol use, even if a single dose, is strongly associated with circadian rhythm and melatonin disruptions, with more alcohol causing more disruptions. Hence, avoid alcohol. If you are already dependent on alcohol, targeting improvements of your circadian rhythm and sleep by using melatonin can yield improvements or even remission of alcohol use disorder.
- If you find it difficult to not eat at all for hours before your bedtime, then you can try to eat a ketogenic meal (ie, lipid/fat based meal), as theoretically this should not produce an insulin nor glucose response and hence should have less impact on the circadian rhythm (although the guts will still have to process food, hence being active and this may disturb sleep - but theoretically without the detrimental metabolic interaction between insulin and melatonin).
- Optimize sleep preparations and quality:
- Use a black silk eye mask and outer ear plugs in silicon (and/or a white/pink noise machine) to reduce the highly detrimental effect of noise and disturbances on your sleep efficiency. This is NOT optional, having any disturbance during your sleep will annihilate any effort you can undertake otherwise! Sleep disturbances can indeed have a very strong detrimental effect on sleep quality and the circadian rhythm that can carry over several days after the disturbances happened. Even with the eyes closed, a light of only 5-10 lux can phase shift the circadian rhythm and inhibit melatonin, and this phenomenon is actually used for "light masks", a kind of light therapy. Noise can cause paranoia and delirium, just like prolonged sleep deprivation, which can be improved by ear plugs. Night-time disturbances are the major factor causing sleep inertia (aka brainfog), more than sleep deprivation, and particularly when the disturbances happens during the deep (slow wave) sleep stage. Slightly bright light (100lux) during sleep impairs cardiometabolic health. Hence, these tools are crucial and should be considered as extensions of dark therapy, to ensure there is no unwanted shift in your circadian rhythm by uncontrolled factors. An inexpensive silk black eye mask will do, and outer earplugs in silicon are advised as they are much more comfortable than inner earplugs (they reduce the proliferation of bacteria and hence itchy ears). The combination of both an eye mask and ear plugs is more effective than any alone by increasing both deep sleep and sleep efficiency.
- If your eye mask is not covering enough and let light slip through, then it's not a good one, get another one. If the mask is not comfortable to wear or itchy, it's also a sign it's bad quality (it's not in silk). Good silk black eye masks are inexpensive, usually costing $10 to $20. But below $10 it's unlikely to be of good quality. If the eye mask is of a standard shape as shown below, the fit (and comfort) can be significantly improved by cutting a straight line in the middle to serve as a nose hole.
- Use a black silk eye mask and outer ear plugs in silicon (and/or a white/pink noise machine) to reduce the highly detrimental effect of noise and disturbances on your sleep efficiency. This is NOT optional, having any disturbance during your sleep will annihilate any effort you can undertake otherwise! Sleep disturbances can indeed have a very strong detrimental effect on sleep quality and the circadian rhythm that can carry over several days after the disturbances happened. Even with the eyes closed, a light of only 5-10 lux can phase shift the circadian rhythm and inhibit melatonin, and this phenomenon is actually used for "light masks", a kind of light therapy. Noise can cause paranoia and delirium, just like prolonged sleep deprivation, which can be improved by ear plugs. Night-time disturbances are the major factor causing sleep inertia (aka brainfog), more than sleep deprivation, and particularly when the disturbances happens during the deep (slow wave) sleep stage. Slightly bright light (100lux) during sleep impairs cardiometabolic health. Hence, these tools are crucial and should be considered as extensions of dark therapy, to ensure there is no unwanted shift in your circadian rhythm by uncontrolled factors. An inexpensive silk black eye mask will do, and outer earplugs in silicon are advised as they are much more comfortable than inner earplugs (they reduce the proliferation of bacteria and hence itchy ears). The combination of both an eye mask and ear plugs is more effective than any alone by increasing both deep sleep and sleep efficiency.

- For the ear plugs for sleep, the most comfortable are outer ear plugs in silicon such as Medigrade or Mack's. But they do not work well during winter, as the cold temperature makes it hard to stick to the outer ear. During these periods, switch to Howard Leight Laser-Lite (Honeywell 3301105) earplugs, which are very soft foam inner ear plugs, they are less comfortable than outer ear plugs but they are much more comfortable than any other type in the author's experience (a lot of brands and types were tested!). Nevertheless, it will regularly happen that the ears get itchy and so you can't stand having earplugs in your ears for the night, or unconsciously rip them off during your sleep. This is to be expected from time to time, but if it happens most of the time, try other brands/material of earplugs, and make sure to clean your ears with cotton tips before sleep.
 earplugs.jpg)
- Very-low-profile sleep earmuffs such as the Hibermate can be used alone or in combination with in-earplugs, providing excellent portable isolation to noisy environments. Using the Hibermate, alone (for more ear comfort, less risks of ear canal itchiness) or in combination with in-earplugs (for more noise isolation) is highly recommended, this works very well in practice, including for side sleepers. The sleep earmuff will take the user's head shape after some weeks of use.
- Using music as a non-drug stimulant during the day can be a good strategy. However, earbuds will make it more likely to experience itchiness at nighttime, and it's more important to be able to use night time earplugs for sleep quality. An alternative is to use bone conduction headsets, which do not put anything in-ear. The sound quality is more mediocre than with earbuds though, but still good enough to enjoy, and there are several other major advantages: silent to others, and the ability to hear and react to every surrounding sounds since the ears aren't filled with earbuds. They are hence perfect for use at night or in silent environments. Bone conduction headphones can be used in combination with in-ear plugs. AfterShockz is the leader in bone conduction headphones, but mimicking brands at a much lower price are available with an acceptable audio quality. They can also be comfortably worn simultaneously with Luminette light therapy glasses. Tip: after each use, cross the branches so that the headphone keeps its tight fitting form to maintain good contact with the skin, which is necessary for good sound quality.

- Mind the ultradian cycles (20 minutes "sleep gates" every 1h30) and the dopaminergic forbidden zone of sleeping. When you feel sleepy, you can expect this feeling to last only 20 min, and then to go away. The next sleep gate, where the tiredness feeling will reappear for 20 min, will be about 1h30 after the last gate. The gates aren't all equal, there is one with a maximum sleepiness feeling, and the others will have a reduced feeling. Trying to sleep at these 20min sleep gates allow to fall asleep fast, sleeping outside may be possible but will be more difficult. See the relevant subsection below for more information on sleep gates.
- If you can't sleep (eg, missed the ultradian cycle window), then wake up or sit and do something else than trying to sleep until the next window to sleep.
- Identify any factor that can impair your sleep and buy the necessary workarounds. Anything that can prevent disturbances on your sleep is well worth it. If it's too warm temperature, buy a fan. If it's mosquitos, buy an anti-mosquito net for beds and/or a anti-mosquito lamp.
- We are the product of our environment. If your environment is not adequate for good sleep, such as noisy neighbors, no tools can completely fix it. Consider moving to another place if you can.
- If you snore regularly, this means your airways are obstructed, and that your sleep quality is impaired. Regular snoring is never normal. Consider getting a sleep study for sleep apnea. Meanwhile, you can try:
- to sleep more on your sides by removing your pillows (as we are more likely to snore on our backs). Indeed, position-related snoring is a known phenomenon and is why military soldiers keep their backpacks while sleeping during missions, to avoid sleeping on their backs which increases the likelihood of snoring and hence of giving their position to their enemies. Furthermore, tribal populations have shown that they sleep without any pillow but on their arms, hence on their sides, and the researchers noticed they have a much lower prevalence of musculoskelettal disorders in addition to no snoring issues.
- to use nasal sprays to clean the airways before sleep. This is an advice given by a nurse in a sleep study, this claim needs to be tested and double-checked.
- If you have other afflictions that impair your sleep or blatantly wake you up in the middle of your sleep, then treat them. This is especially important for comorbid disorders that have a circadian pattern (ie, they trigger more often during the circadian night, such as RLS and PLMD and night legs cramps). The VLiDACMel protocol prevents entrainment, but having a restful night requires more than entrainment, it requires uninterrupted sleep.
- Optional but strongly advised: take multivitamins and minerals, including vitamin B12, vitamin A and magnesium supplementation every mornings. B12 vitamin is known to amplify the magnitude of the circadian rhythm shift of light therapies (see also here) and B12 supplementation entrained a few individuals with non24 (see also here and here). Vitamin A is necessary to synthesize all opsins in the eyes, including the melanopsin pigment necessary for ipRGC cells and entrainment to bright light to work, hence it is necessary to ensure adequate levels of vitamin A, via supplementation if needed. Magnesium also affects the circadian rhythm (studies here and here). Vitamin D deficiencies can affect the circadian rhythm and it interacts with at least 2 clock genes, and vitamin D appears to inhibit melatonin, as the body does not expect to get exposed to Vitamin D unless there is skin exposure to sunlight with UVs, so that Vitamin D supplements should preferably be taken in the circadian morning rather than the evening to avoid fragmenting sleep. Vitamin B6 helps with serotonin and melatonin secretion. Supplements are not going to fix your circadian rhythm, but deficiencies can have a detrimental effect on it, although it's not always a fix as shown by the ineffectiveness of B12 in a placebo controlled trial on DSPD. These vitamin deficiencies may be caused by gut microbiota dysbiosis, such as bacterial overgrowth (ie, candida albicans). Furthermore, deficiencies in other vitamins and minerals may impact mood (eg, magnesium), neurology (eg, vitamin B6, B12) and the circadian rhythm (magnesium and B12), so by taking a multivitamins and minerals supplementation you eliminate these potential factors on your sleep easily. Plus, if you do a strict ketogenic diet, this supplementation will help avoid electrolytes imbalance (but you may also need to supplement in salt). For B12 supplementation, use cyanocobalamin form, as it can be converted by the body into both forms of B12 (methylcobalamin or adenosylcobalamin). Some Discord users with diagnosed chronic B12 deficiencies influencing their circadian rhythm reported the B12 shots are more effective than the pills. Vitamins B3 and B5 are necessary for the production of cortisol, the hormone that prepare the body to fight stress and which interacts with the circadian rhythm, and which release can be boosted with bright blue light therapy. Over supplementation in vitamin D may inhibit melatonin which is hypothesized to result from a cross side effect of vitamin D production, which is triggered by skin exposure to UV, so the eyes are often also concurrently exposed to bright light, so a inhibiting pathway between vitamin D and the circadian rhythm may have hence developed as a byproduct of light exposure, although other individuals with non-24 and DSPD reported that treating their vitamin D deficiency significantly improved their sleep and circadian rhythm stability, so it seems there needs to be not too little but not too much vitamin D to avoid impairing sleep.
- Although optional for entrainment, vitamins and minerals supplementation can be necessary for some individuals in order to sleep, as in the author's case. Indeed, without supplementation over 40 days, the author experienced limbs swelling, hands cramps, muscular weakness and joints and chest and limbs pain, to the point where these symptoms prevented sleeping more than 1h30 in one go, which was of course unsustainable. These symptoms were signs of a peripheral neuropathy, due to vitamins or minerals deficiency. Although the exact deficiency could not be determined yet, it was likely a combination of genetic predisposition in Vitamin B12 deficiency (the author possesses the AG variant in the FUT2 rs602662 location), which is known to be capable of causing peripheral neuropathy (although other vitamins deficiencies can also be responsible — see also here), and a too restrictive diet (due to this experiment, a very precise diet was devised and used everyday to reduce the effect of food composition on the circadian rhythm). Vitamin-induced peripheral neuropathy is a serious disease that can cause permanent nerves damages. There are anecdotal reports from discord members with the non24 disorder that intravenous administration of vitamin B12 was much more effective than oral supplementation, similarly to how intravenous iron supplementation seem to be more effective at treating RLS than oral iron supplementation.
- Leverage the increased availability of rare and specific products in online stores such as Amazon. Although these stores certainly have a detrimental impact on local commercial shops, there is certainly an advantage in allowing rare products to be available worldwide and able to reach the target audience, whereas before such specific products were impossible to obtain even in big cities.
- The author took multivitamins everyday in the morning (at wake up or later if forgot) for the whole duration of the experiment.
- If you don't know what to choose as a multivitamins supplement, try to get a vitamins+minerals supplement with as many different vitamins/minerals as possible, and with each vitamin/mineral fulfilling only a fraction of the daily needs, eg, at least 15% and not more than 50-60%. This way, you are more than likely within safe bounds even if you include vitamins and minerals intake from food. This works because to avoid vitamins/minerals deficiencies, you don't need 100%, you just need more than 0-10% of daily needs, enough for the body's essential processes to work. Generally, you want metals to remain low, because they have a higher risk of overdosing with deleterious side effects, especially since some is ingested from food. Prefer pills or tablets, but not chewable gummies, because the latter have poorer quality control, and dosage can vary wildly from what is written on the package.
- Plan out how to handle the sleepless nights and premature wake ups. Sleepless nights and premature wake ups will always continue to randomly happen due to non-24, as there is unfortunately an uncurable insomnia component, even when under an effective entrainment therapy. In fact, these sleepless nights and premature wake ups become even more apparent during entrainment, as it becomes clear it's not due to the circadian rhythm. Most individuals with non-24 lose invaluable time trying to desperately sleep for hours, without succeeding. Alone in the dark for hours, with nothing to do but think, this creates the perfect opportunity for the brain to generate running thoughts, as nobody is able to suppress all thoughts for hours. This can ultimately cause a depressive feeling of powerlessness. That's why the core of sleep hygiene is to avoid staying in bed if you can't sleep. Hence, it's important to plan what to do during these sleepless nights.
- A good strategy is to strike a deal with oneself to always put one's sleep first, but to allow to get up and do activities if unable to sleep for more than 30min. If you can't sleep and do not feel tired after 30min of trying, get up and do something. But whenever you feel tired, try to go back to sleep/nap as your top priority. If again it does not work after 30min, you can get back up and do activities. This is similar to the core tenets of sleep hygiene, do not stay in bed for too long if you cannot sleep.
- Make sure to avoid getting exposed to bright light (ie, use dark therapy) during sleepless nights. Hence, screens are allowed, but only dimmed to the minimum and with a blue light filtering software.
- Although not a therapy per se, planning out how to handle sleepless nights is definitely the most liberating realization for individuals with non-24. Being unable to sleep is already a huge handicap, but losing this time altogether by not doing any activity is even worse. Being sleepless does not mean you are not allowed to enjoy yourself and the activities you like.
Continue this combination of therapy strictly (respect the hours and use melatonin and light therapy everyday!) for at least 10 days. Indeed, because of carry-over effects such as photic history and gut microbiota adaptation, it takes several days to a few weeks for the body to adapt to both light and dietary (including melatonin) changes. This means that when starting a circadian rhythm therapy, it will take about 10 days for the full effects to be seen, and it will take as much time when stopping the therapy for the effects to wear off. But you should already see some effects a few days in, such as mood and vigilance boost from the first use of light therapy, and some circadian rhythm phase advance after 2 days of light therapy.
In practice, the first week you should see a reduction of your phase delay, and the next week your circadian rhythm (in particular your wake-up time) should be somewhat stable, and it will get more stable along time as you continue with the therapy. The time windows need to be respected, but a slight change of +-30min from day to day is OK in my experience (eg, taking melatonin 30min later or earlier, and there is more room for food timing as long as you do not eat past the melatonin intake time).
In case there is some unexpected event and you miss the therapy one day or use an alarm clock to wake up to get to an appointment, keep in mind that long napping is allowed and advised, as it reduces most of the health issues that sleep deprivation causes.
It should be emphasized that it is crucial that all the non-optional steps should be followed for the therapy to be effective, at least at first. Indeed, this therapy combines multiple approaches to increase the likelihood and robustness of entrainment. Once entrainment is achieved, the patient can try to eliminate some steps as they see fit if they can stay entrained. Indeed, some patients with a less treatment-resistant form of non-24 can be entrained with solely using melatonin pills, others with the ketogenic diet alone, and others with light therapy glasses alone as evidenced by several anecdotal patients accounts.
Maintenance: Once entrainment is achieved, the treatment must be continued as-is with the parameters (ie, light therapy timing and duration, melatonin timing and dosage, dark therapy setup, etc) you found effective for maintenance of benefits. Indeed, the therapy is effective only as long as it is used, otherwise effects are lost under a few weeks, but the therapy can always be restarted later. Note however that the therapy is not 100% effective, it cannot totally freeze the circadian rhythm in place (due to disturbances in daily life that prevent following consistently the therapy, and varying uncontrollable external factors such as sunlight intensity and duration exposure), hence expect to get misaligned from time to time: for individuals with non-24, simply discontinue therapy temporarily to freerun a few weeks until the ideal time is reached again; for DSPD, increase the exposure duration, or discontinue therapy temporarily to find where your circadian phase is, to re-time properly light therapy.
Monitoring
- To optimize dark therapy, you can use a free "lux meter" app on your smartphone, with the screen directed at the light source to check the light intensity (lux). Lux varies with placement, orientation and distance, so it is important to orient the light sensor on the smartphone screen (usually at the top) directed at the light source, at eye level and at the distance you will usually be from the lamp. What lux intensity is low enough for dark therapy? The lower the better, but below 40lux should be fine, below 20 lux is great. If a light source (eg, lamp) is too intense, try playing with distance by placing the light source further from where you will be in your room when you'll use the lamp. If you have a Luminette, you can calibrate your lux meter app by measuring the Luminette 3 settings, the readings should be 500 lux, 1000 lux and 1500 lux. Remember that light intensity is only one factor, the other being the light color, which should be as red as possible (to reduce/eliminate blue and green colored light). Spectral sensors exist but not in smartphones unfortunately so just use your eyes: if a light source is red, it's fine; if it's yellow, it's good enough but not ideal.
- body temperature monitoring as a proxy for the circadian rhythm even when not sleeping or sleeping in circadian misalignment (eg, such as when using alarm clocks due to appointments/work), in which cases the sleep diary is unreliable but temperature monitoring is reliable to reflect the circadian rhythm. TODO: expand this section when milestone 2 is completed.
Safety-Risk analysis
- Apart from the detrimental interaction with insulin and glucose, melatonin is generally considered a safe molecule, being a major antioxydant of the body along with many other health maintenance roles such as male reproduction and also female reproduction (see also here, here and here with not only melatonin but circadian cycle impact on fetal and placenta physiology), and is known to be a major neuroprotector and likely a major anti-cancerigen. It also isn't addictive. It's impossible in practice to overdose with melatonin, no lethal dose was ever found, since even enormous doses up to 6600mg/day for 35 days did not produce any severe toxicity in 11 participants.
- Blue light in blue therapy glasses should be safe in physically healthy, unmedicated people. Most of them filter UV light, so this is not an issue. Also, Luminette and a few others constraint the blue light spectrum to a reduced spectrum and intensity that should not be dangerous at all, and these glasses are certified by the stringent european union safety standards.
- Long term meat-based ketogenic diet may lead to cardiovascular issues and premature death, but vegetarian/plant-based ketogenic diet seems to be on the contrary slightly positive for health.
- Timed meals are shown to be good to regulate glucose and reduce the risk of metabolic diseases such as obesity and cardiovascular diseases. Reducing or avoiding eating when melatonin levels are high was shown to reduce insulin intolerance and hence risks of metabolic diseases.
- See the related sections in Troubleshooting for a more detailed discussion for each tool.
Limitations
Although this therapy can allow for a robust entrainment, entrainment can still be lost under some circumstances, which reminds us that this is not a cure:
- During winter, the shortening and intensity reduction in sunlight can cause a desynchronization. Increasing the duration of artificial light therapy may compensate but may not be sufficient.
- Any illness lasting for more than a week is likely to cause a desynchronization. Some acute severe diseases can as well. Anesthesia also impairs the circadian rhythm. Restless Legs Syndrome (PLMD), PLMD and night legs cramps can also significantly impair sleep and indirectly cause a loss of entrainment, especially since their acute (painful) symptoms show a circadian pattern, which means they are more likely to appear during the circadian night, which naturally push the individual to sleep outside of their circadian night.
- The effect of artificial bright light therapy on the circadian rhythm is variable on a day-to-day basis due to various factors (eg, sunlight exposure in addition to artificial light, and artificial light exposure in the evening, other environmental factors). Hence, light therapy may start producing less phase advance than expected and required at some point, even if the user did not change their usage. This would cause a progressive desynchronization. Likewise, light therapy may start producing more phase advance than expected, and this would cause a desynchronization as well. If noticed early enough, this can be adjust by varying the duration of exposure to light therapy, but in practice it's difficult to assess what direction the desynchronization is happening, as the primary sign is a reduction of sleep duration and efficiency (ie, more fragmentation, lower restorative quality), which provides no information about whether it's because the circadian rhythm shifted later or too much earlier. The introduction of wearable monitoring devices could tremendously improve, or solve, this issue.
- For some individuals, the therapy can be too effective and cause a too short sleep, by phase advancing the wake up time too much compared to the fall asleep time. Here are some tips to reduce the therapy's effectiveness.
- Why is it so hard to treat non-24, and why does it seem like non-24 require much longer or intense light therapy to be temporarily entrained, whereas DSPD seem to require much less? There are logical reasons to assume this holds true, because of the differences in the therapeutic goals. DSPD need a phase advance, which can always be achieved with light therapy, the intensity and duration only modulating how much phase advance is obtained. Non24 on the other hand aims for entrainment, which also involves phase advance but what is required is a stable phase advance, and this changes everything. If stable phase advancing cannot be achieved, it doesn't matter how much phase advance you get, you won't be entrained. https://www.reddit.com/r/DSPD/comments/pvsfjy/comment/hec7o5h/?utm_source=share&utm_medium=web2x&context=3
This is one of the first protocols I tried, and failed, and why I devised the VLiDACMel protocol, which is the only protocol that recommends to use light therapy at the natural wake up time, instead of a set time with an alarm clock. And the results have so far been much better. Not only because it is more effective (if you use light therapy too early, you can phase DELAY and hence worsen your DSPD, instead of phase advance), but also much easier because you suffer from less sleep deprivation, so there is more compliance.
Of course, a combination of multiple factors (eg, getting sick during winter) increases drastically further the likelihood of losing entrainment compared to any single disturbance.
Useful equipment
Some suggestions of useful equipments to monitor or manage circadian rhythm sleep-wake disorders.
Equipment for circadian rhythm entrainment
- Light therapy tools: Luminette 3 (229€). Will be the primary treatment AND a calibration tool to evaluate other light emitting devices (to avoid them during dark therapy). It has a 30-days money back warranty (+ of course a 2 years warranty for defects in European Union), so you can test it for a month to see if it affects your circadian rhythm (it should work under 2 weeks of daily use), and if not you can send it back for free.
- Alternative: use a computer screen at maximum brightness at wake-up for several hours.
- Complement: Lux meter app on smartphones using the smartphone light sensor (free), such as Photometer Pro. For objective assessment of sunlight exposure whether indoors or outdoors (to discriminate what devices are OK before bedtime). Consider anything below 2000 lux to be insufficient for light therapy, except if it's the Luminette, because it is enriched in blue colored light to increase stimulation with lower intensity of light.
- Alternative: A more expensive but more accurate alternative is to use a dedicated, necklace worn melanopic lux (mLux) sensor such as the LYS button. Melanopic Lux accounts for the different effect of colors, so that blue light is assessed as more impactful on the circadian rhythm than light without blue-green color.
- Alternative: Ayo light therapy glasses, not tested by this document's author, but there is some evidence that blue light alone may be much more effective than blue-enriched white light due to circadian light subadditivity, although blue-only light therapy should be avoided by albino indvidiuals or always dilated pupils, white light can be less phototoxic then.
- Dark therapy tools:
- If the expected use is only to filter evening/night time artificial light: Blue blocker glasses such as UVEX with the SCT Orange coating glasses (there are multiple models: Skyper, Astro OTG, Protégé and Ultra-spec 2000, the latter being the best model to wear with other glasses below) ;
- If the expected use is to filter daytime sunlight, whether indoors or outdoors: wrap-around blue blocker sunglasses such as hair removal laser safety amber/red tinted glasses filtering wavelengths in the range 400-550nm (example), as these laser safety glasses are made to ensure protection against the much more dangerous lasers and hence also include a VLT (light dimming) filter in addition to a color wavelength filter.
- Complement: use blue light filtering softwares such as f.lux or LightBulb on computers and Twilight on smartphones, in addition to screen dimmers such as Nelson Pires Dimmer v2 on Windows or Twilight on Android smartphones.
- Complement: buy RGB smart LED bulbs that can be controlled by wifi or a remote. These can usually be programmed to automatically switch to a dimmed red light at a specific time every day, hence automating dark therapy at home. One inexpensive example is Yeelight 1S, which costs about 15 euros to 20 euros apiece.
- Alternative: Add clip-on or VLT shading films to dim down light sources, these can be added on any normal glasses, but usually they do not filter much and do not adapt well.
- Optional: SwitchBot automatic curtains opener. SwitchBot is a brand that specializes in domotic automatization of non connected furnitures. The automatic curtains opener is programmable with a smartphone app and has several variants depending on the type of fixture: U-rail, I-rail or rod, and it has an optional solar panel to avoid the need to recharge. It is relatively affordable, at about 80 euros one device, plus 20 euros for one solar panel. This can be a great alternative to sunrise lamps, or be useful to automatically take care of pets.
- Melatonin instant-release <= 3mg. The author of this document strongly recommends the sublingual Valdispert Melatonin 1.9mg Instant Release (mirror) (it's the one with pure melatonin only, not the one with "4 actions" because of including other compounds). This will help with stabilizing the circadian rhythm at the time you wish and feel sleepiness. Always prefer sublingual (orodispersible in french) tablets with no other compound than melatonin (besides of course a few conservative or flavor additives that are always present), as sublingual pure melatonin tablets are of higher quality in general, particularly for over-the-counter melatonin.
- Another person with non-24 recommended "Vitality NUTRITIONALS" which are sublingual instant release melatonin like Valdispert's, distributed by VitaminExpress www.vitaminexpress.org Made in the Netherlands, dosed at 1.5mg. However this is a bottle, not packaged in individually sealed blister pack, hence this may degrade after a week of opening the bottle. Another untested product is Chronobiane 1mg.
- In UK, Pharma Nord Melatonine 3mg (not Melatonin Complex) was licensed in the UK for the treatment of jet lag, and hence should be appropriate for circadian rhythm disorders too.
- In the USA, there are only a few recommendable pure sublingual melatonin tablets in blister packs. One is Major Melatonin Tablets, 3 mg, 100 Count as sold by Walmart, another is webber naturals Melatonin 3 mg - 15 Tablet Blister Pack sometimes sold in 2 packs (30 days = 1 month). Alternatively, search for "melatonin blister pack sublingual tablet" or if no blister pack is available, at least search for "melatonin usp sublingual" (the USP label being a self-validation process). Do not worry about instant release or prolonged release, both should work anyway and if you take a sublingual tablet, it can only be an instant release since it's the coating that makes a prolonged release, hence prolonged release melatonin is always presented as an ingestible pill that will dissolves slowly in the guts. The author did not try these products. Another product is Herbatonin 0.3mg, also in blister packs.
- Optional: DNA testing using Nebula Genomics, if MT2 receptor mutation, then also need to control food/glucose and melatonin mixup?
- Optional: smart alarm / chronobiological alarm clock, such as Sleeptracker Pro watches (discontinued) or FitBit, or smartphone apps such as Sleep As Android, which can vibrate when it detects that the user is in a light sleep stage via actigraphy, it's very efficient to forcefully wake up despite sleep deprivation. Indeed, humans are highly sensitive and alert to touch and vibration, much more than sound. In addition, waking up during a light sleep stage is highly effective as to the user it feels like they are already awake when the alarm triggers, so that they are much less likely to fall back asleep or snooze. The window of detection of the light sleep stage is configurable, usually the default is 30min, which means that at the earliest, the smart alarm triggers 30min before the programmed alarm time if a light sleep stage is detected, and at worst at the programmed alarm time if no light sleep stage is detected. The combination of both features (smart alarm + vibration) is hence highly effective to wake up, and has the distinct advantage over sound that it doesn't bother partners living close by or in the same bed (which would induce sleep deprivation for them, a common but avoidable side effect that partners of individuals with circadian rhythm disorders often experience). However, they do not fix sleep deprivation so the tiredness and cognitive impairments will still appear during the day. Alternatives include Axbo and Sleep As Android app on Android smartphones (but apps are less accurate than wearables). Avoid faradic stimulation (electrically induced pain) devices such as Pavlok which are not supported modern science and will only increase sleep deprivation and pain. Combine with napping whenever possible to reduce sleep deprivation.
Tools for circadian rhythm disorder management
- Sleepmeter Free on Android and its widget, to log a sleep diary and generate sleep charts. Just tap the Sleepmeter Widget before sleep and tap again when you wake up, it will record your sleep timing and duration, and optionally you can add tags and comments after if you want to add more infos for yourself. In early 2021, Sleepmeter mysteriously disappeared from the Play Store and the developer is unresponsive to sollicitations, but the app can still be downloaded on APK Pure, its widget also.
- Sleepmeter Free can also be used on computers (Windows, MacOS and Linux) via BlueStacks 4, an Android emulator. Simply install BlueStacks, then download Sleepmeter Free APK (APK = installation file for Android app), and simply double click on the downloaded APK. BlueStacks should automatically install the app and it should show up in "My Games" tab inside BlueStacks.
- Note: Sleepmeter Widget does not work correctly on Android 10+: if it doesn't switch from sleep to wake or inversely when tapping on the widget, then it's necessary to open the app, then minimize it and then try to tap again, then switching states should work. This appears to be because newer Android versions do not allow widgets to update data in sqlite databases. It appears the app will stop functioning sooner or later. If you know how to program in javascrit/typescrit/react-native, please help us make an open-source digital sleep diary app to replace Sleepmeter, see the circalog project for more infos, a sketchboard and a relational database graph are available.
- On Android 14+, SleepMeter cannot normally be installed, but it is possible to force installation (thanks to eatnerdsgetshredded for the tip!): enable USB debugging on your phone (you need to enable Developer Mode, no root needed), then connect your phone to a PC with USB debugging, and then you can use the following ADB command: `adb install --bypass-low-target-sdk-block app.apk`
- Paper sleep diary alternative: the AASM sleep diary / sleep graph template is recommended (mirror here, 2021 updated version here).
- Here is an unofficial digital spreadsheet version of the AASM sleep diary template, which presents two major advantages: 1) can store unlimited data and hence eases assessment of chronicity of sleep patterns, 2) easier to carry everywhere in a smartphone or computer and hence input data at any time and anywhere and hence reduces misrecalls and data loss.
- Alternative opensource free electronic diary with a similar tapping system: https://andrew-sayers.github.io/sleep-diary/
- Another alternative opensource electronic diary for Android is Plees-Tracker: https://github.com/vmiklos/plees-tracker
- Another alternative for Windows PC computers is SleepChart, by the developer of SuperMemo, who has non-24. The main advantage of this app is the ease to input data from a paper sleep diary for example, simply click on the chart to draw the sleep graph.
- Alternative: For sleep tracker wearables users such as Fitbit wristbands, the data from these sensors can be converted to a sleep graph using Kizari's tool: https://sleepcharter.z13.web.core.windows.net/
- Alternative: For individuals with sleep apnea, the CPAP machine may be able to record usage data, which can then be converted to a sleep diary using the opensource tool OSCAR, as was done by DSPD reddit member EarendilStar.
- Alternative: Sleep Diary Dashboard by Andrew Sayers, another wonderful opensource tool that can convert various sleep diary formats including Sleepmeter, Plees-tracker and custom excel sheets into a generic sleep diary format and then generate a standard medical sleep report to present to doctors.
- Alternative: for owners of FitBit devices, sleep graphs can be generated very easily using this open-source software: https://fitbit-sleep-vis.netlify.app/ (sourcecode is here: https://github.com/carrotflakes/fitbit-sleep-vis )
- Alternative: not free, but this is a baby tracker which can generate a nice sleep graph but also tracks other things such as meal times, etc: Huckleberry.
- Alternative: Track & Graph, an open-source Android app to track various factors that are completely customizable by the user, with customizable graphs and data import/export as csv files (thanks to Yetscrape for the tip!): https://github.com/SamAmco/track-and-graph
- Lux meter apps on smartphones. Any free lux meter app will do, what matters is the light sensor included in the smartphone, but nowadays most light sensors mimic pretty well the eyes responsivity to bright light, so the measurement is quite accurate between 5 lux to 30k lux usually.
- Online Actogram by Barrett Davis, an opensource preliminary python tool to potentially massively screen for circadian rhythm disorders using browser's history (examples here).
- Noice, an opensource multisounds whitenoise generator for Android. I recommend enabling a mix of pink noise, brown noise and a natural noise (such as gentle raindrops) to efficiently mask out external noises. Here is a preset the current document's author made for newborns but which also works for adults: InfantWombSim for Noice v1.3.3, WombSimulator fo Noice v2.
- Other opensource white noise generators on Android include Chroma Doze and White Noise Plus.
- An equivalent non-free app for iOS/iPhone is Dark Noise by Charlie Chapman, but the source-code is available so maybe it can be installed for free from the app files?
- HabitLab to ease sleep hygiene (reduce disruption from social apps).
- LightBulb, an opensource blue light filter and brightness dimmer for Windows
- An alternative on Linux is GammaStep, which automatically change the gamma (brightness and color) of the screen depending on your surroundings (acquired from the webcam). An awesome idea that partially automates dark therapy.
- Dark modes for various apps are highly recommended, they can greatly reduce eye strain and the potential impact of screens on the circadian rhythm while improving readability.
- A dark mode is when the background is made dark or black, and the text or interesting features in light color or white. Since the background represents most of the displayed space, this greatly reduces the total emitted lux, without losing any content visibility. A basic dark mode can often be implemented by simply inverting colors, but this does not always work (eg, pictures should not get color inverted but icons should), hence good dark modes are tailored for each app/websites.
- Set the OS into a dark mode, not only the windows bars will be darker, but also apps that support native dark mode will also switch automatically.
- Most web browsers now offer a dark mode, either as an app setting or depending on whether the OS is set to a dark mode, such as Chrome and Firefox on desktop, or Firefox and Opera Touch on mobile.
- Adobe Reader offers a native dark mode to read PDFs on desktop, Xodo PDF offers the same on mobiles (Android and iOS).
- DarkReader web plugin allows to open online PDFs in Chrome with a dark background.
- A lot of text editors offer a dark mode: Notepad++, Zim Desktop Wiki, Zettlr, etc.
- Some apps provide more dynamic features for context-specific dark modes, such as dimming more the unnecessary background on websites such as YouTube or even unfocused apps, such as the opensource Turn Off The Lights browser extension and app.
- The book Sleep Misfits: The reality of Delayed Sleep Phase Syndrome & Non-24 by Sally Cat is highly recommended, being the only book currently written compiling the experience specific to patients living with non-24 and DSPD handicaps. Reviews note that this book can help in validating one's own experience, and help with acceptance and coping with the handicap.
- Rotime is a web app available for desktop (Windows, Linux, MacOSX) and mobile (Android, iOS) specifically tailored for people with non-24 and other circadian rhythm disorders. The main demographic is workers with circadian rhythm disorders or shift work, but it can be used by anyone who want to schedule their days on a dynamic non-24 timeframe. You can enter your tasks, their durations, and then the app allows to easily rotate the time you start these tasks. It's a great app for designing a work schedule in general, but not for specific tasks. There is a free version for one day, or an older free version all the time in WebArchive, or a paid version with more features and to support further development.
Wearable circadian rhythm monitor
TODO: This section is a work-in-progress, come back later for updates.
Although there is currently a "wearables revolution", it is still hard to find devices that can continuously (24/7) record vital signs with a sufficient quality (sampling rate, low noise) to be considered adequate for research or medical purposes. However, there are a few, which we could use and determine as adequate for the purpose of ambulatory circadian rhythm monitoring, potentially by the patients themselves.
The two major sensors that are the most informative to monitor the circadian rhythm are the temperature sensors and the ECG (with an accelerometer). The total for one iButton and a Polar H10 is less than 200 euros, and these devices will last for several years.
The following is a practical, hands-on summary for the more complete setup instructions found in the Wearadian project: https://circadiaware.github.io/wearadian/docs/SleepNon24BiologicalMeasures.html
Usage advices: A critically important technique to properly wear these devices is to move them regularly to let the skin breathe, so as to avoid rashes (caused by sweat and warmness but not allergies since they are made of cotton and silicon), it's necessary to move the sensors (and belt) up and down to slightly different body sites to let the skin breathe. Hence, the sensors should be moved at least twice a day (once at wake up, once before sleep), and they should also be moved as soon as there is a feeling of itchiness. For example, the chest belt can be placed above the solar plexus during the day, and below (overlapping with the belly) before sleep and for the duration of the night. Cleaning the sensors and belt with a tissues imbibed with alcohol everytime after shower and every half week is also a good practice.
Also, it's important to avoid any wearable that requires the use of medical tape or sticky gel patches/electrodes, as they may feel comfortable at first but will invariable produce a skin reaction after some time (usually a few days). For continuous wear, it's necessary to use wearables that can be in contact with the skin without any adhesive, such as by strapping a belt.
- Wrist skin temperature sensor for circadian rhythm phase assessment: Maxim Thermocron iButton DS1925EVKIT (Starter kit including one DS1925L iButton and the DS9490R and DS1402D-DR8+ connectors to retrieve the iButton's data on computers via USB as a CSV file). Cost: about ~$100. If DS1925L is unavailable, can also use DS1922L but the internal memory and battery are much shorter (4 years for DS1925L with 5min sampling rate (setup 300s in the config), 6 months for DS1922L for 2min sampling rate). The iButton does not need to be recharged, but unfortunately once it runs out of battery, it cannot be replaced (although there is one academic paper which shows how to replace an iButton's battery, but this is not an official instruction, do it at your own risk, and of course the device will be much less airtight after).
- Advantages: Wrist skin temperature has no delay, it shows the current state of the circadian phase because it directly captures changes in body heat flux, whereas core body temperature lags behind by a hour or two due to thermoregulation lag.
- Disadvantages: rarely painful on the wrist especially when using a brand new cotton sport wristband until it loosen with use, clunky software suite (no smartphone app, transfert is wired via USB, need to export to CSV and then plot yourself the data or use Circalizer), prone to bias (the cotton wristband insulates the iButton from environment but still skin temperature can be biased by a lot of environmental factors, and it's just more noisy by nature compared to core body temperature).
- Interpretation tips: opposite of the core body temperature: a steep increase of wrist skin temperature signals the start of the circadian night, while a slow decrease signals its end and the start of the circadian morning and day. There is no time lag. The data needs to be smoothed because it is very noisy.
- Tips for usage:
- Buy a cotton sports wrist band such as is used for tennis ("sweat bands"). The Under Armour wristbands are recommended, they exist in various lengths, even tiny ones which are great during summer.
- Buy also velcro stripes (hooks type, not loop) with adhesive, to glue on the iButton and then attach it on the inside of the cotton sports wrist band (the velcro hooks will hook well on the cotton as it naturally forms loops).
- How to wear: Wear the cotton wristband + iButton on the non-dominant arm, and place the iButton on contact with the skin, positioned at about the middle of the width of the arm to be on the radial artery. Technically, the radiar artery runs from the wrist to the elbow's interior, so you can place the iButton anywhere on the length of the forearm. In fact, you should regularly move the wristband up and down your forearm, to avoid the sweat and warmth accumulation from itching and damaging the skin (move twice a day at least, and whenever it's itching). If necessary, the wristband can be placed on the dominant arm for some time if you need the non-dominant one to rest.
- This setup provides true 24/7 skin temperature monitoring setup, as the iButton DS1925L can last 4 years with its integrated battery (then need to buy another one or can try to manually replace the battery as some researchers have done).
- Data need to be transferred to a computer every 6 months (for the DS1925L, the interval is much smaller with DS1922L, about 10 days), and the device memory (mission) needs to be reset, otherwise it will stop recording new records.
- ECG (heart rate and heart rhythm) + 3-axis accelerometer (chest actigraphy): Polar H10. Costs about 80€. Comes with a chest strap. Battery: 16.5 days with one button (CR2320) battery.
- Advantages: ECG is useful to assess sleep quality and discriminate out of phase sleep (ie, naps) vs in phase sleep sessions (ie, biological night sleep sessions), by observing the heart rate. ECG is also useful to evaluate some health parameters especially cardiac, and can allow to pre-screen for further assessments by a cardiologist using ECG devices with more channels. The accelerometer can be used as an actigraphic diagnostic tool, although being on the chest means it is less accurate than on the wrist (as laying down can be sufficient to appear as being asleep). The accelerometer can foremost be used to regress motion artefacts.
- Disadvantages: Wearing a chest belt can be uncomfortable until you get accustomed to it ; requires a phone that is always connected to the ECG (ie, you will likely need to buy a dedicated phone and wear it at all times) ; storage consumption (ECG generates a LOT of data).
- Interpretation tips: Heart rate is generally higher during high wakefulness phases of the circadian rhythm, and lower during the circadian night. Posture also plays a role, with laying down decreasing the heart rate (just like core body temperature), but not as much as the circadian night. Heart rate variability may also be interesting to observe but this needs to be calculated manually.
- Tips for usage:
- The Polar H10 chest strap sensor was selected because it's the only chest strap ECG available with a long battery (2 weeks!), and all other consumer devices can only capture heart rate. Furthermore, chest strap ECG is more reliable for long-term ECG acquisition than wet electrodes or other systems, because there is no wires and hence chest strap ECG is the only ECG technology reliable enough to capture ECG during motion (ie, cheststrap ECG is robust to motion artifacts), as motion is unavoidable in continuous 24/7 use (and especially during the biological night for sleep acquisition!).
- The Polar H10 sensor needs to be paired via Bluetooth to a smartphone, and an app needs to be used at all times to record the ECG (because only heart rate can be stored on the internal memory). No cloud service registration required, all data is stored locally.
- Use the Polar Sensor Logger app on Android by Jukka Happonen to log both the ECG and accelerometer data with the sampling rate of your choice (up to 200Hz/8G for the accelerometer and 130Hz for the ECG). It also saves the Heart Rate in a separate CSV file, and the extra columns represent the RR-interval in milliseconds. The timestamp format is in nanoseconds and the epoch is 1.1.2000. Note that the app requires both bluetooth and location (GPS), hence to save battery, the phone can be set to Plane mode and wi-fi can be disabled, everything can be disabled except bluetooth and location, and the screen can be turned off during data collection. Data is stored in realtime in csv files in the sensorDataLogs folder at the device's root, so that even if the logging is interrupted due to a bug or the device being out of battery, the last logging session won't be lost.
- Note that although the app can work with only bluetooth, it won't be able to seek and automatically reconnect to the Polar H10 sensor in case of disconnection without location (GPS) enabled.
- Also to ensure automatic reconnection, it's necessary to enable the dual Bluetooth stream/pairing on the Polar H10 after each change of battery (the memory is flushed then) using the official Polar Beats app. Note that this app requires enabling wi-fi temporarily (in addition to Bluetooth and GPS location) to pair with the Polar H10. This can be a good opportunity to also disable GymLink and ANT+ to extend the H10's battery life.
- It might be a good idea to buy a dedicated Android phone with a long battery, which can allow to continuously record up to 10 days with a single charge (the generated data with accelerometer set to 100Hz and 2G is 400KB/min total for accelerometer+ECG+Heart Rate, so this makes for 6GB for 10 days, or 17GB/month, or 200GB/year of data, so it all fits in any modern smartphone's internal memory).
- Using as a bluetooth receiver the Realme 6i and its 5000mAh battery (cost about 170 euros), the battery consumption rate is 10%/24h, hence up to 9-10 days can be acquired with one full charge.
- To achieve this and avoid background app kill, the Realme 6i with Android 10 needs to be setup as follows: set Background Processes Limit to 4 instead of standard limit in Developers Options ; disable Do Not Disturb mode ; in battery optimizations options (specific to Realme UI), disable all optimizations except for screen optimizations and standby optimizations ; finally, launch the Polar Sensor Logger app and Lock it (show the list of apps and then click on the 3 dots in the corner to see the Lock option), then launch the acquisition, setup 100Hz/4G for the accelerometer, then click on the Graph tab and click on the Pause button (after checking that the graphs were alright). Now switch back to the Main tab and you can turn off the screen and let the acquisition run. The data will be continuously saved in CSV files in the sensorDataLogs folder, even if the app or phone crashes at some point.
- Alternative to get an even longer battery bluetooth receptor: make an Arduino-based bluetooth low energy (BLE = BT 4.0) logger to microsd card. Some developers already made heart rate loggers for Arduino and Polar H7 chest bands (see also here and here), but not the ECG, although the SDK is open so that should also be possible to do.
- Using the Polar Beat app, the H10 sensor can be configured to have a dual Bluetooth stream, so that it can send data to two different devices/apps simultaneously. This can be used advantageously to concurrently continuously record the ECG data on one device, and use another device (the day-to-day smartphone) for when you want to visualize your current heart rhythm in real-time. To do this, install the Polar Beat app, pair the sensor, then go to the settings, click on the sensor and the sensor's options will show up, and then you can enable the "2 BLE receptors" option.
- Using the Polar Beat app, disable GymLink and ANT+ to significantly increase battery duration.
- Combined with the EliteHRV Android and iOS app, the Polar H10 can be used for breathing relaxation exercises (fundamental resonance breathing etc), and also be used as a biofeedback tool.
- Big advantage of this setup compared to others: it really allows for continuous ECG, since there is no need to transfer data to restart a new session, as the phone's memory is used and it's vast. So only the smartphone's battery is the limitation, but it can be recharged while the acquisition continues, and even the data can be transferred concurrently using FTP or similar apps. This is a true 24/7 continuous ECG monitoring setup.
- Main limitation: a receiver bluetooth smartphone needs to ALWAYS be in range to capture the data. Since the bitrate necessary to transfer ECG is quite high, and the signal is greatly attenuated by going through objects, it's easy for the ECG signal to be lost. Fortunately, the Polar Sensor Logger app by Jukka Happonen was kindly updated following the author's feedbacks to automatically reconnect on signal loss, as long as GPS is enabled (due to a limitation of how Bluetooth is managed by Android OS).
Innovations of this protocol
Key innovations compared to other circadian or light-based therapies:
- Very long exposure to low-to-moderate intensity of light therapy. Very long means from 4-5h up to the same duration as sunlight (10+ hours). Continuous bright light therapy is here used, but intermittent light therapy can be an alternative, although this will cause a lesser inhibition of melatonin and lesser increase in cortisol (hence more daytime sleepiness). Technically, light therapy can be used anytime and all the time during the circadian morning and circadian day, and can be started later than natural wake-up, but must be stopped before the circadian evening.
- This is in contrast to all other light therapeutic protocols that recommend a much shorter exposure duration of 20min-1h/day.
- Wearable light therapy device (eg, light therapy glasses) to ensure a consistent regiment of light therapy exposure at the retinal level, and to increase user compliance by increased comfort and mobility (necessary for long exposure). Light boxes are to be avoided absolutely as they are often ineffective in practice.
- This is in contrast to current recommendations that place the same or more weight on fixed light boxes.
- Therapy starts at natural wake-up time (not at target wake-up time). Alarm clocks are to be avoided, and even if used, light therapy should be used only after the usual or predicted natural wake-up time (or the circadian morning as assessed by melatonin sampling). There is no gradual advancement of the starting time, and no forced early wake-up, the therapy's starting time should shift only along with the natural wake-up time.
- This is in contrast to current recommendations that always start light therapy at the target fixed time, or start from the current natural wake up time and shifts 15-30min earlier every couple of days, requiring a forced wake-up, and unpersonalized to the user's circadian phase (and circadian phase shifting capabilities). Here, the light therapy should be started at natural wake-up, including when natural wake-up is variable. There should be no forced wake-up.
- Should be combined with dark therapy during the circadian evening and circadian night. This avoids losing the benefits of light therapy during the circadian morning and day.
- Other parameters are irrelevant or have a negligible effect in comparison (for circadian entrainment and shifting): beliefs, social factors, physical exercise, food intake (but food intake timing and composition is still important for general health!).
Other innovations of this protocol:
- Does not require prior sleep deprivation nor behavioral constraints (ie, a "strict sleep schedule"), which was shown to reduce the effectiveness of light therapy (due to adenosine buildup) and has highly deleterious consequences.
- Why don't we use a gradual wake up time forced by an alarm clock, eg, asking the patient to forcefully wake up 15min earlier each day to start light therapy? Firstly, because this does not work for individuals with non-24, as the goal here is to shorten the circadian period, and not phase advance, which is way too difficult to achieve anyway with currently available technologies (we simply don't get enough phase shifting to allow for a reliable consisteent phase advance with non-24). Secondly, and this is more applicable to DSPD, as those with DSPD can indeed get a phase advance, but not if they are exposed too early: indeed, if they get exposed before the core body temperature minimum, which can very well happen late in the objective morning due to their delayed phase, then light therapy will actually DELAY their phase further and hence worsen their DSPD symptoms, instead of phase advancing as wished. Although advancing by 15min each day should not apriori cause this issue, it will after long enough, because advancing by 15min/day is MORE advance than the circadian phase advance that can be obtained by circadian shifting therapies, so after 2 weeks or so, the individual will forcefully wake up ~3.5h before their natural wake up time, and it may very well be before their minimum core body temperature point. In fact, most clinicians (incorrectly) recommend their DSPD patients to start light therapy before their CURRENT wake up time, which is ver likely a restricted wake up and hence happening much earlier than their natural wake up time, ond so this further reduces the margin with the core body temperature minimum, and hence starting light therapy just 1h before the restricted wake up time can very well already fall on the phase delay part of the bright light PRC curve. Thirdly, non-24 and DSPD patients who seek therapies are most likely already chronically sleep deprived, hence asking them to further restrict their sleep by subtracting an additional 15min/day of their already short sleep runs the high risk of uncompliance and increases dropout rates, hence it's not surprising that studies using such a protocol always display high dropout rates. Fourthly, the only viable reason to suggest this scheme is because with the effect of light therapy, we can assume that the patient's phase will progressively advance, and hence to optimize and get the most phase advance we have to adapt light therapy's administration timing to be earlier, moving alongside the patient's circadian phase. However, this assumption is flawed in practice, because: 1) 15/day is way too much, bright light therapy does not move the circadian phase this fast, 2) there is a delay (called photic history) of several days up to a week for bright light therapy to affect the circadian rhythm, 3) it is unnecessary, because if light therapy is effective, the patient will naturally wake up earlier and earlier, and so they can simply be instructed to start light therapy as soon as they naturally wake up, which is not only easier and increases compliance, but also allows the therapy to be more individualized as then we are more guaranteed that the patient is not at risk of starting the therapy too early, they will start earlier and earlier but at the pace of their circadian rhythm phase advances, 4) there is still a risk of mistiming and getting exposed too early, because there is a sudden reversal at the core body temperature minimum point, so it's much safer to ensure we get exposed AFTER (to get a phase advance for sure), even if the phase advance magnitude is a bit reduced, we can just increase light therapy intensity and/or duration to compensate; hence in practice even this viable reason is not worth it. Also, don't be mistaken, clinicians don't prescribe this 15min/day scheme to optimize administration timing relative to the PRC curve, tmost have no idea and don't even consider the PRC curve at all, their reasoning is much more simplistic, as they simply assume that we wake up when we get exposed to bright sunlight, so you simply need to get exposed to bright light earlier, and you will magically wake up earlier! But that's not how the circadian rhythm works, it moves with specific and strange rules depending on where you get exposed, it does NOT just synchronize to the time you get exposed to bright light. In summary, there are only drawbacks, and no positive reason to require the patient to follow a scheme of forcefully waking up 15min earlier each day to start bright light therapy.
- Allows not only for entrainment but for sleep schedule correction by waking up earlier and earlier by using very long light therapy with the duration as a variable of adjustment. This allows to adjust the wake up time after entrainment, which no other therapy on non24 could achieve a constantly earlier wake up, the subjects having to freerun until they get in phase again with their ideal sleep schedule, which is highly impractical.
- A previous study by Czeisler's team conducted a study in 2012 on 14 healthy men with a very long bright light therapy regimen of 5-8h of bright light exposure everyday for 5 days, which allowed them to achieve 8h of phase advance on average. This landmark and unfortunately poorly known study shows the viability of very long bright light therapy to produce significant phase advances in a short time span on the human circadian rhythm, in line with a subsequent study by the same lab and the current document's author's experiments results, which were found independently before finding these sources. However, one difference is that Czeisler's study required a concomittant sleep restriction scheme with a behaviorally progressively advanced wake up time (ie, using an alarm clock), whereas the present protocol does not. Also, the present protocol is the first to use very long bright light therapy as a treatment for circadian rhythm disorders. Another difference is the use of Luminette, a portable light therapy device, instead of constraining the patient to a light-filled room. A final difference is that the experiment spanned only one week, whereas for circadian rhythm disorders it's crucial to ensure stability of the new sleep-wake schedule over the long term.
- A step-by-step comprehensive multi-system approach to entrain not only the central clock but also key peripheral clocks, and with multiple treatments with complementary effects to increase the likelihood and robustness of successful entrainment. The goal is to gain control of as many important zeitgebers as possible, while staying implementable in an at-home setting by non medically trained patients.
- Another multi cases study also provided a combined therapy with melatonin and bright light therapy timed relatively to the circadian rhythm phase of non24 patients. However, as in nearly all studies, this was combined with sleep restriction (ie, forced progressively earlier wake up time by an alarm clock) which reduces effectiveness of light therapy, and hence is likely the reason of the high relapse rate. However, the study does not describe meals timing.
- A focus on exogenous factor that can shift the circadian rhythm while accounting for endogenous specificities (eg, photosensitivity, timing of light therapy and melatonin relatively to current circadian rhythm phase, etc). Mental states and behaviors are disregarded as the author found them irrelevant.
- Designed for at-home use in a realistic setting, not in a controlled lab environment.
- Conception of an at-home circadian monitoring protocol, similarly to diabetes insulin and glucose monitoring devices, for the patient to make informed decisions and adequately time the therapies.
- A complete cohesive therapy that can be directly applied in the clinical setting or at home under medical supervision. All the necessary tools and the margin of adjustments (eg, light therapy duration) are described along with the scientific justifications. All other currently available therapies only use one or 2 tools at most and with very precise values for the parameters as the purpose was to study their effects, there is no currently available protocol to use in the clinical practice.
- Easier to implement and follow but also more flexible for the patient in practice than other combination therapies (see also here).
- Low burden diagnostic and monitoring solution through temperature for the patient, more applicable in practice.
- Designed and assessed for long-term use, whereas most circadian shifting interventions are only monitored for one week up to one month rarely.
Associated dataset
A continuously updated dataset covering experiments prior and during this protocol is available including a continuously maintained and annotated electronic sleep diary continuously and a record of a set of vital parameters including core body temperature (via dual-heat-flux method), skin temperature (via Thermocron iButtons), 6-axis actigraphy (using Axivity AX6), light intensity and spectral composition (using in-house recorder, open hardware details to be published) and ECG. This public dataset is available at https://github.com/lrq3000/non24article/tree/master/analysis . It will continue to be expanded as the experiment progresses over time, in order to provide for an as exhaustive as possible view of an individual's circadian rhythm. MRI (3T structural, functional and diffusion) and 30x whole-genome sequence are available upon request by academics.
Going further
- Join an online community to share coping tips and tricks or just your complaints. Medical doctors recommendations do not cover coping tips, that's why patients communities can be very helpful. The r/N24 subreddit is a good place to start. There is also a chatroom on Discord.
Protocol variants
This section contains variants of the VLiDACMel therapy repurposing the same tools and principles but for other aims.
Backward cycling therapy
WIP section: this is a work-in-progress, the content may change at anytime. Please consider this section to be experimental and requires further testing and validation.
The VLiDACMel therapy can be slightly modified to cycle the circadian rhythm backward, in other words to produce so much phase advance that the non-24 individual will wake up earlier and earlier (instead of later and later naturally). In fact, a previous study by Czeisler et al in 2012 did the same with a 5-8h/day regimen of bright light therapy, which achieved 8h of phase advance under 5 days. However, this was never tested before on individuals with non-24, no therapy ever demonstrated such a significant phase advance in their core body temperature. But in the current document's author's experience, this is possible.
To do so, use the VLiDACMel therapy as indicated above for entrainment, and modify the following points:
- Use a very long duration of exposure to blue light therapy glasses, such as 5h or more during summer and 8h or more during winter solstice. This is the key component to cycle backward, increase the duration as much as needed, with longer duration allowing for a nearly proportionally increased phase advance and hence faster backward cycling.
- Expect to sleep less than the full night, because the fall asleep time (melatonin onset) shifts with a delay whereas the wake up time (melatonin offset) instantly reflects the new circadian rhythm phase shifted by light therapy. But it can still be expected to sleep more than when completely out of phase (ie, for adults who on average need 7-8h of sleep, the user of this therapy can expect to still be able to sleep 6-7h/day if they find they can monitor when their circadian rhythm night is and follow their sleep schedule along).
- To reduce the delay between melatonin onset and melatonin offset, one can use melatonin pills 1-2h before the estimated start of the circadian night. Taking exogenous melatonin this late will not result in a circadian phase advance, but this is already taken care of by very long light therapy. Instead, exogenous melatonin is here used very close to the expected fall asleep time to induce sleep a bit earlier and consolidate sleep.
- Avoid eating meals several hours before the biological night. Since it's difficult to estimate especially during backward cycling since the circadian night will be always moving, ideally the user should rather eat a breakfast and lunch (can be eaten later, in the middle of the day, instead of just a few hours after breakfast) and completely skip dinner. This will help avoid digestive issues and the melatonin-insulin-carbohydrates interaction that can slow down the backward cycling and fragment sleep.
- Always prioritize reducing sleep deprivation, as it significantly reduces light therapy and melatonin efficacy: if the individual slept less than 1 ultradian cycle away from what they ideally need should be considered sleep deprivation (eg, if ideal sleep duration is 7-8h, then any sleep session less than 6h is sleep deprivation as it is 1 ultradian cycle = 1h30 to 2h less than ideal sleep duration). If sleep deprived, always take a nap whenever possible to reduce sleep deprivation. The individual can start light therapy after, and for as long as they stop light therapy 3-4h before their (estimated) circadian night. In the author's experience, having trying both avoiding naps and doing naps whenever possible, the latter always improved the backward cycling therapy and made it much faster. Use a sleep eye mask and ear plugs to isolate from environmental sleep disturbances such as sound noises.
- If wearing a body temperature monitoring wearable, avoid bright light and eating if wake up earlier than the end of the circadian night. Wait until the circadian night ends (core body temperature raises above the lower phase, limb temperature: lowers below higher phase).
From preliminary results, the author observed a possibility to backward cycle 1 to 2h/day by using 9h during winter solstice and 5h/day in February. At the time of this writing, the author tried this strategy 5 times and it worked 3 times. However, for the 2 failed attempts, it still allowed to entrain the circadian phase to a stable schedule.

Effect of very long bright light therapy (6-8h/daily) using Luminette v3 on the sleep-wake pattern. On the right, we can see the backward cycling effect thanks to the great phase advance produced by bright light therapy.
A reddit member of the N24 subreddit also reported similar results with 6-8h of artificial bright light therapy daily using Luminette, see here for a chart.
Daylight simulation, an extreme but simpler bright light therapy protocol
This variant protocol is an extreme form of bright light therapy, where we don't just do artificial light therapy to complement sunlight, but to replace it completely. In other words, using this variant, we are not reliant on sunlight anymore for entrainment. This may be a potential strategy to overcome the loss of entrainment during winter and the reduced sunlight exposure, as happens commonly to individuals with non-24 and DSPD. Timing light therapy administration with this protocol is also easier.
Instructions:
- Simply do at least 5h of light therapy, but closer to 10h is better, during the 14h of wakefulness after wake up, as these 14h are your circadian day. Light therapy can be started and ended at any time during these 14 hours. The hours after 14h of wakefulness are to be assumed to be the circadian evening and night, when dark therapy needs to be used instead (ie, staying in the dark, dimming all light sources and filtering blue and green colors to keep red or orange lights).
- If we don't care about the exact timing of the circadian night and wake up time but we just want a stable, consistent sleep schedule, this variant can be started right away, at any point in the circadian phase, no need to wait to be in phase.
This is what the author of the present document is using, and has used several times in the past. This allows to "freeze" the circadian rhythm in place at any timing, it can hence allow non-24 individuals to mimic any chronotype, such as ASPD, morning lark, evening owl, or even DSPD. There are also side benefits such as mood and energy level increases thanks to the anti-depressant effects of bright light exposure, which increase productivity compared to freerunning, even when the circadian rhythm is frozen in an extreme DSPD-like chronotype (eg, sleeping at 8am and waking up at 4pm). This does not however fix all issues and energy levels dysregulations and irrepressible naps/siesta, nor the weird insomnia phenomenon, nor the reliability of the therapy and variability of wake up time is still an issue (ie, the wake up time will still vary in a 1-3h window between different days, but will not delay further, it will remain in this window).
A recent study published in 2022 on patients having developed a non-24 like disorder following severe brain injury found that the use of very long bright light therapy to simulate a whole artificial day allowed to reduce the freerunning period by 3h, which is a staggering amount! Although not directly targeted at true non-24 disorder patients, this paper is the first to demonstrate in practice the clinical usefulness of daylight simulation, and hence is a first step towards a potential validation.
Phase-delay bright light therapy (true chronotherapy)
WARNING: Avoid this protocol if you have DSPD! Indeed, there may be a possibility for some individuals that freerunning can't be stopped once it is started, since there are some case reports of individuals with DSPD turning into Non24 after doing (behavioral) chronotherapy! Phase-delay bright light therapy works differently but still has the same effect of causing a freerunning, albeit more controlled, so that the same risks likely apply!
In the case the entrainment fails at some point and freerunning restarts, it is possible to use bright light therapy in the circadian evening and night, instead of the circadian morning and day, to accelerate the daily phase delay and hence the freerunning period. This can be advantageously used to reduce the periods in inverse phase with the day-night cycle (ie, nightwalking) and restart the VLiDACMel entrainment therapy earlier. In the author's experience, this usually allows for a 2x up to 3x freerunning speed increase (and hence it shortens the time to complete a circadian phase reversal, ie, a 12h phase shift, by the same amount).
This is in effect what behavioral chronotherapy was designed to do, but unsuccessfully in practice, as sleep deprivation does not affect the circadian rhythm but only the sleep homeostat, so that the freerunning observed with chronotherapy can only be caused by the uncontrolled and inoptimal exposure to sunlight. Indeed, sunlight rise time and intensity cannot be controlled. Whereas here with bright light therapy in the evening and night, the circadian rhythm is directly manipulated at the optimal timing to cause a (near) maximal phase delay. There is another major difference: whereas chronotherapy relies on willful sleep deprivation by staying later and later everyday by sheer will, this tiresome procedure is unnecessary with phase-delay bright light therapy, as the circadian rhythm will shift thanks to the exposure to bright light, the individual can simply sleep whenever they feel tired. They should feel tired later and later everyday, with no effort required. In addition, they can use this additional time when they still feel energized to do activities and hence productivity loss is reduced contrary to chronotherapy.
To summarize the steps of phase-delay bright light therapy:
- Get exposed to bright light during the circadian evening and the first part of the circadian night. Do not continue in the second part as there are risks of getting exposed after the minimum core body temperature point, which would phase advance (instead of phase delay, because of exposure before the minimum core body temperature point, as is the objective here).
- Use dark therapy in the circadian morning, which means that at wake up, you can use red tinted "blue blocking" glasses and/or blackout curtains to avoid exposure to bright light (both artificial and sunlight).
- Avoid pulling all-nighters (complete sleep deprivation), instead, try to nap during the circadian siesta and sleep during the second part of the circadian night (ie, partial sleep deprivation, since exposure to bright light during the circadian evening and first part of the circadian night necessarily reduces the sleep window opportunity, as only the second part of the circadian night can be slept, and in addition melatonin is inhibited which delays sleep onset and impairs sleep quality). Keep in mind that total sleep deprivation is not necessary to shift the circadian rhythm, napping during the circadian siesta and sleeping during the remainder of the circadian night are allowed, this won't slow down the freerunning since sleeping (or not) has no effect on the circadian rhythm. Keep in mind that when naps are done, the sleep homeostat gets partially reset, so that it can be expected that the sleep session during the next circadian night will be shorter.
- (Optional, do this for faster freerunning but at the expense of impaired mood and decreased daytime energy): avoid bright light, including sunlight, in the circadian morning and day, ie, do dark therapy during the circadian day. Circadian rhythm shifting is kind of a zero-sum game: the phase delay obtained by being exposed to bright light during the circadian evening and night will be reduced by the phase advance obtained during the circadian morning and day, hence a greater phase delay can be obtained by reducing phase advances.
- Stop when your wake up time is a few hours before your target ideal wake up time. Eg, after 10 days of phase-delay bright light therapy, I will be waking up at 4am, whereas I would prefer 7-9am. But I stop before to have some days for the photic history of evening bright light therapy to wear off, otherwise I may overshoot the time I target.
This procedure was used by the current document's author more than 4 times and systematically allowed for a faster cycling back into phase with the objective day-night cycle compared to waiting for the natural phase-delay freerunning (no therapy) to achieve the same.
Given circadian plasticity is disproven by empirical results of the VLiDACMel protocol, in that phase advancing/period shortening using bright light therapy in the advance phase of the light PRC curve does not produce long lasting changes of the individual's circadian rhythm after therapy's discontinuation, it is highly unlikely that phase-delay bright light therapy can neither affect the circadian rhythm with long lasting changes. Rather, it is much more likely that any effect of phase delay bright light therapy also disappear following therapy's discontinuation (after a few days of photic history washout period), which is the current document's author's experience.
Furthermore, the author of this document could successfully switch to the VLiDACMel entrainment therapy right after 2 weeks of phase-delay bright light therapy, with the entrainment building up over about 10 days as usual, hence this suggests that phase-advance bright light therapy (such as VLiDACMel) can be started right away after discontinuing phase-delay bright light therapy.
Note that this variant protocol of using bright light therapy in the phase delaying part of the PRC curve is the standardly recommended treatment for Advanced Sleep-Phase Disorder (ASPD) by both the AASM and the french sleep health organization, since it was discovered in a case study by Czeisler et al. Indeed, the treatment for ASPD is very well defined, it's simply the opposite of the treatment for DSPD, but it is usually much more effective because it's easier to phase delay than to phase advance (because our circadian rhythm reacts in an asymmetrical way - more scientifically, we say that the PRC curve amplitude of bright light is asymmetrical).
In practice, to treat ASPD, just try to get exposed to bright light in your circadian evening, ie, 2-3h before the time you fall asleep usually, and then stop 2-3h before the time you want to fall asleep. You can use bright room light, a computer screen at max intensity, or if you need a stronger effect you can buy a Luminette. Some sleep researchers hypothesized that melatonin may also be administered inversely to DSPD, during the circadian morning instead of evening, to get a phase advance, but this was not confirmed in practice, and the sleep drowsiness inducing effect of melatonin (type 1 receptors) are also not conducive to being used during daytime. Lastly, even though bright light therapy is a well defined effective treatment for ASPD, in practice it still forces the individual to stretch their circadian day and hence cause circadian misalignment side effects common to other circadian rhythm disorders similarly to jetlag, such as headache, brainfog, fatigue, cognitive issue, cardiometabolic dysregulations in the evening, etc... as well as specific issues such as melatonin inhibition due to bright light therapy exposure in the circadian evening and first part of the circadian night. This may be circumvented by using intermittent bright light therapy instead of continuous bright light therapy, which produces as much circadian shifting but only a fraction of melatonin suppression.
Mood, energy and motivation regulation
If what matters to you is not the time at which you are entrained but your productivity, whatever time you sleep or are awake, then light therapy can always be used, even through the periods when there is some freerunning (eg, winter). Indeed, several studies and systematic reviews demonstrated that bright light therapy is as effective as antidepressants to regulate mood and motivation. Furthermore, several studies demonstrated that sleep deprivation and circadian misalignment independently cause depressive-like symptoms in healthy individuals. Blue light therapy also reduces the circadian siesta dip performance decrease and triggers cortisol production, one of the major hormones of wakefulness.
Hence, even when blue light therapy cannot achieve complete entrainment, it can still allow to be productive thanks to its mood, energy and motivation regulating effects.
In the present document's author's experience, the two most important factors for motivation and mood regulations are:
- long bright light exposure
- sleeping sufficiently long every day (and in circadian alignment)
For example, if sleeping long enough but without exposure to bright light (eg, freerunning but staying mostly indoors), then the individual will stay in a motivation-less zombie-like state most of the time and is more likely to need to sleep during the siesta (ie, biphasic sleep). If exposed to long bright light but not sleeping sufficiently long or in circadian misalignment, the individual will retain some if not most motivation although it can be variable and with variable mood, napping can then be necessary to recover sufficient energy levels to work. Only when both conditions are satisfied can motivation be fully restored.
Intermittent bright light therapy
WIP: this is an experimental protocol. It needs more validation before being recommendable.
Intermittent bright light therapy (IBL) consists in getting exposed to bright light therapy intermittently instead of continuously (Continuous bright light therapy - CBL). For example, instead of being exposed to bright light for 4h, one can get exposed to bright light for 15min every hour, and repeat 4 times over the 4h, which in total would equal to just a hour of bright light exposure over 4h. Yet, despite the total exposure duration being shorter than with continuous exposure, intermittent bright light exposure was demonstrated by a study by circadian rhythm science pioneers Czeisler et al to be nearly as effective as continuous bright light exposure to shift the circadian phase, with a similar dose-dependent response. In other words, whether you get exposed to 4h of bright light exposure or 15min every hour 4x will produce nearly the same phase shift, and you can get more phase shift by repeating intermittent bright light therapy more (eg, more than 4x), just like extending continuous bright light. The main difference of effect between IBL and CBL is in the suppression of melatonin: whereas CBL almost completely suppresses melatonin, IBL suppress only a fraction, about 20-30%, of melatonin.
Given the above, IBL may potentially be useful in some cases:
- since it can phase shift without suppressing melatonin, it is less effective than CBL to suppress sleep inertia and brain fog, but on the contrary it can be helpful when used as a phase delaying bright light therapy for individuals with ASPD, since they can then get exposed to IBL during their circadian evening without fully inhibiting their endogenously secreted melatonin.
- when the user requires a strong phase shift, and hence a very long duration of bright light, but more than the battery of their device can provide. For example, Luminette can provide 11h of continuous bright light therapy at the lowest intensity setting of 500 lux, but some individuals may need a stronger phase shift and hence a stronger intensity. When using Luminette on the highest intensity setting of 1500 lux, the battery lasts only 3h. Some users reported they then had to resort to using a light therapy lamp to complement their bright light therapy session. Using IBL could allow to obtain as much shift while saving on battery, by getting exposed intermittently, such as 20min as the automatic switch off duration setting on the highest intensity of Luminette, and then keep the device switched off for the rest of the hour, then restart next hour, etc. This would eliminate the need to use a light therapy lamp, while still theoretically getting as much phase shift, but less melatonin inhibition, although the latter should not be a problem since we can assume that the individual is unlikely to secrete melatonin during their circadian day, which is when individuals with non-24 and DSPD likely would like to get exposed to bright light therapy (and individuals with ASPD need much less light therapy since the human circadian rhythm is much more exquisitively sensitive to phase delays compared to phase advances, the PRC curve being asymmetrical).
- for comfort for those who cannot use bright light therapy for so long as they would need. For example, those who experience dizziness and migraines (then combining with a blue-only light therapy glasses such as Ayo could further reduce side effects caused by the sudden exposure to bright white light), or simply those who are at work or in situations where wearing a bright light therapy device such as Luminette can appear as socially awkward.
The VLiDACMel protocol can be straightforwardly converted to use intermittent bright light exposure instead of continuous bright light exposure. This may greatly reduce the dropout rate and increase compliance, but potentially at the expense of losing some of the benefits such as less energizing/wakefulness promotion and less mood improvements with IBL compared to CBL (because of a reduction in melatonin suppression).
Note that the current document's author did not yet try the IBL protocol, so I have no personally acquired empirical evidence of whether this is really as effective or not as the study above claims.
Weaker forms of bright light therapy in case of hyperphotosensitivity
Some people are hyperphotosensitive, which means that they are more sensitive to bright light than others. In most cases, this is not pathological, as they can still get exposed to sunlight without much side effects, but they feel uncomfortable side effects such as migraines to artificial bright light therapy. There are however more extreme forms of hyperphotosensitivity, such as sunlight photophobia, where exposure to sunlight is also painful.
In case of hyperphotosensitivity, there are alternative forms of bright light therapy that can produce weaker but still effective circadian manipulation:
- Reduce the duration and intensity of bright light therapy. Hyperphotosensitivity can indicate a higher sensitivity to the circadian rhythm modulating effects of bright light too, so that it can be unnecessary to use very long bright light therapy, a short light therapy of less than 60min/day can be amply sufficient.
- A blue-only light therapy glasses, such as Ayo, because blue only light may cause less side effects than blue-enriched white light (such as Luminette) while providing the same circadian manipulation effects. A member of r/DSPD or r/N24 who use this model recently told me it seems what I describe above hold true in practice, although they could not directly compare to a Luminette which they don't own.
- A green light therapy glasses, such as ReTimer, as green light not only cause less side effects than blue and white light, but it is even used to reduce pain. The drawback is that it is also less effective at circadian phase shifting than blue light, and it does not cause cortisol secretion, so green light does not clear brain fog / sleep inertia unfortunately, and you may need to use green light longer than blue light to get a similar amount of phase shifting. There is some feedback from reddit that this indeed reduces side effects while still being effective.
- If you already possess a Luminette, it emits white light, which includes all other colors, so if you want to try blue-only or green light therapy, you can tape on the Luminette's LEDs a colored gel filter such as those sold for VJing, they are usually inexpensive and can be found anywhere including on Amazon. Note that the wavelength will not be as optimized as you would get with a green or blue-only light therapy glasses, but it should still have an effect similar to these glasses. Doing the opposite (ie, trying to emulate a Luminette white light enriched with blue light out of a blue-only or green-only light therapy glasses) is not possible.
- A light therapy lamp, so you can position your head or orient it further away to reduce the lux your eyes receive.
- Use light therapy glasses with an Intermittent Bright Light (IBL) pattern instead of Continuous Bright Light. IBL involves using the light therapy device only a fraction of the time, eg, the first 20min of every hours, instead of the full hour. So for example, if to get the phase advance you wish you needed 3h of continuous bright light, you can instead get exposed to 20min each hour and you repeat x3. Studies have shown that IBL produces nearly as much phase shifts as continuous bright light, but it suppresses melatonin less, so you can expect less energizing/wakefulness-promotion effect, but the circadian rhythm shifting effect should be equivalent. For more infos on IBL, see the dedicated subsection above.
- Check whether some of your medications may cause or increase your photosensitivity. In particular, ADHD drugs often increase photosensitivity. If this is the case, you may want to discuss with your doctor to try other replacement drugs with similar effects but no hyperphotosensitivity.
- Avoid light therapy altogether and only use dark therapy. Circadian entrainmen works when our eyes detect a contrast between light and dark phases. By increasing the contrast during the dark phases, we also increase the efficacy of entrainment to the light phases even without using light therapy. Then, sunlight or simple room lights can be used.
Before using any of the possibilities listed above, please first talk with a physician if this is safe for you, especially if you have a more extreme, pathological form of hyperphotosensitivity, such as sunlight photophobia.
TROUBLESHOOTING
Introduction - A workbook of how sleep works, and how it can be manipulated to manage circadian sleep-wake disorders
Sleep is a very intuitive, natural process for most living beings, and is essential for survival. Yet, it is a widespread assumption that sleep can be controlled by will. In reality, science has now well demonstrated that sleep is an unconscious and mostly incontrollable process: our bodies are genetically programmed to sleep during some periods, and be awake at others, with most of this sleep-wake programming being regulated by what is called the circadian rhythm. Every living organisms need to sleep, and, interestingly, every living organisms possess their very own circadian rhythms: some particularities are shared across the specie (e.g., nocturnal rodents tend to be awake at night and sleep during the day), while other sleep parameters vary from individual to individual in a random, bell-shaped manner and which can be shared across species but not by all individuals (e.g., some individuals will sleep and wake up later than others, sometimes much later, usually called the night owl phenotype, and it exists in all species).
Anybody can conduct their own experiment to check the validity of this statement, from the safety and coziness of their very own bed: simply try to sleep all the time you can during 24h, and write down the periods you were asleep, and those you were awake. For nearly all adults, this should invariably result in either 1 or 2 periods of sleep per 24h, since human sleep is naturally biphasic or monophasic depending on the duration of bright light exposure. These two sleep periods can vary in terms of duration and of timing, but they tend to happen during two precise periods defined by the circadian rhythm: the circadian night, which is when the body expects to sleep the longest and do most of its restorative antioxydative magic, and the circadian siesta, a shorter sleep window opportunity for those who could not sleep to their fullest during their circadian night.
A sleep period is defined by the sleep onset (when we fall asleep) and the sleep offset (when we wake up, also called the "wake up time"). Both parameters are very much controlled by the circadian rhythm: assuming no sleep pressure (more on that later), it is nearly impossible to fall asleep before one of the two sleep windows, and nearly impossible to stay asleep past the wake up time of the sleep windows. The at-home experiment outlined previously is a clear demonstration of this hard fact.
The rest of this document is essentially a workbook that describes what we know about these circadian sleep-wake parameters, what we may know, what we don't know, how to manipulate them especially in the context of circadian sleep-wake disorders management, and some personal suggestions on the matter.
A few notes on the design choices before you read on
The rest of this document is designed in a way as to both provide technical details with scientific references for any non-trivial statement, while also trying to answer several common and less common but crucial questions about circadian rhythm phenomena, disorders and potential treatments or strategies to manage.
There are two kinds of solutions usually: either change your environment, or wear gadgets to isolate from the environment. This guide focuses on wearables, because although the results may be similar, wearables are much more comfortable and controllable, and hence more reliable than modifying the environment. Indeed, it's much easier to just put on your light therapy glasses and do your things such as preparing yourself for work, than standing in front of a light therapy lamp for 1-3h without doing anything else. You can even bring your light therapy glasses with you to work and use it while commuting if you don't have enough time beforehand.
Also, wearables are a lot less expensive than older solutions. A proper light therapy setup used to cost thousands of dollars to get enough lux (most cheap lamps don't provide enough lux unless you are literally on the nose with the lamp). Nowadays, light therapy glasses cost only $100-200. For other things such as dark therapy, blue blocker glasses cost only $10. That's why for example we prefer an eye mask and ear plugs instead of using cardboard on the window and soundproofing the walls. We also prefer using blue blocker sunglasses and light therapy glasses such as Luminette instead of advising to buy bright light neon fixtures that you'll need to setup up all around your house at the correct orientation for you or to go outside for a walk early in the morning: it's far easier to just put some glasses on your nose when your alarm rings.
The content in this section initially aimed to be a shorter version of an older 200+ page document, but has grown into its own, much longer thing (500+ pages if we count the references!), with a lot more information and less approximations than the original document (which was written when I had much less knowledge and experience on the topic).
If you have a circadian rhythm disorder (non24 or DSPD or other), you can continue reading with the next section. Otherwise, you can jump directly to the "Introduction to zeitgebers" section to learn more about the foundations of circadian sleep-wake rhythm science. Then, depending on your interests, you can read the "Light and dark therapy", "Melatonin" and "Food timing, diet composition and meal size" sections for more information about specific zeitgebers.
General informations about the non24 disorder
What is the non24 sleep-wake circadian rhythm disorder?
The non24 sleep-wake circadian rhythm disorder, also called freerunning sleep-wake pattern or hypernycthemeral syndrome, is a severe and rare intrinsic circadian rhythm sleep-wake disorder (CRSWD) with no cure currently known. A hallmark of circadian rhythm disorders is experiencing difficulties or inability to follow socially acceptable sleep schedules in the long run. Non24 can appear since birth or later in life, as the circadian rhythm changes with age. Although very common in blind individuals (two thirds are affected), it also affects more rarely some sighted individuals. It is a severely disabling, debilitating disease characterized by an inability to sleep and wake up on a 24-hour schedule, and which impacts not only sleep but also wakefulness, hence why this disorder belongs to the family of circadian rhythm sleep-wake disorders. Indeed, if untreated, this disorder produces constant sleep deprivation which compounds with circadian misalignment, which in turn causes excessive sleepiness during wakeful periods, accompanied by near constant brain fog, reduced cognitive abilities, slower reaction time and health issues such as cardiovascular diseases and metabolic disorders such as diabetes and obesity. This is in addition to the social exclusion directly caused by this irregular schedule. This worsen comorbid organic and mental disorders such as autism, adhd and depression. Unfortunately, no cure is known, and management therapies rarely work, with some commonly prescribed treatments such as benzodiazepines (sleeping pills) and modafinil worsening the condition and its associated chronic sleep deprivation, which in turns can cause chronic insomnia. Although insomnia is the most common complaint accompanying the non24 disorder, hypersomnia complaints are also possible.
Although all humans naturally have a non24 circadian rhythm when in isolation from external timecues (~24.2h - bigger estimates were false), the non24 disorder presents a necessary and unpreventable freerunning (ie, their circadian night delays later and later, which in practice means they wake up later and later each next day) despite exposure to external timecues (ref) (ie, zeitgebers, such as sunlight). Indeed, humans circadian clock, which defines different periods of activities such as the circadian day for wakefulness and the circadian night for sleep, is usually synchronized with the objective day-night cycle, mostly via sunlight, but in individuals with non-24, the circadian clock cannot align and hence the circadian night and day are ever shifting and misaligned with the objective day-night cycle. In practice, this means that individuals with non-24 cannot control not only their ideal sleep time, but (more problematically) neither their body's wake up time, even when they try to control their bedtime. If they do sleep earlier, they will simply take a nap that will be shorter and less restorative than a circadian night sleep (eg, often lasting only 2h — which is one ultradian cycle — even if they sleep during the objective night). Likewise, if they try to sleep later, they will wake up at their natural wake up time, no matter how short their sleep is (eg, if their current circadian night is from 8pm to 4pm, sleeping at midnight will see them incontrollably wake up at 4pm anyway, for a total sleep duration of 4h, which can appear to the untrained eye to be a form of insomnia, when it simply is the consequence of sleeping in circadian misalignment). These principles are governed by the generic sleep processes all animals share, but they are more apparent with circadian rhythm disorders such as non-24 due to the circadian misalignment with the day-night cycle.
The clinical signs characterizing the non-24 disorder is, when the individual is left free to choose their own schedule, to experience days longer than 24h (with some having an extreme form with days longer than 32h) with longer wakefulness periods than typical sleepers experience (eg, 8h of ideal sleep duration and 18h of wakefulness period instead of 16h). In practice, an individual with non-24 will feel more refreshed under a 24+h sleep-wake schedule, whereas a typical sleeper would feel a gradually more unbearable pain and decrease in sleep quality. The circadian night duration can be of similar length to typical sleepers of the same age group or there can be hypersomnia (including daytime drowsiness or long naps sessions), although individuals with non-24 often sleep less than they need to due to sleep restriction because of circadian misalignment or social/work obligations. Although the non-24 disorder is often just described as "having a day longer than 24h", this is a layman description of a lengthened circadian period, the latter being the scientifically accurate description of non-24, as it is the lengthened circadian period that in turns increases the length of the behaioral sleep-wake period. In practice, the sleeping pattern can be highly variable and unpredictable from one day to the next, even in the absence of external disturbances, due to naturally reoccurring endogenous transient changes in the circadian rhythm, scientifically termed "relative coordination and transient (dis-)entrainment", as sleep bouts can happen at any random unexpected times, as well as experiencing premature wake-ups. A common example experienced by individuals with non-24 is to see their circadian rhythm delay faster when they are awake at night than when they are awake during the day (ie, see the "relative coordination" phenomenon). This shows that the circadian rhythm is always changing, and hence, a "steady freerunning sleep pattern" should not be expected but rather an average freerunning pattern with chaotic noise and disturbances. Non-24 is sometimes incorrectly labelled as a chronotype, when more accurately, non-24 is precisely the lack of any chronotype, as the timing of the circadian night progressively changes all the time and will revolve around the clock, covering all possible timings after a while. Since non-24 is the lack of a preferential chronotype, there cannot be a concurrent diagnosis of another chronotype (such as DSPD) as this would be antinomic to the very definition of non-24, although the individual may be temporarily/transiently entrained to a schedule during specific and limited periods of time due to relative coordination to sunlight or to a medical therapy, or appear as a delayed or an irregular sleep-wake pattern when restricted by duties and alarm clocks.
Although there exists a few therapeutic options for management, there is no permanent cure, and a sizeable part of this population appears to be treatment-resistant, especially those with some comorbidities that interact with the circadian rhythm and contraindicate melatoninergic therapies, such as restless legs syndrome (RLS) and periodic limbs movement disorder (PLMD).
The non-24 circadian sleep disorder was first clinically documented in 1971 in a sighted individual, and only later in 1977 in a blind individual (see also here), hence predating the advent of ubiquitous exposure to screens and bright artificial lighting. Although it was initially suspected to be associated with mental disorders, studies have since then failed to demonstrate specific associations (eg, bipolar disorder), although there is a documented higher prevalence of circadian rhythm disorders in individuals with neurodigergence such as autism and adhd, or mood disorders such as major depression.
Since their circadian rhythm, and hence ideal sleep period as dictated by their biology, is often in mismatch with societal needs and the day-night cycle, individuals with non-24 are often chronically sleep deprived or at best living in circadian misalignment. At first, disregarding their circadian rhythm will cause the same transient symptoms as for jet lag: foggy thoughts, headaches, digestive issues, daytime tiredness, chronic fatigue, insomnia (inability to sleep when allowed to). If this circadian misalignment becomes chronic over years, more serious health issues will appear, such as cardiovascular diseases, metabolic disorders like diabetes type 2 and obesity, cancer as well as severe depression among other diseases that can be caused or worsened by severe chronic sleep deprivation and circadian misalignment. These health issues are not specific to non-24, but are the consequences of of chronic circadian misalignment and/or chronic sleep deprivation, and hence are observed in all circadian rhythm disorders, including exogenous circadian rhythm disorders (ie, disorders that are caused by external constraints such as work obligations, as opposed to endogenous circadian rhythm disorders which are caused by internal, presumably biological causes) such as night shift work disorder (ie, the circadian misalignment caused by night shift work).
Maintaining social obligations can worsen the symptoms of individuals with non-24, as this can not only lead to further sleep deprivation worsening all cognitive and mood capacities, but also additional disruption of their circadian rhythm from external cues (eg, unwanted light exposure mistimed with their circadian rhythm) depending on the time of the activity. Having non-24 is not inherently unhealthy, but trying to restrict sleep to fit into social expectations is, due to the chronic circadian misalignment and sleep deprivation. Furthermore, research has demonstrated that it's crucial to sleep in phase with one's own circadian rhythm, especially during childhood, to prevent the development of other diseases, such as metabolic disorders.
Unfortunately, even without social obligations, individuals with non-24 have no means of monitoring reliably their circadian rhythm, and hence often sleep in circadian misalignment and hence sleep too little. This makes them live in a nearly always exhausted state, only waiting for the next opportunity to sleep (since they cannot sleep just whenever they wish to due to the homeostatic sleep pressure and circadian process regulating the sleep-wake schedule), and when they finally fall asleep at a random unexpected time, they often get a sleep too short to be reparative, waking up prematurely because of sleeping in circadian misalignment, so that they continue to be exhausted and the cycle repeats. Hence, individuals with non-24 are often prone to undersleeping due to the lack of circadian rhythm monitoring tools, and sometimes oversleeping in proportion with the time they last spent awake or when sleeping in partial alignment with the circadian rhythm. On top of these endogenous issues, their sleep can often be impaired by external disturbances such as noise, sunlight, warm temperatures. Although there are unavoidable social constraints on sleep when living in society, it is worth noting that forcing someone else to wake up repeatedly despite knowing they need to sleep is physical and mental abuse, and is as such an established and practiced torture method.
Non24 and other circadian rhythm disorders are invisible diseases, with sleep-shaming being a common occurrence. Sleep shaming is due to the general public's misconceptions about how sleep works, which is not specific to sleep disorders but also affects typical sleepers doing night shifts. This also affects typical sleepers working on usual 9-5 office hours, with an endemic chronic sleep loss due to voluntary bedtime restriction to fit in the 24h society, which was termed "social jet lag". More generally, the general ableism culture does not help with the recognition of chronic diseases. This even leads some scientists, who clearly lack an expertise in circadian rhythm science, to suggest to avoid prolonging the work career of evening chronotypes, despite the latter being much more manageable than non-24. Michael Reed wrote for Metro.co.uk an excellent first-hand account of what it's like to live with the non-24 circadian rhythm disorder. See also this Youtube video by Leslie Exp: Non-24 Hour Sleep-Wake Disorder: My Experience.
Although non24 can be easily diagnosed with clear guidelines, medical and in particular psychiatric misdiagnosis is unfortunately a common iatrogenic occurrence for individuals with a circadian rhythm disorder, including for children, which can be very distressing and cause further harm. Indeed, although being so common that the non-24 disorder is the norm for blind individuals, the awareness about the existence of this disorder is still very low among the public, even more so for sighted non-24. That's why November 24th was chosen as the International Non-24 Disorder Awareness Day, with the "Think Zebras" theme of the 2015 edition as a reference to the common but error-prone medical saying to "diagnose common diseases first", as this has the unfortunate side consequence of increasing the rate of misdiagnosis of rare diseases such as non-24.
Often, non-24 is a multifactorial and complex disorder, which means that it is suspected to be in a similar class of disorders as autism, where multiple causes can lead to developing a non-24 disorder. This is also hinted at by the fact that some individuals are born with the disorder inherited from parents, such as is the case for the author of this document, while others seem to develop it later in life (although this may be due to misdiagnosis in childhood).
An excellent description of what non-24 is and what it entails is presented by Dr. Helene Emsellem for The Balacing Act TV show: https://www.youtube.com/watch?v=wOqwRik-WpU
Are circadian rhythm disorders real diseases and disabilities?
Are non-24 and other circadian rhythm disorders considered to be disabilities?
Technically, non-24, among with other circadian rhythm disorders, are "lifelong untreatable pathology of the circadian time structure" which are functionally chronically affecting the individual in their everyday life and tasks to an extent that is in the accepted definition of what is a disability. Some authors argue for another definition of disability, in that since these disorders are currently not curable (only merely manageable for some individuals), these treatment-resistant individuals with a circadian rhythm disorder should be recognized as disabilities. And even for those who are responsive, having effective management therapies does not change the fact that circadian rhythm disorders are lifelong disabilities with no cure that require constant management and merit accommodations.
Is non-24 and other circadian rhythm disorders diseases?
Although there is no generally accepted definition on what constitutes a disease, one definition consists in conditions that can be improved with treatments, which is the case for circadian rhythm disorders. Another consensual approach to defining what constitutes a disease is whether the condition is included in a well established medical corpus of diseases, such as in the World Health Organization's International Classification of Diseases, which is the case for both non-24 and DSPD as well as other circadian rhythm disorders. Yet another definition is whether the condition reduces life expectancy or increases the risk of fatal outcomes, such as cardiovascular diseases or car accidents or cancer, which is again the case for circadian rhythm disorders (see more details below in the relevant section on "Health issues of a circadian rhythm disorder"). Another common definition is whether it significantly impairs day to day functions and the possibilities to get employed, which is evident given the testimonials from individuals with non-24 and DSPD (including for business owners, not just employees). Finally, another definition can be whether the condition can be artificially mimicked by the patient or not, demonstrating whether the patient lacks control over the condition, in which case thi could be deemed a disease. In this case, it was demonstrated in numerous studies on highly trained crews such as the NASA Mars crew and submarines military crews that forcing a non-24 sleep-wake schedule onto individuals without the disorder fails very fast, with the personnel systematically deeming such a schedule inhuman after a few weeks of attempting it under natural living conditions (see also the "Malingering the non24 disorder subsection" below). Anecdotally, most individuals with non-24 live and sleep in a shared environment with relatives, who usually do not display the non-24 sleep-wake schedule, again demonstrating on a case-by-case basis that non-24 is an endogenous uncontrollable condition and hence a disease.
Hence, whatever the definition chosen, the non-24 circadian sleep-wake disorder fits the criteria of a disease.
Non-24 is a well established disease, this is not a "fashion illness", as it is recognized internationally as a disability by the World Health Organization's International Classification of Diseases (WHO ICD) since ICD-9 (1975 see also here) and up to the latest ICD-11 as of this writing. The WHO ICD is the international standard for the classification and billing of all diseases and disorders. Essentially, if it's in the WHO ICD, it's a disease. You don't need to know what the WHO ICD is or how it works, but your doctor should (and if they don't, run away!).
The WHO ICD codes of non-24 for each version are as follow:
- ICD-9-CM: 327.34—"Circadian rhythm sleep disorder, free-running type"
- ICD-10-CM: G47.24—"Circadian rhythm sleep disorder, free running type"
- ICD-11: 7A63—"Non-24 hour sleep-wake rhythm disorder", "Circadian rhythm sleep-wake disorder, non-entrained disorder type", "Circadian rhythm sleep-wake disorder, non-24 hour type"
There are similar codes and recognition for DSPD (ICD-10 code: G47.21). See for example this french redditor who got their DSPD officially recognized by their country's social security system or this other one.
Organic vs non-organic in DSM, recognized and billable in ICD-10. TODO: update the above using: https://www.reddit.com/r/N24/comments/h8tnco/does_it_make_sense_to_try_to_get_a_diagnosis/
Sighted non-24 is considered an orphan disease (a rare and commercially unattractive disease). Non-24 is also included in Orphanet and NORD classifications of orphan and rare diseases respectively. It is a billable disease in all countries where ICD-10 or DSM is used for billing medical disorders, and hence open to disabilities accomodation, especially in USA under the Disabilities Act.
Disabilities recognition and accommodations should be available in most countries in the world, as 164 countries ratified the United Nations' Convention on the Rights of Persons with Disabilities (CRPD), although these disabilities rights are often poorly communicated about or are slow to be implemented by governments despite the convention existing since 2006 (see also here). Reading the convention is highly recommended to disabled individuals to understand their rights, and easy read versions are available here and here. The list of countries that ratified the convention can be found here. However, since this is only a convention, not a law, and hence not legally binding, it amounts to a commitment that states who ratified it make towards their citizens. The convention can however be part of a legal argument and may be considered by judges, but it cannot constitute the sole argument and it may be ignored. The convention can also potentially be a legally binding argument in constitutional courts. Note also that several countries made laws to implement the convention when they ratified it, such as Belgium, so it is worth looking at national laws to see if there are some that are in link with the CRPD.
Given that the AASM, the leading american and worldwide scientific organization on sleep science, has published a position statement in 2021 stating that "sleep is essential to health", with an importance on par with healthy nutrition or physical exercise, that was endorsed by more than 25 medical, scientific, patients and safety organizations, there is a case to be made that sleep disorders can qualify as disabilities, as without accommodations this would amount to deny the individual from his right to live healthily.
(NB: It could maybe even be argued that discrimination based on sleep patterns can be similar to preventing access to facilities by not providing adapted access to people with mobility issues, with both being inherent and hardly changeable with current medical technology.)
At the individual level, non-24 brings many burdens that undoubtedly qualify it as a disability. On a personal level, living with a chronic disease such as non-24 is extremely straining. Being unable to do what others can do not only easily but unconsciously, such as sleeping, is hard to accept and live with, which requires a long grief process, as the individual must grieve the loss of their past self and future plans.
On a professional level, employment is certainly more difficult to access for those with a non morning chronotype, such as DSPD and non-24, which could be framed as a chronotype discrimination. This discrimination has very real and far reaching consequences at a societal level, impacting all citizens beyond those with a different chronotype, and also those who have a typical chronotype but must do a night shift work. There is no better example than healthcare, where the morning chronotype mindset ("the early bird catch the worm") reigns supreme, which is barring entry to medical candidates who get rejected because of their chronotype or circadian rhythm disorder. On top of that, night staff is almost always shunned and put aside, considered as a lesser group than the daytime staff, and is often forgotten from invitation to staff meetings. This is unfortunate, as healthcare is always necessary, day and night. Currently, clinical workers with non-evening chronotypes are required to work during their biological night as part of the night staff, as a way to "iron them out" (a nonsensical notion), which is undoubtedly the reason why iatrogenic events (medical errors) and accidents occur more often during night shifts compared to daytime healthcare (see also here, here, here and here), especially when there is increased sleep deprivation (see also here) although circadian misalignment also plays a key role (see also here). Integrating workers with an evening or night (DSPD) chronotype would allow to reduce these errors as these workers would be at their peak efficacy during night shifts, just like morning chronotypes have their peak during daytime.
This example shows that with increased awareness and integration of different chronotypes into the workforce, costs and accidents can be reduced and productivity increased, at little to no cost for society or the company, as this simply requires accounting for the staff chronotypes and offering alternative organizations (eg, remote or recorded meeting sessions, asynchronous communication between teams by e-mail - which is already the case between dayshift and nightshift staff). Due to the detrimental health effect of shift work on misaligned circadian rhythms and the decreased performance and increased risk of work accidents, some scientists call to match employees chronotypes to shift work positions (see also here). Indeed, a study demonstrated that aligning work schedules with chronotypes allowed for clinically significantly improved workers sleep and mood, including 0.5h more sleep on work days and 1h less social jet lag, and that evening chronotypes better tolerated frequent night shifts. In 2021, a large-scale study demonstrated that mismatching the work schedule with the individual's genetical chronotype led to decreased well-being and increased anxiety and depression, in line with a previous study showing that mismatching chronotype and work schedule leads to a much more variable sleep schedule and increased circadian misalignment. Unfortunately, night shift schedules are still poorly regarded, despite their necessity in our 24/7 society and whether they are the result of occupational requirements or the endogenous rhythm of the individual. Furthermore, employers often apply a chronotype discrimination for their hiring process, by looking for morning owl chronotype individuals even when the job schedule is at night. This ideological prejudice of the immorality of night schedules can be traced back centuries ago up to modern day, despite being disproven by several studies, with immorality increasing when the subject needs to perform a task when in circadian misalignment, regardless of the genetic chronotype (source studies here).
Smart business owners with wide office hours (ie, night shift) or where teleworking is possible could flexibly organize employees schedules based on their circadian alignment to optimize production and reduce work accidents, with "sleepiness surpassing alcohol and drugs as the greatest identifiable and preventable cause of accidents in all modes of transport", as indeed driving accidents are much more likely for night shift workers with a misaligned circadian rhythm. There is also ample evidence that shift work increases the risks of developing cardiovascular and metabolic diseases likely due to circadian misalignment. Preliminary evidence indeed suggests that aligning the work schedule of shift workers with their circadian rhythm can reduce health issues and increase productivity. Indeed, morning larks perform badly and lack attention in the evening or worse at night (despite subjectively thinking they have the same performance), whereas night owls perform better in the evening and DSPDs during the night. Furthermore, non-evening chronotypes working on nightshift often suffer from depression as a result of the mismatch between their work schedule and their circadian rhythm and also the lack of bright light exposure as accounts from nightshift workers show, whereas individuals with DSPDs instead are more than happy to get such jobs. In addition, individuals with DSPD would arguably have reduced risks of health complications as they would not suffer from shift work disorder, which theoretically can result in a much lower economical burden of illness vacancies of shift workers, which would here be replaced by permanent night workers. Hence, with some societal recognition and work culture evolutions, the variety of chronotypes could be leveraged to ensure optimal work conditions and productivity with minimal risks to health in our already 24/7 society, at no cost for neither companies nor the wider society as it would only necessitate a rescheduling of employees work hours depending on their respective chronotypes. Given the notable advantages with no downside, it rather strikes as peculiar that in our 24/7 society, where everything is expected to be available at all time, society as a whole reject the very people who can make this omnidisponibility possible.
Here is a saddening compilation of personal stories of adults with DSPD who had to drop out of school or their work career due to missing accommodations and recognition of their DSPD disorder. All fields of work, and all education degrees, including healthcare, are affected. The r/shiftwork reddit is also full of sleep shaming stories recounting how their work and out of office time is systematically disregarded by higher ups, who often are not shift working themselves and hence lack the understanding of what night shift working entails (ie, that everybody need to sleep sometime, and work done at night is still work done, even if the higher ups were sleeping at the time). Unfortunately, sleep shaming and sleep deprivation appear to only be worsening societal issues between 2010 and 2018 in the USA.
The challenge to employment and schooling is far greater for non-24, which can be considered as a non-chronotype, with no preferential and stable sleep-wake schedule, it changes from one day to the next. Since there are hardly any job, even with accomodations, that can allow for unplanned flexible schedules, which would require minimal to no social interactions (ie, clients business relationships, work meetings, etc.), it's highly likely that most individuals with non-24 remain unemployed for most of their lives. Furthermore, although societal awareness of different chronotypes could certainly reduce or eliminate most issues for DSPD and similar delayed disorders, it would not help non-24 employment, as the issue with non-24 is the lack of any regular schedule and hence of any possibility to predict, in addition to the consequent constant sleep deprivation due to unpreventable sleep interruptions by external factors making energy levels and cognitive performance (and risk of accidents) highly variable from one day to the next. Hence, although employment data are lacking, it is safe to assume given the volume of testimonies that most individuals with non-24 remain unemployed. Indeed, there are only a handful of jobs where a constantly shifting schedule would be an advantage, such as NASA's Mars monitoring missions which require ideally a martian schedule of 24.6h, and maybe healthcare with an adapted rotating schedule with smaller increments. On the off chance that someone with non-24 lands a job with a typical 9-5 daytime work schedule, they will end up missing more and more appointments as time goes by and their circadian rhythm continues to shift and hence are more and more unable to sleep when required, then doing overwork to try to compensate, to finally end up either burnout by the overwork or by the sleep deprivation (or a combination of both). Making one's own company is not any better, as although the office hours are certainly more flexible, the necessary customer relationship will still dictate a mostly diurnal working schedule. Working with remote companies will also enforce a regular although delayed working schedule, which again cannot fit with the non-24 circadian rhythm disorder.
In addition to these employment issues, individuals with non-24 can be qualified as unlucky. The most obvious reason is the acquisition of, or being born with, an crippling inheritable chronic disorder that is non-24. But beyond that, individuals with non-24 get much less opportunities than those with a 24h circadian rhythm: they are (almost) never there at the right time. Opportunities are mostly a product of interpersonal connections, which means that the more interpersonal interactions one can get, the more likely they will get opportunities. Hence, these opportunities happen mostly when most of the rest of the world is awake. Mechanically, the always changing schedule of non-24 bars the individual from a lot of these opportunities to only the short period of time (days/weeks) where the individual's circadian rhythm is in phases with the day-night cycle of their timezone (or timezone of interaction if working remotely). This lower probability of experiencing opportunities due to the always changing mismatched circadian rhythm is what makes individuals with non24 objectively unlucky.
It's crucial to understand that constraining someone with non-24 to fit within a fixed schedule does not just imply they will sleep less than optimally, as for typical sleepers, but that they will not sleep or so little this will be unsustainable healthwise. Sleep is an essential need for all living animals to survive, just like food and water. Hence, accommodations are not a convenience to increase the quality of life, they are a necessity for individuals with non-24 to live and preserve their dignity as human beings. Unfortunately, it is unclear what accommodations are required, since flexible schedules still need to be planned in advance and hence cannot accommodate the chaotic schedules of non-24, and the same goes for appointments and meetings. Asynchronous work and schooling, whenever needed depending on the current circadian phase of the individual, may be a potential, but partial, accommodation.
Even at home, circadian rhythm disorders are very debilitating. Due to the mismatch with social expectations, the individuals are drastically limited as to which activities they can carry out at night without bothering neighbors or violating laws about night noise, or simply by the opening hours of commodities and services. Some patients describe this situation as being "encaged" and "walking permenanently on eggshells", with computers with internet access being their only daily window to a lively world.
For a more in-depth discussion about non-24 and disabilities, watch this excellent video by Leslie Exp (textual script here) and this critique of the 24/7 society by Johnathan Crary (summary here).
Finally, seeking the help of disabilities associations can be very fruitful, these associations can help with the formalities to get the disability recognized and they likely won't judge the patients.
TODO: add info about remote work from: https://www.reddit.com/r/N24/comments/jewt6z/what_accommodations_have_you_successfully_got/
Diagnosis and sleep diary
Why seek a diagnosis for sighted non24 (or DSPD), when it's a rare and hence costly disorder to diagnose, and with no known cure? Because non-24 is a severe handicap, with undiagnosed non-24 typically causing an involuntary and uncontrollable absenteeism that, without adequate diagnosis and institutional accommodations and support, cause professional or scholastic failure for adults and children respectively, with on top an unwarranted blaming and shaming for being unable to control the consequences of a severe handicap they have no knowledge of. The failure of these individuals is a failure of medical support and institutional handicap integration laws, not the individual's.
Furthermore, even beside institutional recognition and accommodations of the handicap, knowing the accurate diagnosis allows the patient to better understand their constraints and adapt their lifestyle around it to improve by themselves their quality of life and alleviate unwarranted guilt. This also opens the access of the patient to more therapies and to accommodations, which are known to be crucial steps for handicaps caused by any chronic disorder to reduce the impact of the pathology on their quality of life.
DSPD is less disabling than sighted non-24, but it is still a disorder that is poorly acknowledged, accommodated and underdiagnosed, despite having a significantly detrimental impact on daily life, wellbeing and relationships, especially when unmanaged.
Hence, the next subsections will describe how the diagnosis for sighted non-24 is done in practice, what are the steps, and a later section describe how to get accommodations once a medical diagnosis has been obtained.
How can I get diagnosed of non-24 or DSPD?
Two things are needed: a sleep diary over 2+ weeks of unrestricted sleep (no alarm clock), and to know that non-24 and DSPD are recognized as diseases in the World Health's International Classification of Diseases manual (WHO ICD) as it means that both sleep disorders should be recognized by insurances and workplaces as diseases that open rights to disabilities accommodations. According to current medical guidelines as of 2022, for non-24, the sleep diary should show at least 2 weeks of freerunning sleep (staircase pattern), whereas for DSPD, one week of delayed sleep is sufficient for diagnosis. However, in practice, at least one month of a sleep diary is required for doctors to ensure it is not a temporary sleep pattern.
Curating a sleep diary (also called a sleep log) is the basic and most essential tool for the diagnosis and management of any circadian rhythm disorder, especially for individuals with a non-24 disorder as their sleep schedule constantly changes. The curation of a sleep diary (also called a sleep log) is hence not only strongly recommended for diagnosis, but should also be continually done even long after as a self-management tool. Writing a sleep diary consists of writing when we fall asleep and when we wake up, including naps, for at least 2 weeks for diagnosis, and preferably to continue after for self-management. More exhaustive sleep diaries also include the bedtime (so that the sleep latency, aka the time taken to fall asleep, can be estimated) and the standing up time (ie, at what time the individual gets out of bed), as well as the timing of various compounds such as melatonin, caffeine, alcohol, etc. This sleep diary will not only be helpful to you to better understand your sleep patterns (ie, determine the circadian misalignment), but also can be used for diagnosis with a sleep specialist according to the official USA (executive summary here) and UK guidelines and also is necessary to correctly time the only few treatment options (see also here and here and here) that exist currently, as sleep diaries are reliable estimators of the circadian rhythm. Indeed, due to the unstable nature of the sleep schedule of individuals with non-24, it may be necessary to adjust the timing of the therapies from day-to-day, or sometimes reduce/increase the dosage (eg, of melatonin) or duration (of light therapy). Curating a sleep diary does not only allow to keep track of bedtime and wake up times, but also of sleep duration, which is a strong predictor of cognitive performance and mood during the day, as well as sleep pressure the next day/night, as too little sleep strongly indicates sleep deprivation, whereas a too long sleep indicates the body attempted to correct for previous sleep deprivation. Interestingly, a sleep diary over at least 2 weeks but preferably longer as a management tool is also the gold standard assessment of insomnia, hence sleep diaries should be systematically be requested in any sleep study updated to the latest medical guidelines.
It is crucial to try to write the sleep diary, at least for some weeks, with an unrestricted sleep schedule (no alarm clocks nor appointments requiring to forcefully wake up at a specific time). Indeed, restricted sleep will not only hide the freerunning pattern characteristic of non-24, but can also cause sleep deprivation which may produce chaoticity in the sleep patterns. It is also crucial to log all sleep sessions, including naps, however short they are.
The author strongly recommends the app Sleepmeter Free on Android (mirror) and its widget to write a digital sleep log which will produce nice sleep graphs that are easier for humans to read and hence for doctors to diagnose. It can also be installed on a computer using the Bluestacks emulator. A paper sleep diary is also fine, and there are plenty of templates available online, but the AASM sleep diary / sleep graph template is recommended (mirror here, 2021 updated version here , or the unofficial digital spreadsheet version). A sleep graph such as with this template or software is much preferable over simply writing the fall asleep and wake up times, as a graph is much more legible for observers including sleep doctors, this will drastically reduce the rate of misdiagnosis. Write down your last sleep session as soon as you wake up, to avoid forgetting the accurate timing after you go on with your day. Other standard sleep diary templates (but not sleep graphs) include the Consensus Sleep Diary and its 3 variants.
Once you have a sleep diary over at least 2 weeks, or preferably 1 month or more to get a clearer pattern, look for a sleep specialist experienced with circadian rhythm disorders or a chronobiology clinic (not a sleep clinic) to diagnose your circadian rhythm disorder, preferably a chronobiologist with a background in neurology. Alternatively, psychiatrists with a well established research experience in circadian rhythm science, and hence who also are chronobiologists, can also diagnose non24. Sleep clinics often only test for sleep apnea and narcolepsy, not circadian rhythm disorders, although they should according to guidelines. Sleep specialists cover a wide range of background, but pneumologists, psychiatrists, psychologists and psychotherapists should generally be avoided unless they have a recognized experience in circadian rhythm disorders as they are highly prone to misdiagnosis and mistreatment due to systematic issues in the practice of psy* clinicians, such as the unfounded "secondary" classification of sleep disorders and circadian rhythm disorders¹. Indeed, sleep disorders, including insomnia and circadian rhythm disorders, should always be treated as they rarely resolve on their own and always with specific treatments for the sleep disorder, irrespective of any co-morbid psychological condition, which is why the DSM-5 dropped the terms "secondary" and "primary" insomnia to only keep "insomnia disorder", as the assumption that sleep disorders stem from psychological disorders (ie, is secondary to psychological disorders) is not supported by empirical evidence for the reasons reviewed here. Pneumologists focus on sleep apnea, and while it's good to be tested for this too, these clinicians usually disregard circadian rhythm disorders. Indeed, most sleep clinics will only test for sleep apnea and narcolepsy, ignoring any circadian rhythm disorder as a side effect (see also here), despite there being no evidence supporting this view, as indeed the comorbid circadian rhythm disorders usually do not resolve with sleep apnea treatments, both disorders appear to require independent therapies.
To find a chronobiologist doctor, who will be properly trained or experienced with circadian rhythm disorders, the Circadian Sleep Disorders Network patients association curates a list of chronobiology doctors from recommendations by previously diagnosed DSPD and non-24 patients:
https://www.circadiansleepdisorders.org/doctors.php
If you find a competent doctor who could diagnose you with a circadian rhythm disorder and isn't on this list please send the Circadian Sleep Disorders Network association an email to add your doctor in the list, this will help your future peers.
If you are still having difficulties in getting diagnosed, you may try to find a local/national association partnering with the International Rare Diseases Research Consortium (IRDiRC), such as EspeRare, as the IRDiRC aims to allow all patients with a rare disease to get diagnosed under one year of coming to medical attention.
If in this list there is no doctor in your area, either travel to one if possible or if really too far, try to look by yourself for a specialist in circadian rhythm disorders. A good method is to look for scientific publications using scholar.google.com and then contact the authors that are in your region (eg, local university or hospital).
An especially excellent chronobiology center of worldwide reputation for non-24 diagnosis is the Centre for Chronobiology in Basel, Switzerland, they should be recommended especially for children diagnosis.
If you find a properly trained doctor who successfully diagnose you, make sure to ask them to write down a description of your diagnosis and an accommodation letter describing how it impairs your daily functioning, this will help later on to continue to get your treatment (eg, melatonin) if your doctor goes out of business and you need to find someone else, and to get disability recognition and work/school accommodations. Indeed, given that these disorders are uncurable, accommodations will become a necessity at some point, and asking for it as soon as you get your diagnosis will save you a lot of trouble later on. Example letters of accommodations for DSPD are available in the members section of the Circadian Sleep Disorders Network website (for those in hardship, membership only costs $5).
In some countries, you may have an institution that oversees sleep medicine facilities and provide a standard path of care. For example in France, In France, there are two partnering medical associations that are relevant for sleep disorders medical care : SFRMS and Réseau Morphée. SFRMS is a medical facility certification body that also produces diagnostic and treatment guidelines for circadian rhythm disorders such as recommending the diagnosis of non-24 with the sampling of melatonin at two timepoints separated by 2-4 weeks, and they provide a national map of all sleep medicine centers that are certified for the diagnosis of all sleep disorders including circadian rhythm disorders. Réseau Morphée is a network of sleep research professionals (instead of institutions), and it provides additional resources that complement SFRMS resources. They designed a standard path of care for people who suspect they have a sleep disorder: First complete the online questionnaire on sleep disorders by the Réseau Morphée, you will get as a result a report PDF and an ID you can communicate to any SFRMS sleep doctor for them to access your results directly online. Secondly, find a SFRMS certified medical sleep center close to you. Thirdly, call them to confirm whether they can diagnose the non24 disorder by sampling urinary or blood melatonin twice at 2-4 weeks interval as is the standard procedure defined by the SFRMS guidelines, if unsure that the center you found can perform this test, then call another SFRMS certified center. Fourthly, after clinical assessment of your sleep which needs to include melatonin sampling and may include a sleep chart and a polysomnography, if a non24 disorder is indeed diagnosed, ensure or ask the doctors to write a report confirming you have the non24 disorder, as a written trace of your disorders is crucial for you and future medical professionals you will see to manage your healthcare and also eventually for public social support if necessary such as handicap recognition or indemnity.
Whichever doctor you meet, specialized or not, they should never shrug off your sleep issues as a side issue. It is a primary disorder that always requires independent treatment (it's NOT secondary to another psychological issue). Patient generated health data should always be considered by clinicians, including your sleep diary. As the non-24 disorder, especially among sighted individuals, is poorly known, it is necessary for patients to go online and collect information by themselves to come armed with knowledge to share with their physicians. But even then, there is no guarantee physicians will consider the patient's complaint seriously, altthough they should according to medical guidelines.
If the clinician is not considering your complaint seriously, you can mention that non-24 is recognized by the WHO ICD. This stands for the World Health Organization's International Classification of Disease. The WHO ICD is the international standard for the classification and billing of all diseases and disorders. Basically, if it's in the WHO ICD, it's a disease. You don't need to understand what the WHO ICD is or how it works, but your doctor should (and if they don't, run away!).
The WHO ICD codes of non-24 for each version are as follow:
- ICD-9-CM: 327.34—"Circadian rhythm sleep disorder, free-running type"
- ICD-10-CM: G47.24—"Circadian rhythm sleep disorder, free running type"
- ICD-11: 7A63—"Non-24 hour sleep-wake rhythm disorder", "Circadian rhythm sleep-wake disorder, non-entrained disorder type", "Circadian rhythm sleep-wake disorder, non-24 hour type"
The non-24 disorder is not a newly recognized disorder, it has been recognized since 1979 as the Non-24-Hour Sleep–Wake Syndrome code: C.2.d in the DCSAD, and as the Non-24-Hour Sleep–Wake Rhythm Disorder (no code) in the ICSD-3 (International Classification Of Sleep Disorders) released in 2014. While the American Psychiatric Association recognizes the DSM-5 since 2013 the disorder as "Circadian rhythm sleep–wake disorders, Non-24-hour sleep–wake type", it recommends to use the ICD-9-CM code 307.45, or ICD-10-CM code G47.24 when it goes into effect (see p390 of DSM-5).
You can also mention that the non-24 is not rare at all in blind people, as it's estimated that 2/3rd of blind people suffer from non-24. It's rarer in sighted individuals, but this is the same disorder. So if your doctor ever saw a blind individual, chances are they had non-24 (although they were likely undiagnosed if your doctor doesn't know about non-24...).
Unfortunately, with sighted non-24 being a rare orphan disorder, the standard care pathway is often made more difficult by untrained clinicians than it should be. This is unfortunately a common issue with chronic illnesses, even those that are not rare (see for example r/ChronicIllness), but the rarity causes additional struggles.
¹ Indeed, both sleep disorders such as insomnia and circadian rhythm disorders are often ignored and left untreated as these fields assume that sleep issues are secondary to other psychiatric disorders, which means that the psy practitioners will try to diagnose and treat any other disorder except the sleep issues. This distinction was criticized as being unfounded and detrimental to the patients' proper treatment since at least 2001 for insomnia and more recently in 2020 for circadian rhythm disorders, but the unfounded primary/secondary insomnia distinction still perseveres in the clinical practice, so it's unlikely that there will be any change soon for circadian rhythm disorders either. See the subsection about misdiagnosis below for more information.
Diagnosis methods for circadian rhythm disorders
This section describes more technically the diagnostic methods and tools for non-24, which are also applicable with little adaptation to other circadian rhythm disorders such as DSPD.
Circadian rhythm disorders are common in the clinical practice, despite being underdiagnosed. Nevertheless, diagnosis is theoretically easy and inexpensive. According to the latest medical guidelines, sleep diaries over at least 2 weeks are the main diagnostic tool for circadian rhythm disorders (see also here) and insomnia (see also here) as explained in the previous section. However, due to potential masking biases, it is recommended to curate a longer sleep diary over at least 1 month or even more, until a clear pattern emerges.
Specifically, non-24 is characterized by staircase-like freerunning circadian phase and sleep-wake pattern typical of non-24, whereas DSPD is characterized by a delayed but relatively stable circadian phase and sleep-wake pattern especially during week-ends for workers (social jet lag) or every days if sleep is not restricted. In practice, it's necessary for the patient to sleep unrestricted (no alarm clock, going to sleep when feeling tired and waking up naturally) over at least 2 weeks, and preferably longer as relative coordination to sunlight can mask the freerunning pattern by creating an illusory and temporary entrainment and to continue monitoring and manage the sleep disorder whenever there is a relapse, as is recommended for insomnia.
Although current medical guidelines only state freerunning over 2 weeks as the sole and sufficient criteria to diagnose non-24, the current document's author recommends to use a stricter criterion:
We propose the following updated non24 diagnosis criteria: Given either a sleep diary or circadian phase proxy measures (melatonin sampling, core body temperature) over at least 2 weeks, if a freerunning pattern is observed despite periodic zeitgebers exposure and unrestricted sleep opportunities, then this should be sufficient to diagnose non24.
Indeed, all humans naturally freerun when left in a completely dark environment void of zeitgebers, especially light. But if the individual is exposed to zeitgebers on a daily basis, especially sunlight, and still display a freerunning pattern (eg, with alarm clocks), then this demonstrate a robust freerunning drive resistant to zeitgebers exposure and hence this condition would clearly demonstrate an endogenous non-24 disorder. The criterion of being sufficiently exposed to zeitgebers was already proposed by a 2015 review, however the proposition of allowing the individual to sleep unrestricted by alarm clocks nor obligations is a new criterion.
The AASM states in its latest meta-analysis of behavioral therapies for insomnia some of the reasons why sleep diaries have become the standard assessment tool for sleep disorders, preferred over behavioral questionnaires, as sleep diaries allow for a more accurate tracking of the sleep patterns and also allow to measure a wide array of sleep metrics and their daily variability:
> In the study of insomnia treatments, nighttime sleep and insomnia symptoms are most commonly measured with daily sleep diaries,29 which capture information about the timing of sleep (bedtime, rise time) in addition to individual sleep parameters, such as sleep latency (time to fall asleep initially), wake after sleep onset (WASO; duration of nighttime wakefulness), and early morning awakenings (waking in advance of the desired rise time) that are commonly the primary symptoms targeted in insomnia treatments. Additional summary metrics commonly derived from daily sleep diaries include total sleep time and sleep efficiency (total sleep time/time in bed*100%). Daytime napping/sleeping behaviors are also commonly tracked in daily diaries when delivering treatment. The primary advantage of sleep diaries is that they allow for the daily collection of information on nighttime symptoms, making them less subject to recall bias than questionnaires. Treatment effects are most commonly assessed with aggregated mean-level changes in individual sleep diary parameters across time, generally every 1 or 2 weeks, but increasingly, the variability of these parameters across days is also being viewed as clinically important.
In other words, humans are very bad in estimating their own sleep patterns without an adequate measurement instrument. We are prone to overestimating the amount of time we spend asleep and underestimate the time spent in bed awake trying to sleep.
As written earlier, the clinical signs characterizing the non-24 disorder is to experience days longer than 24h, with longer wakefulness periods than typical sleepers experience (eg, 8h of ideal sleep duration and 18h of wakefulness period). Hence, patients complaining of a "too long day" and of "sleeping later and later" should tip the clinician on to proceed to instruct the patient to curate a sleep diary in order to assess the presence of a circadian rhythm disorder. It is important to do a differential diagnosing by recognizing and excluding other circadian rhythm disorders, such as delayed sleep phase disorder (DSPD) with a delayed sleep schedule but not abnormally long wakefulness period nor sleep period, hypersomnia with a longer sleep period but standard wakefulness period duration, and advanced sleep phase disorder (ASPD) with an earlier sleep schedule (much rarer and usually only observed in elders). Other sleep disorders such as sleep apnea should also be investigated, as treating them can improve or more rarely resolve the circadian rhythm disorder, although a diagnosis for a sleep disorder does not preclude the diagnosis of a circadian rhythm disorder, they are not mutually exclusive.
TODO: add figure showing prototypical sleep graphs for each circadian rhythm disorder.
A longer sleep diary with no constraint on sleep is preferred, as the non-24 disorder can be missed or misdiagnosed with DSPD due to sleep restriction and relative coordination (transient entrainment) to sunlight or other zeitgebers. However, for the trained eye, it can be possible to suspect non-24 with a restricted sleep schedule, see for example this figure kindly provided by Kieran Wood and annotated by the current document's author (note that age in the following figures is defined at the end point of the sleep diary):
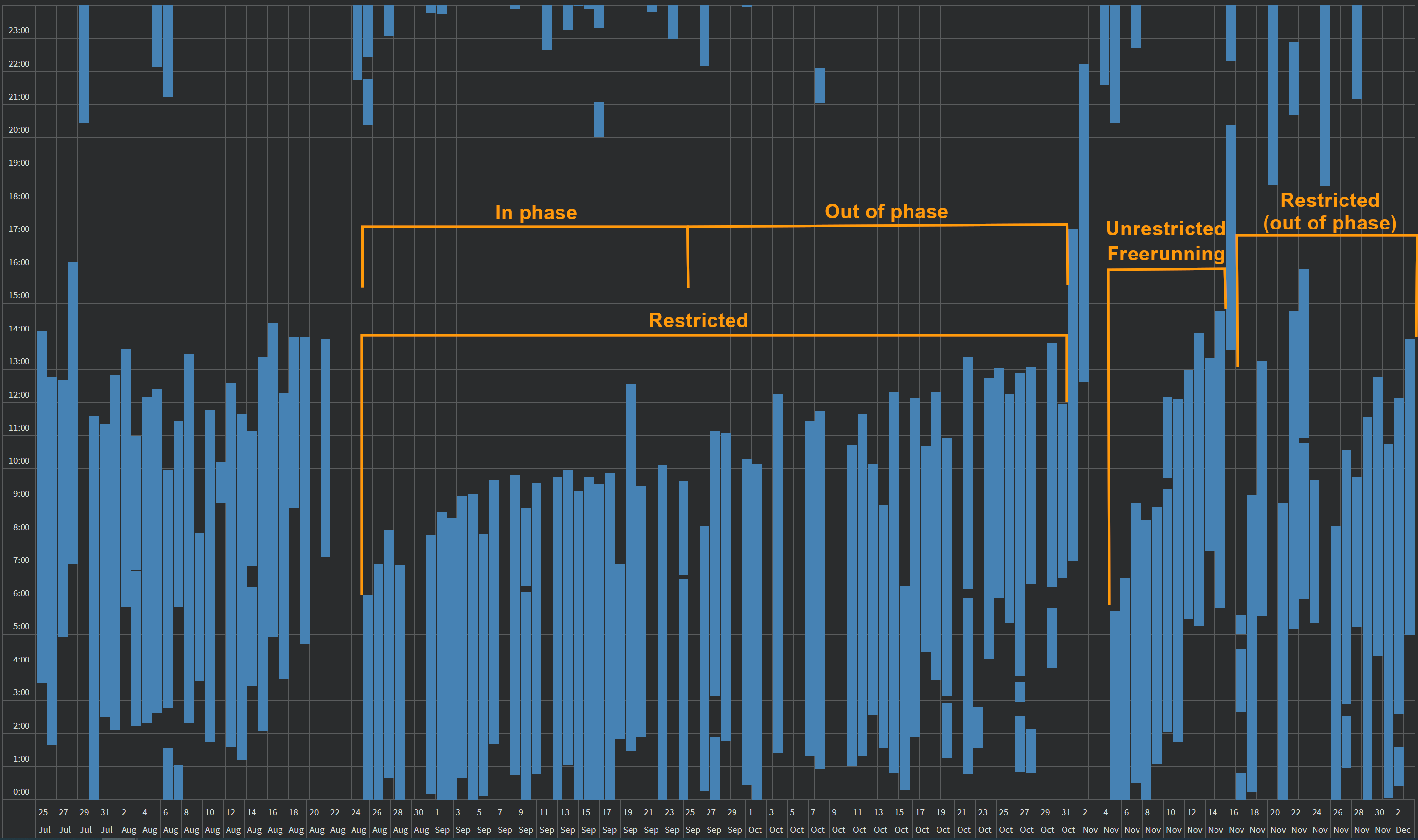
This is an actigraphic sleep graph of a sighted non24 man of ~25 years old over 4 months, acquired using a Samsung Galaxy Watch wearable sports band. The individual's freerunning period is ~27min, which means that a full circadian revolution is completed under ~2 months. We can see the staircase-like pattern typical of freerunning during the period of unrestricted sleep. On both sides, we can see restricted sleep patterns. On the left, there is a period of restricted sleep, starting in phase with the day-night cycle for about 1 month, and progressively becoming chaotic as the individual's circadian rhythm continued to freerun and become out of phase (up to being in total opposition) with the day-night cycle during the 2nd month. However, due to the sleep restriction (work commitments, which required using alarm clocks), the freerunning pattern of the circadian rhythm was masked and hence is not apparent in the sleep pattern, but it was still happening and was expressed as chaotic sleep patterns during the out of phase period, with alternating patterns of short sleep (duration < 5h), hypersomnia (>9h), drastically varying fall asleep and wake up times by several hours, and missed sleep (all-nighters). The alternance between "stable" sleep for one period and chaotic sleep for the other period is a sign of (restricted) non24 disorder. The period of chaotic sleep is usually accompanied by other health symptoms such as more frequent illnesses. Although this graph was generated out of actigraphic data, the same patterns can be observed on manually curated sleep diaries.
Such a restricted sleep-wake schedule may appear as a very slow freerunning phase on the surface (ie, 1h of phase delay per month), but there are gaps, nights without any sleep, that must not be ignored. Very very slow freerunning phase delay is unheard of, this is most likely an estimation error, as in this case as demonstrated by the much faster freerunning phase delay when unrestricted (~25h) but without sleep gaps. More likely, the masking due to the restricted wake up time makes it look like the delay is very slow, but in fact the circadian phase is running around the clock. There are a few potential reasons:
- When the fall asleep time seems to "reset" backward suddenly, it's because of the sleep pressure buildup due to accumulated sleep deprivation.
- Another thing that is happening is that the siesta, which start is 12h apart from the circadian night start, also delays progressively forward, until it matches with the current sleep schedule. Assuming a 45min of daily phase delay, this would mean that a full freerunning cycle is completed under 32 days, and half a cycle, a 12 hour shift, is completed under 16 days. This means that every 16 days, the individual can sleep at, let's say, 3 am, at first because of alignment with the circadian night, then 16 days later at the same time because of alignment with the siesta (but then the duration is limited to about 5h), then 16 days later it's again in alignment with the circadian night, etc. In-between, the subject cannot sleep at 3am, but will either sleep later or not sleep at all if too much in circadian misalignment with either the circadian night and the siesta (ie, trying to sleep in the middle between both, during the wakefulness period of the circadian phase).
- Finally, there is the effect of relative coordination which can increase or slow down the freerunning phase delay.
Here is another example of a restricted non-24 sleep-wake schedule:
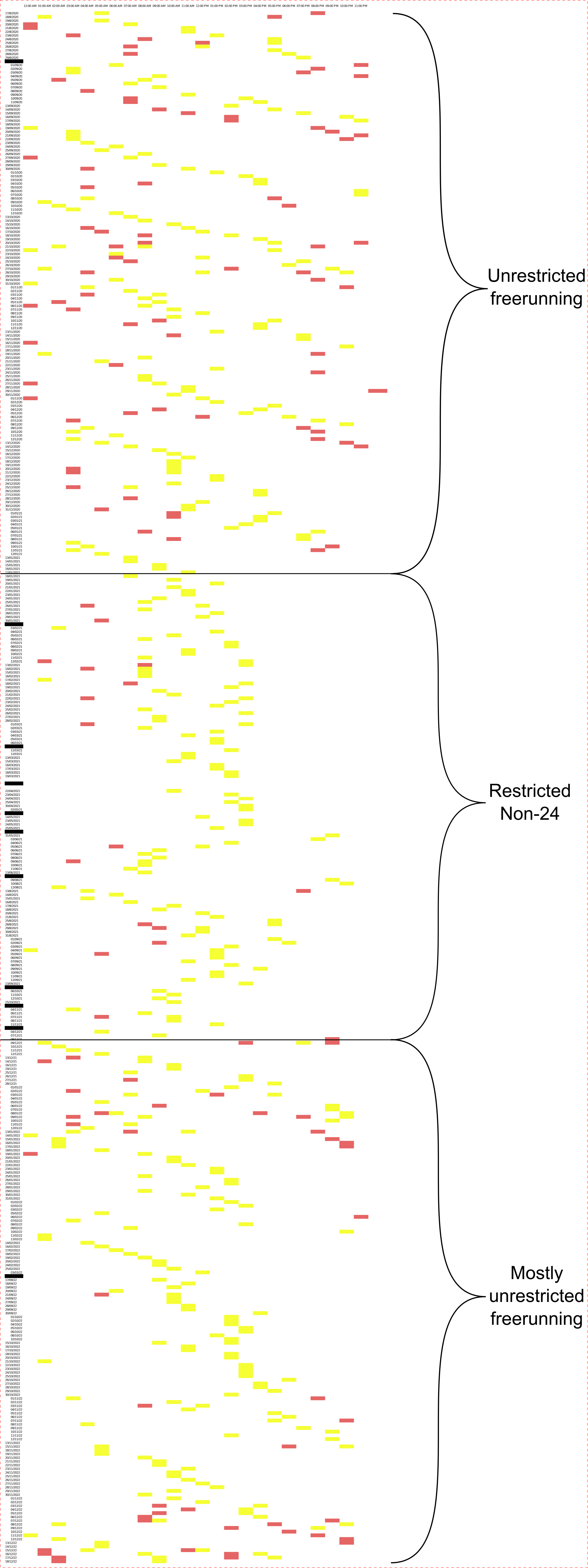
Sleep diary over 1.5 years of a 31 years-old man (2 years post female-to-male hormonal replacement therapy) with sighted non-24. Yellow blocks represent the wake up times, red blocks the sleep times. This sleep diary was recorded before the individual knew about the existence of the non-24 disorder or that he was affected, and hence the sleep diary contains gaps of a few days, and then a 6 months hiatus (right-click to open the image in full resolution to see the dates in more details). The middle section shows a restricted non-24 sleep pattern, when the individual had to restrict their sleep due to their social duties, trying to target a 8-9 am wake up time. We can observe in the middle section that the individual appears at first to succeed at the expense of reduced sleep duration (chronic sleep deprivation), but ends up failing to maintain such a schedule even at the expense of reduced sleep duration towards the end, with the innate freerunning sleep-wake pattern reappearing progressively. We also observe high variability in the sleep and wake up times, which is not due to uncompliance and lack of rigor, but is a hallmark of a restricted non-24 sleep-wake schedule, caused by the misalignment between the always shifting endogenous circadian phase and the day-night cycle. Sections before and after show a clear typical non-24 "staircase" sleep-wake pattern when the individual could sleep freely at his own natural schedule. The wake times, which is known to be a much more reliable proxy measure of the circadian phase and is more difficult to influence, reveals the staircase sleep pattern typical of non-24 much more clearly than the sleep times. Original data can be found here.

Five sleep diary excerpts over 35 days each at different points in time over 2 years, of a sighted 27 years-old man (under continuous female-to-male hormonal replacement therapy started years before, with male hormonal levels in the range of male born individuals during the covered period). Magenta blocks represent sleep periods, gray blocks represents periods of missing data or days when the individual did not sleep at all for at least 24h (between 12am and 12am). The top sleep diary excerpt shows the individual's sleep-wake pattern when fully unrestricted, when they were completely free of defining their own schedule during the COVID-19 lockdown of 2020, and displaying a staircase-like freerunning sleep-wake pattern, very typical of non-24. The three sleep diary excerpts in the middle show their sleep-wake patterns at different points over 2 years when their sleep is semi-restricted, which is when their sleep is regularly being disrupted by various obligations such as work schedules, doctors appointments, errands, etc. The last sleep diary excerpt at the bottom shows their sleep-wake pattern when their sleep-wake pattern is fully restricted by an obligation to constantly wake up at the same time for work. We can observe that during unrestricted sleep, although there is a constant daily phase delay due to endogenous circadian freerunning, sleep duration and sleep timing is relatively consistent and predictable, the daily offset being almost constant. However, during semi-restricted periods, a pattern emerges: sleep duration shortens, likely as the individual tries to maintain a constant wake up time despite their endogenous clock freerunning which makes their sleep onset happen later and later, until sleep duration becomes so short that they either do not sleep (all-nighter) or experience a sleep so short that next days their sleep-wake pattern becomes very chaotic, with both abnormally long and mistimed sleep periods. Finally, the fully restricted period shows similar phenomena than semi-restricted periods, but happening more frequently, with in addition the individual developing workaround strategies such as late afternoon or even evening naps to cope with the chronic sleep deprivation, and their sleep is also much more fragmented, even when they manage to fall asleep relatively early, they unwillingly wake up in the middle of the night due to the misalignment of their circadian clock (their circadian clock likely experiencing a siesta during the night, and hence a low drive to maintain sleep, and their real circadian night likely happening later during the day, when the individual is at work). Given the variability of their wake up time (despite trying to forcefully maintain a constant wake up time), they were likely unwillingly frequently late or absent, impacting their moral and mood on top of the effect chronic sleep deprivation already has on mood. This example clearly shows how sleep-wake pattern regularity and predictability is directly in inverse relationship with restriction.
Note that in all of the above examples, none of the displayed periods involved any chronobiological/circadian therapy. Restricted and semi-restricted sleep-wake patterns involve the use of alarm clocks and other behavioral interventions with the goal of trying to forcefully maintain a constant sleep-wake pattern. Circadian waveform manipulation via therapies such as zeitgebers-based are not here considered restrictive, as they aim to manipulate the endogenous circadian rhythm, without directly restricting the sleep-wake pattern itself.
The above examples demonstrate that while the staircase pattern is typical of freerunning, it is not necessarily observable as it can be masked by sleep restriction, despite the presence of the non-24 disorder. The staircase pattern is also insufficient to diagnose non24. Indeed, any human can freerun when isolated from zeitgebers influence. Hence, the proper diagnosis of non24 must also take into account whether the individual is sufficiently exposed to zeitgebers, and still freeruns, and also if the individual sleeps better while freerunning than when not. A reliable measure is the sleep duration. Individuals who freerun but do not sleep well while doing so are likely not non24, and may rather be experiencing a temporary freerunning period consequently to a loss of entrainment, as it can regularly happen to DSPD individuals, especially during seasons changes (ie, winter, DST time change, etc).
TODO: a new study (see also here) released in 2020 explored the criteria to diagnose "latent circadian rhythm sleep-wake disorders" or LCRSWD, which are an alternative name for what we above name "restricted sleep" or "covert sleep".
It's worth noting that there are various forms of non-24. The example graphs above cover the arguably more common cases of non-24, with a circadian period slightly above 24h but less than 27h. However, there are more extreme forms of non-24, with a 30h+ circadian period. These extreme forms usually involve hypersomnia, with a much longer sleep period than typical (eg, 10-15h/circadian period), while the wakefulness period remains in a typical range (16-18h) for adults. These cases are more likely to be misdiagnosed as irregular sleep pattern when they are restricted, as these individuals often need to fragment their ever-moving extended sleep periods.
TODO: add an example graph of restricted vs unrestricted non-24 + hypersomnia.
This review outlines a standardized approach to diagnose circadian rhythm disorders solely from sleep diary and patient history.
Alternatively to a sleep diary, medical-grade actigraphy can be used for diagnosis (see also here), although this does not replace a polysomnography. Actigraphy is essentially a way to automatically generate a sleep diary. Consumer-grade actigraphy (eg, fitbit) cannot be used for diagnosis, but clinicians should still take into account patient-generated health data to investigate further. Actigraphs are generally wrist-worn on the non-dominant arm, but for children and toddlers it can be waist-worn on cloths, although a slight overestimation of total sleep time and sleep efficiency is to be expected.
In the clinical setting, dim-light melatonin onset (DLMO) salivary sampling is the gold standard, preferably over a period of time longer than 24h, such as by sampling at one appointment and then sampling melatonin again 2-4 weeks later to observe if there is a shift between the two melatonin profiles as recommended by the SFRMS (french sleep medicine association), and diagnosis is done just like for sleep diaries by looking for a specific pattern (freerunning, delay, advance, etc), but this test is unfortunately seldom used due to constraints and cost (see also here), most clinics not being equipped to do that and this procedure not being reimbursed by health insurances in most European countries, and hence can be a high financial burden for the patient, with an estimated cost of up to $US10 per sample to assay in 2003. It is also highly cumbersome for the patient, as it requires the patient to be maintained in a dim lit environment to avoid melatonin suppression by light and with samples being taken every 30 min to 1h during at least 6h at night but preferably >= 24h especially for circadian rhythm disorders since the rhythm can be variable between days, causing further sleep disruptions. Hence, individuals' DLMO remain largely undetermined in the clinical setting. However, DLMO can be reliably estimated from sleep diaries, especially when using the sleep midpoint or wake up time. An alternative is to measure melatonin metabolites (6-sulfatoxymelatonin) from urine (see also here). Most currently available objective diagnostic methods are of a high burden to the patient.
Sometimes, salivary sampling over 24h is proposed at home with a home kit (see also here for Europe, recommended in this clinical review on DSPD in children, and here for USA). This setting is a very great proposition, but the instructions sometimes fail to account for circadian rhythm disorders. To properly do a home test of salivary sampling, you need to test every x hours as instructed (if you have 12 sampling units, you do one sample every 2h) over 24h, and you need to stay in a black room all day long. Hence, you need to prepare up beforehand, try to do a test day & night without using the sampling kit yet but measure the lux: cover light sources including sunlight as much as possible, check with an lux meter app on Android or iPhone to check that your room is illuminated with less than 10lux during daytime - and of course it's forbidden to light up any artificial light source at any time during 24h! Indeed, to be representative of your circadian rhythm and not have any masking artifact, it's necessary to stay in a dark room during the whole sampling period (usually 24h) as any light source, even low light such as a computer screen, can inhibit melatonin, and here you want to sample your natural melatonin rhythm free of any confounding factor that could influence or inhibit it, especially light.
Clinicians should be aware of the limitations of DLMO sampling, especially that DLMO will vary for a few weeks after melatonin discontinuation. Hence, if the individual is already using melatonin, DLMO sampling should be scheduled no earlier than 4-6 weeks after the patient stopped melatonin intake.
For example, the excellent Centre for Chronobiology in Basel offer at-home melatonin sampling kits, with a protocol involving 8 samples throughout the day and night while staying in a darkened environment, over 2 days that are one week apart (which makes for 16 samples in total), for a cost of about 230 euros in 2021. This melatonin sampling protocol allows to objectively assess a phase delay between the timing of the circadian night sampled on the first day versus the second day a week later, and hence objectively confirm a freerunning circadian rhythm (not just the sleep-wake pattern) and hence a diagnosis of non-24. Hopefully, this testing protocol for non-24 can get generalized in the future, the design is very elegant and efficient.
Novel very promising diagnostic methods that could be faster or used at home include non-invasive core body temperature monitoring which should be the most accurate measure of the circadian rhythm given that body temperature modulation is how cells clocks are synchronized to the circadian rhythm throughout the body, wrist skin temperature monitoring. New non-invasive devices based on heat-flux (zero-heat-flux and dual-heat-flux) technology allow to non-invasively monitor the circadian rhythm continuously, which would allow for a cheaper objective assessment of circadian rhythm disorders such as non-24, as the device can then be re used, and could easily be sent at home for a week for the patient to collect the data themselves. Heat flux devices include the 3M Spot-On (zero-heat-flux) and experimental dual-heat-flux as well as other brands. The difference between zero heat flux and dual heat flux is that dual heat flux uses two heat flux sensors and consumes much less energy, making it perfect for wearables, whereas zero heat flux needs to be plugged to an electrical outlet due to the higher energy consumption. Core body temperature can be used for diagnosis similarly to sleep diaries, by looking at the patterns of the low phases (freerunning, delayed phase, advanced phase, etc), with the advantage of being less biased by the sleep homeostat, behavior and other factors compared to sleep diaries.
Another novel and very promising diagnostic method is testing the pupil's contraction reflex speed in response to bright white light, which was found to be a reliable discrimination method for DSPD, by assessing the response of both the ipRGC cells but also the cones and rods to bright light. There is however evidence that the pupillary light response (PLR) to bright white light is not necessarily associated with objective sleepiness, the latter being closely linked to circadian dysregulations. An alternative is to test the PLR to only blue light, often called the circadian light, exposure, which is the cause of the maximum post-illumination pupil response (PIPR) test, which can be done either after blue light exposure with varying light intensities or via a chemically induced photosensitivity test. Pupillary diameter and reaction time to bright light exposure can also reflect ultradian cycles. Photic history does not appear to impact PIPR and hence pupillary light response, but supine position (laid down) does increase PIPR, hence being laid down may increase photosensitivity. A 2021 study found that individuals with DSPD have a reduced PIPR, in other words a reduced pupillary diameter in response to blue light exposure, compared to controls, and individuals with sighted non24 had an extremely reduced PIPR and pupillary diameter response (0.88 ± 0.58 mm for sighted non-24 vs 1.82 ± 1.44 mm for DSPD, vs 2.05 ± 1.04 mm for controls). However, these methods are not yet officially accepted for medical diagnosis, and their novelty makes them hard to find at a local clinician in practice. Two known places where they are conducted are Northwestern University and Monash University according to Dr Jackie Lane, both being research facilities, not clinical labs. She also mentioned the Reflex app, a PLR testing app (but not PIPR).
In the future, a blood test may also allow to diagnose circadian rhythm disorders as accurately as melatonin sampling, but since melatonin levels are only an imperfect proxy to measure the circadian rhythm (see also here), the blood test, just like melatonin sampling, can get a negative result for some individuals who do have a circadian rhythm disorder, but when the test is positive, it can be expected to be accurate, and hence provide a less expensive alternative to melatonin sampling for medical practitioners inexperienced with circadian rhythm disorders.
Sleep clinics offer to do a sleep study, which consists of a set of tests usually including polysomnography, either at home with a home kit or at the clinic (the latter meaning it's necessary to sleep at the clinic). A sleep study allows to mostly assess if there is a sleep disorder and especially sleep apnea and narcolepsy, but they rarely investigate circadian rhythm disorders since they are done only for one night, and circadian rhythm disorders are only revealed in multi-days patterns of sleep. According to a sleep lab technician (see also here), circadian rhythm disorders are not usually assessed during sleep studies as they are considered a diagnosis of exclusion from sleep studies, which is unfortunate since circadian rhythm disorders are a separate clinical entity that is not mutually exclusive with other sleep disorders and can be as disruptive. In fact, most sleep studies often require the subjects to phase advance their circadian phase to ensure the subject will sleep enough during the study, but hence biasing totally all potential results about the circadian rhythm. It is not uncommon for individuals to have both sleep apnea and a circadian rhythm disorder such as DSPD or non-24, and the treatment of sleep apnea usually does not improve the circadian rhythm disorder.
In addition, due to the "first night effect" (ie difficulty in sleeping in an unfamiliar environment), activity and sleep based measures won't be reliable since they only reflect the participant's sleep and hence will be majorly biased by the first night effect, they can only be used if the participant sleeps at home or multiple nights at the clinic and the first night's measures are discarded. Furthermore, sleep clinics often have a defined schedule, so the patient has to fit in and come to sleep only under specific hours, which may not align with the patient's circadian rhythm's current phase, prevent the patient from sleeping when allowed to by the staff and appointment time, although sometimes some sleep clinics can agree to accommodate different schedules so it's worth asking.
Hence, at-home sleep studies are more indicated to diagnose circadian rhythm disorders, and they can also diagnose sleep apnea if present, and this eliminates the risk of not sleeping during the sleep study (the "first night effect"). The only advantage of at-clinic sleep study is to allow to differenciate between the different kinds of sleep apnea (obstructive sleep apnea - where the respiratory tract is mechanically obstructed - versus central sleep apnea - where the cause for sleep apnea is neurological). The best course of action in case of suspected circadian rhythm disorder is hence to first make the patient write a sleep diary, then if a sleep study is required to eliminate other causes of sleep disorders such as sleep apnea, an at-home sleep study should be done first and then only if sleep apnea is detected, an at-clinic sleep study can be done to discriminate the type of sleep apnea.
Doing an at-clinic sleep study as a first indication is a nonsence for circadian rhythm disorders which can only logically result in a lot of misdiagnoses or null results. There is one exception, being at-clinic sleep studies including melatonin sampling and body temperature monitoring under a constantly dim-lit environment, as they can be reliable measures of the circadian rhythm in a one night clinical setting, whereas activity and sleep quality based measures such as EEG, polysomnography or actigraphy cannot (at least in one night). In addition, melatonin and body temperature reflect the circadian rhythm even when not sleeping, so the first night effect has negligible impact on these, but these measures are reliable only when in a constantly dim lit environment, as light can suppress the circadian rhythmicity. However, even with melatonin sampling or body temperature monitoring, a one-night sleep study can only diagnose a delay in circadian phase, hence diagnose DSPD, but not the non-24 disorder since by definition the non-24 disorder needs to display a freerunning pattern over several days. Hence, if a sleep study is required, prefer to conduct it over several days and at-home. Also make sure to ask for an at-home clinic that can be activated by the patient before sleep (and not with a preset time window where the patient needs to sleep - which is unfit to diagnose circadian rhythm disorders in particular non-24).
Note that sleep clinics are fundamentally different from chronobiology clinics, the latter being specialized in treating circadian rhythm disorders, and are hence a good choice for individuals with non-24. But they are rare.
To interpret the results of a sleep study with polysomnography, please refer to this excellent tutorial aimed to primary care physicians but written clearly enough to be interpretable for the general public.
It's worth noting that most studies on animals actually use proxy measures of wake schedules, and infer indirectly the sleep schedule. This is crucial to keep in mind when interpreting animals studies, most are actually not directly studying sleep but wake patterns. Likewise, a lot of studies on human sleep use questionnaires, which are correlated with sleep measures but can be hugely biased since humans are particularly bad at estimating their own sleep-wake patterns.
Multiple Sleep Latency Test (MSLT) is the oldest vigilance test. It is often conducted during sleep studies. This test is mostly used to diagnose narcolepsy and sometimes insomnia, but it has no diagnostic value for circadian rhythm disorders, especially since it does not account for the circadian rhythm: indeed, the test consists in monitoring how long it takes for the subject to fall asleep in "conducive conditions" during the period of observation which usually lasts 7h. Ideally, this period should happen during the individual's circadian night, but there is no indication in the MSLT standards to do that. For example, if the MSLT test was to be conducted during the day, all typical sleepers would fail the test and be diagnosed with insomnia since they would take more than 15 min.
At first, you may have to be screened for sleep disorders of respiratory cause such as sleep apnea. Priori to that, you can yourself do a self-screening using a snoring detection app. The author strongly suggest to use Do I Snore Or Grind app on Android (it also detects sleep stages using sound and/or actigraphy on bed - although the accuracy is debatable). If you cannot find such an app for your device, a simple audio recorder will do, then look at the waveform to find the most loud events recorded during your sleep. Indeed, snoring is always a sign of a respiratory disorder, so if you score high on snoring, this may indicate an issue such as sleep apnea, but it is not necessarily the case, so snoring just indicates that further tests at a medical facility are necessary. However, if you score low or no snoring on several days (as it's normal to snore a bit from time to time), then it's unlikely you have a respiratory sleep disorder. Once you got screened for a respiratory sleep disorder and got a negative result, you need to ask your GP to be referred to a sleep specialist to be tested for "non-respiratory sleep disorder", or better a circadian rhythm disorder, although specialists of circadian rhythm disorders are much rarer than more general sleep specialists. It may be more difficult to get to a sleep specialist depending on the country you live in and whether you need to be referred or whether you can go directly. The UK falls in the first category, you can follow these instructions. For other countries such as France, you may be able to search a sleep specialist by yourself and go directly.
Proxy measures of sleep for self-screening
While the previous section describes the currently accepted or future methods of assessing accurately the human circadian rhythm of individuals, there are proxy methods that can offer instantaneous, low-cost and at-home results, which patients may want to use or may be instructed to do by their sleep clinicians to screen them for possible clues of circadian rhythm disturbances first before orienting them towards a more costly sleep assessment in lab or at-home with adequate, but more expensive, tools.
The first is to manually curate a sleep diary, either electronic or paper based. This is a special case, as it can not only be used for screening but also doubles as a diagnostic tool for circadian rhythm disorders as well as a couple other sleep disorders per current clinical guidelines worldwide. But although current guidelines recommend sleep diaries as the primary and standalone diagnostic method for circadian rhythm disorders, it only assesses sleep-wake patterns directly, and hence remains a proxy of the circadian rhythm. See the previous section for more infos.
A second method is to use computer usage, smartphone usage, and/or browser history, as a proxy of wakeful periods and hence indirect measure of sleep periods. This is a very imperfect proxy, the least reliable, as it is a proxy of wakefulness, not sleep, and themselves being proxies of the circadian rhythm. But it can allow to access weeks if not months of backlogged data instantaneously. A tool called online_actogram to plot computer internet browser usage is already available. Computer usage and smartphone usage (across apps, not just internet browser) can be recorded with the ActivityWatch open-source app, but we don't yet have a tool to plot a sleep-wake diary out of it (help is welcome if you are a developer!).
A third method is to use a proxy method of sleep periods, such as the data that can be recorded from sleep apnea CPAP devices via the open-source OSCAR software. Although this is again an imperfect, proxy measure of sleep periods and hence of the circadian rhythm, the usage of CPAP machines is theoretically more directly associated with sleep periods, and can assumedly produce proxy sleep graphs of similar accuracy to manually curated sleep diaries. In practice, CPAP data through OSCAR can indeed reveal non-24 sleep-wake pattern pretty well.
Experimental simpler self-screening procedure
This produce of diagnosis was devised by this document's author, it was not yet tested nor peer-reviewed.
If you don't have a sleep diary nor the possibility to do a sleep study in a sleep clinic, here is a self-screening procedure:
- Just try going to sleep when you are tired and avoid alarm clocks for one or two weeks if possible. If not possible, try to focus on weekends sleep, when you can sleep in.
- Your natural wake up time is the best indicator of where is your circadian night.
- After a few days without an alarm clock it should either:
- stabilize to a late, afternoon time if you may have DSPD.
- keep on shifting later and later if have non24. This is an average over a week, the change can be more chaotic from one day to the next.
- How to ensure that you slept in circadian alignment with the circadian night and hence that the estimation is accurate:
- Pay attention to the duration of your sleep sessions, if they last less than 5h30 (assuming your optimal sleep duration is 7-8h, otherwise calculate the minimal duration of a circadian night sleep = your optimal sleep duration minus one ultradian cycle which last about 2h ; eg, on average for adults, 8h of optimal sleep needed - 2h = 6h minimum duration for a sleep session to be considered in circadian alignment with the circadian night) and are not fragmented nor interrupted and there is no 2-4h nap during the rest of the day/night, then this is strongly indicative of a sleep in circadian misalignment with the circadian night, and can suggest a circadian rhythm disorder.
- Falling asleep faster is a good sign you are sleeping more in circadian alignment within your circadian night.
- Naps are allowed, but this will reduce the duration of your main circadian night sleep, and can delay the fall asleep time, but the natural wake up time will remain unchanged and hence a good estimator. The rule of how it works is that we generally cannot sleep more than the optimal duration we need under 24h. So if you need 8h of sleep daily optimally, then you can either sleep 8h at once during the circadian night, or 8h minus the duration of naps you did the rest of the day for a combined total of 8h of sleep over 24h. And if you sleep only outside of the circadian night (ie, circadian misalignment), the maximum duration will be 8h minus one ultradian cycle of 2h = 6h over 24h.
If the result of this procedure is that you may have non24 or DSPD, consider trying to get a formal diagnosis by professionals (see the sections above).
Additional signs of a potential circadian rhythm disorder:
- irrepressible daytime sleepiness/chronic fatigue, this is a strong sign. This can manifest as staying in bed most of the day whenever allowed to.
- taking a long time to fall asleep, more than 45min, regularly. When sleeping in circadian phase, falling asleep should take less than 30min, and usually it takes less than 10-15min.
- frequent inability to wake-up with alarm clocks, deep slumber robust to noise. Chronic sleep deprivation induces deeper sleep, whereas chronic good sleep duration and quality can make the individual transition into being a lighter sleeper.
- more frequent than average susceptibility to catching illnesses such as influenza (common flu) or rhinopharyngitis, which is a sign of an immunodepression potentially caused by chronic sleep deprivation and/or circadian misalignment. This is especially applicable to children.
- being often (or almost always) late.
It's worth noting that all of the additional signs above are drastically reduced or eliminated when the circadian rhythm disorder is properly treated or accommodated when there is no effective treatment available, which tends to suggest that these are causal to the circadian rhythm disorder.
Early detection of non-24 in toddlers and infants
As of this writing, there is no documented cases of non-24 in toddlers and infants in the scientific and medical literatures. This does not mean that non-24 cannot affect young children, it just means there is no known information on this question. Hence, there is currently no clinicial who would be willing to diagnose non-24 in a toddler or infant, given there is no medical guideline or even just documented case on how to handle this rare scenario. Nevertheless, given that non-24 can have a genetic inheritance such as in the author's case, it is arguable that non-24 can appear very early. But is it detectable?
A well designed single case study of a newborn monitored from their 2nd week of age to 6 months determined that the circadian rhythm appears very early in life in some infants, with temperature circadian rhythm appearing as soon as birth but with a statistically significant signal at 1 week, and the sleep circadian rhythm at day 56. The study also suggested that newborns are already responsive to sunlight exposure during the day and dark therapy at night. This strongly suggests that circadian rhythm disorders may be detectable very early in life.
A few parents who were suspecting non-24 kindly provided some very valuable data on their toddler and infant's sleep patterns. The author is very grateful for their kind data sharing, with the hopes this will allow researchers and clinicians provide better medical support for infants with non24. The data was anonymized per the common standards in clinical research. Please note the data displayed below is exclusive, this is the first ever documented cases of potential non-24 in toddlers and infants. Feel free to reuse the data, following the requirements of the open-source license that cover this entire documentation.
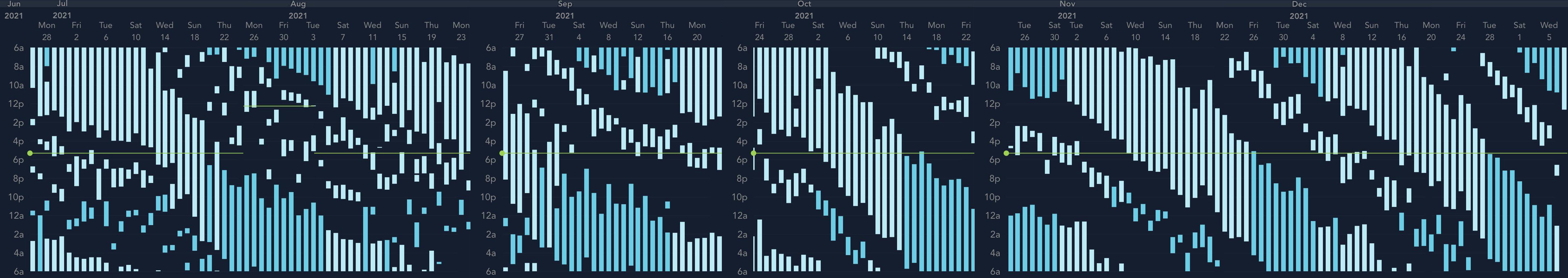
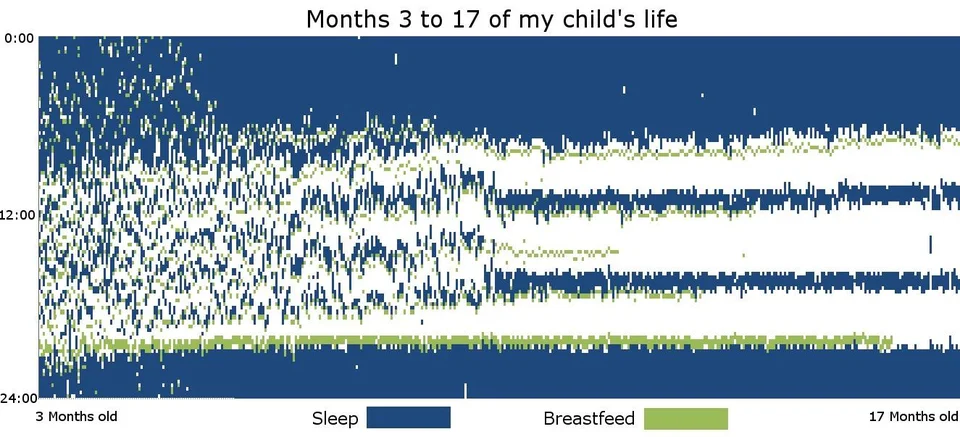
Top image: Six months sleep diary of a 1 year old girl toddler with a clear non24 sleep-wake pattern. The sleep graph was generated with the smartphone app Huckleberry and manually stitched together in The Gimp, with some missing data gaps. The parents and child travelled between July 17 midnight until July 22 noon but stayed in the same timezone. The source is from private communications, the parents wished to remain anonymous. Bottom image: Sleep graph from a sleep diary of a typically sleeping baby from 3 months old to 17 months, collected by u/jitney86. For comparison purposes, only the right side of the right sleep graph will be here considered (ie, at around 1 year of age, when the sleep stabilizes in a triphasic sleep pattern). To view in high resolution, right-click and select "Open image in new tab". For fun, see here the initial image with just 1 month of data, try to see if you could have diagnosed the non24 pattern with less data.
In both cases, we can observe how both toddlers experience a daily occurring major block of long sleep, which is likely aligned with the circadian night, and several shorter bouts of sleeps, which are naps periods. Interestingly, in both cases, there seem to be on average 2 naps periods in addition to the major long sleep period, hence a triphasic sleep pattern. We can observe that not only does the long sleep period (the circadian night) of the non-24 toddler gets inexorably delayed every days, revealing a characteristic staircase pattern typical of the non-24 disorder, but the nap periods also appear to be delayed as well. This data is very precious, and demonstrates that the non-24 sleep-wake patterns can be observed very early in life, as early as 1 year old. This strongly support the hypothesis of an endogenous pathophysiology (ie, that some individuals get born with the non24 disorder, regardless of environmental and psychological factors).

Six months double-plot sleep diary of a 4 year old girl infant (turned 4 year old about in the middle of the graph), logged in a spreadsheet. The x axis (hour) is duplicated to allow to better observe the sleep pattern, as is commonly done in studies of non24. Right-click on the image and choose "Open image in a new tab" to see in full resolution. The blue colored sections represent the sleep pattern under a prescribed therapeutic protocol by sleep doctors, which involved sunlight therapy, melatonin and sleep restriction. During this period, the girl infant cognitively regressed, losing all the developmental progress obtained so far by the parents since the diagnosis of non24 and allowing their children to sleep in freerunning, and the infant suffered from severe narcolepsy-like daytime sleepiness, falling asleep whenever the infant wasn't stimulated (eg, just going to the toilets and coming back, the infant was asleep in plain day). This daytime sleepiness was a clear sign of severe sleep deprivation. The parents decided to stop the protocol and allowed again their infant to sleep as they need, with a freerunning pattern. The developmental progress was then restored and resumed. According to the parents, the daily freerunning delay is on average 45 min, except when the circadian night overlaps with the objective day period, then the freerunning accelerates due to relative coordination with sunlight. The M label in some cells represent the time of exogenous melatonin intake. The source is from private communications, the parents wished to remain anonymous.
Just like the previous one, this sleep graph reveal a clear staircase-like sleep pattern, very typical of the non-24 disorder and its freerunning circadian rhythm phase. But here the child is older, hence with a more consolidated sleep, with much rarer naps at ~4 years old than the child above at ~1 year old. This graph also allow us to observe the sleep pattern over a much longer period of 6 months, which demonstrate how the freerunning sleep pattern is quite stable over time, repeating indefinitely at approximatively equal periods. The sleep disruptions due to environmental disturbances or social (school) requirements are also apparent, especially during the phases when the circadian night is opposite to the day-night cycle (ie, the child sleeping during the day).
The child was also diagnosed with autism, and her cognitive development tremendously progressed when her freerunning sleep was left unrestricted (ie, sleeping whenever she needed to, including naps), which brought praises from the medical staff. The naps also progressively disappeared (this can be already observed towards the end of the above sleep graph, and the trend continued beyond), as the circadian rhythm and sleep processes matured, in line with what can be observed in typically sleeping children. The impressive cognitive development and the natural reduction in naps at the expected age suggest that, at least in this case, letting the child sleep according to their circadian rhythm (including naps) contributed to their natural cognitive and sleep development, contrary to what could have been assumed.
Nevertheless, this is in line with previous research. Indeed, it is now widely recognized that "poor sleep exacerbates problematic daytime behavior", especially for children and adolescents with severe symptoms associated with ASD, as sleep patterns predict with a 81% accuracy worsened behavior in individuals with low-functioning autism. Sleep deficits also lead to difficulties in communication, as well as increased restrictive and repetitive behaviors. The American Academy of Neurology published guidelines in 2020 to recommend to systematically screen autistic individuals for sleep issues, and for sleep to be a primary target of treatment as a major way of improving the quality of life and the symptoms of autism. A 2018 review even suggested that autistic children should be profiled based to design better targeted interventions. Sleep issues are indeed highly prevalent with individuals with ASD: 44% to 83% of children and adolescents with ASD have sleep issues.
These two sleep graphs provide the first recorded evidence that the non-24 circadian rhythm sleep disorder is potentially detectable very early in life, potentially even towards the end of the 1st year of life in toddlers.
Unfortunately, some clinicians confuse non-24 in toddlers and infants with an irregular sleep-wake pattern, which is common for very young toddlers in the first 3 months of life, when the circadian rhythm has not yet matured. But even then, the major, long sleep period in phase with the immature circadian night can be clearly separated from the daytime fragmented sleep-wake periods (ie, lots of daytime naps):
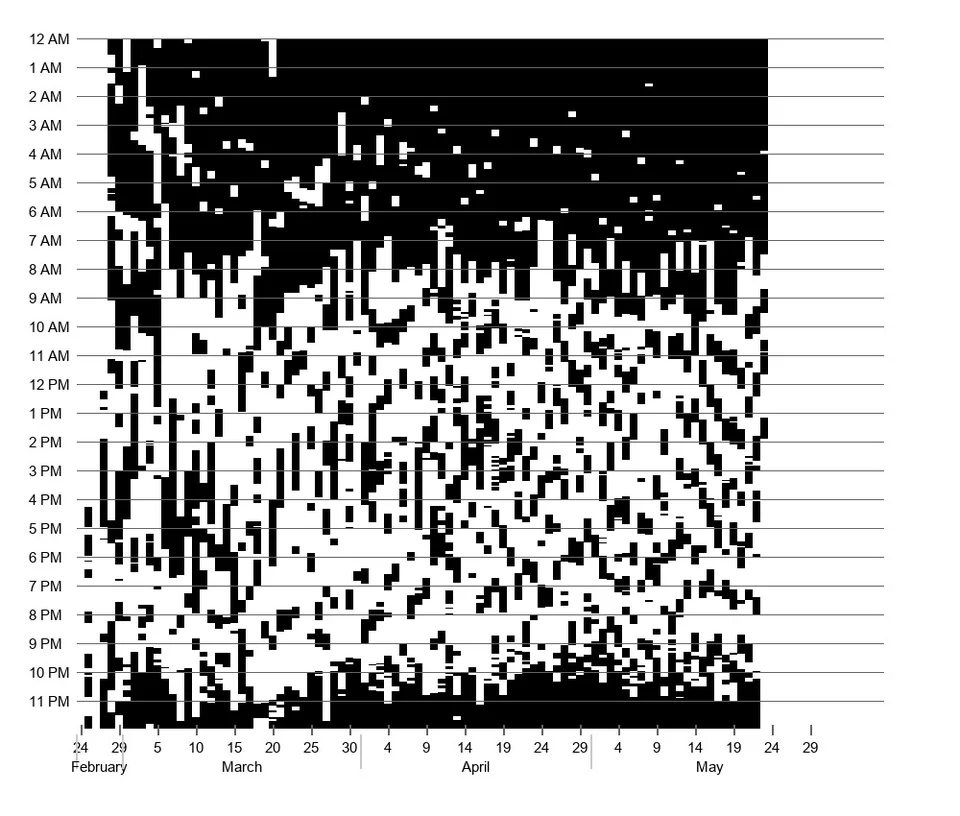
A sleep graph for an even younger infant from 3 days old to 3 months old, showing a irregular sleep pattern due to an immature circadian rhythm system. By u/AtmosChemist.
Although data is lacking for children this young, given the evidence collected above about the non-24 pattern clearly appearing as a staircase-like pattern of the long sleep period, it can be argued that the same staircase-like pattern may appear in sleep diaries or actigraphy logs of even younger children, regardless of the daytime sleep-wake fragmentation.
Although all sleep graphs above were generated manually by the parents curating a sleep diary for their children, it's alternatively possible to use actigraphy to record children's sleep-wake patterns, by attaching the actigraph on their chest using a chest strap, as it is not possible to attach it on their arms since they are so small. This harmless procedure is common in medical studies. However, only clinical-grade actigraphs should be used, since commercially available actigraphs, and even some clinical-grade actigraphs, are unable to reliably record daytime naps, which can explain why most studies on children sleep do not account for naps, and hence they are also unreliable to detect the circadian night of children with non-24 when their phase is opposite to the objective day-night cycle.
Some practitioners, when treating a toddler or infant with non24, prescribe sleep or nap restriction, ie, avoiding naps or even restricting sleep. This is a very unfortunate and uninformed decision that impairs the child's cognitive and neurological development. Indeed, it was demonstrated that napping toddlers retain learnt spatial information, whereas toddlers who remain awake forget. Memory consolidation is crucial in the child's development, as it precedes lexical development, and is mostly done during sleep. But sleep also affects semantic development, since infants who napped, but not those who remained awake, could remember 1.5h after the learning event what was the precise word meaning and moreover how to classify new category exemplars they did not see before, demonstrating a capacity for generalization that infants who avoided naps could not. Another study demonstrated that naps promoted abstraction in language learning of infants, another high level cognitive capacity. In fact, a study shown that 3-years-old infants could only remember a visual stimuli, a cartoon face, 1.5h-2h after presentation only if there was a period of sleep/nap in-between, even if short, hence the authors concluding that even short naps are beneficial for infants memory development (and likely other cognitive functions). Chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if from a young age. In summary, sleep including naps is necessary for children's neurocognitive development. Sleep and nap restriction should hence be avoided. It's worth noting that most of the studies promoting sleep restriction on children were using imprecise actigraphs or behavioral questionnaires, which were so imprecise they couldn't record daytime naps.
Why detect a circadian rhythm disorder so early in life? Early detection of disease is well established to be the main method to obtain more favorable outcomes. Despite widespread assumption that children may outgrow their sleep issues, there is no evidence of that for either non-24 or DSPD, as of course the presence of these disorders in adults demonstrate they do not necessarily disappear with age. An unaccommodated and untreated circadian rhythm disorder cause chronic sleep deprivation and chronic circadian misalignment, both having serious detrimental effects on the health of adults and even more severe effects on the development of children. Chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if from a young age. Chronic sleep deprivation increases the risk of cardiometabolic diseases, even in children. Chronic sleep deprivation greatly impairs academic performance of children and teenagers. Growth hormones are mainly released during sleep, so that it can be expected that chronic sleep deprivation may stunt height growth. Innovative thinking and flexible decision making to adapt to new situations and find new solutions, crucial skills for academic success, are drastically impaired by sleep deprivation. Indeed, sleeping allows to find innovative mathematical solutions and patterns compared to individuals who do not sleep during the time gap between problem presentation and restitution (see here for a layman presentation). Even if the patients have comorbid diseases, clinicians should be cognizant about sleep disruption complaints, especially with children and teenagers, as sleep disruption worsen all risks of comorbidities. It is hence no surprise the AASM released a recent (as of 2021) scientific statement emphasizing sleep as essential to health, especially for children. Indeed, sleep medicine researchers previously called for the recommendation of systematically assessing pediatric sleep disturbances using standardized scales.
Sleep data can be non invasively monitored even in newborns without any loss of comfort, either by manually curating a sleep diary of the infant's sleep by the parents, or by an automatically generated sleep graph from an actigraphic device attached via velcro to the cloths of the infant on their waist level or their wrist when they are older (so that they are unlikely to try ingesting the device) for more reliable data.
Quantifying parameters of a non24 case
(TODO: work-in-progress)
The major characteristic to quantify a non-24 pattern is to calculate the circadian period/length (tau), which is informally the length of the internal day and night for an individual with non-24.
Formula to calculate the circadian period: tau = average of daywise differences of midpoint of sleep or wake up times + 24h.
Step-by-step calculation of the circadian period:
- From a sleep diary, write down the midpoint of sleep for each day. The midpoint of sleep is calculated as latest wake-up time - earliest fall asleep time.
- Start from the latest date, and calculate midpoint_n - midpoint_n-1 (ie, subtract the midpoint time of the day before the last day from the midpoint time of the last day). Then continue with the other days similarly, ie: midpoint_n-1 - midpoint_n-2, etc. You will end up with a serie of n-1 values, which represent the daily freerunning delay, which is the amount of phase shift observed between each day.
- Calculate the median of this serie. This gives the median daily freerunning delay, aka median daily phase shift. An average works too but is more sensitive to noise.
- Finally, add 24h to this average. This should end up with a value greater than 24h if there is a non-24 pattern.
The above is the ideal, most accurate way to calculate the circadian period. It requires data over at least 2 weeks with unrestricted sleep (ie, no alarm clock).
There are other simpler methods to calculate the circadian period:
- By doing the same calculation as detailed above but using the wake up times of the longest sleep session each 24h instead of the midpoint of sleep, as the wake up times are easier to collect.
- By counting the duration of a full freerunning cycle: allow the individual with non24 to make a complete around-the-clock freerunning course and record a sleep diary during this whole period. This means that the individual must wait until they record a sleep session for which the wake up time is close to the wake up time that happened at the start of the freerunning course. In other words, count the number of days it takes for the individual to do a full freerunning cycle, the sleep diary is there to know more precisely when the freerunning course is completed. Then, the circadian period is simply 24h + 24h/(the number of days that separate the two sleep sessions). For example: if it takes 12 days to do a full freerunning course, the individual's circadian period is: 24 + 24/12 = 26h circadian period length. The disadvantage with this method is that if the individual has a short circadian period (ie, 24.3h to 25h), and hence a long freerunning cycle, they will have to wait a long time before being able to calculate their circadian period.
- By calculating the difference between 2 wake up times at least 2 weeks apart (or longer for more accuracy). This method is much less accurate than the others above as it is more prone to noise since there is no averaging, so the estimated period is very imprecise and can change a lot when using different time points (ie, days). The wake up times need to be less than 24h apart, ie, less than a full freerunning cycle between these two timepoints. To calculate: circadian-period = 24h + (last-wake-up-time - earliest-wake-up-time) / number-of-days-between-the-two-timepoints. For example, let's say the circadian phase shifted from 8am to 2pm over the course of 12 days. The circadian period can be calculated as follows (note: 2pm represented in 24h format as 14h): 24h + (14h - 8h)/12 days = 24.5h. Tip: for a much increased accuracy, select only the sleep sessions that are long enough, ie, with a duration close to the ideal sleep duration needed by the individual (look at the excerpt below for reference of average sleep durations by age). This is because a long sleep session is likely an indication of a sleep in phase with the circadian night, so that there is more confidence that a long sleep session does represent the circadian phase of the circadian night at the time, and not a circadian misaligned sleep session that happened only thanks to the sleep homeostat.
- By calculating the difference between the number of internal days versus the number of objective days. This method was devised by reddit member u/non-24 and explained here (and here).
(TODO: average sleep durations per age ref: https://pubmed.ncbi.nlm.nih.gov/29073412/
> Results: The panel agreed that, for healthy individuals with normal sleep, the appropriate sleep duration for newborns is between 14 and 17 hours, infants between 12 and 15 hours, toddlers between 11 and 14 hours, preschoolers between 10 and 13 hours, and school-aged children between 9 and 11 hours. For teenagers, 8 to 10 hours was considered appropriate, 7 to 9 hours for young adults and adults, and 7 to 8 hours of sleep for older adults.
>
> Conclusions: Sufficient sleep duration requirements vary across the lifespan and from person to person. The recommendations reported here represent guidelines for healthy individuals and those not suffering from a sleep disorder. Sleep durations outside the recommended range may be appropriate, but deviating far from the normal range is rare. Individuals who habitually sleep outside the normal range may be exhibiting signs or symptoms of serious health problems or, if done volitionally, may be compromising their health and well-being.)
Hypersomnia and non24, which we could classify as a subcategory of non24, is when the non24 disorder is not caused by a too long period of wakefulness, but when the sleep period is too long for a 24h day.
To know if hypersomnia, (TODO: check criteria, is it sleeping more than 12h?)
Severity can be considered to be proportionate to the length of the circadian period, as indeed stronger phase advance therapies are required to offset more the longer the period. In practice, it can be assumed (and from anecdotal feedback) that periods up to 26h are likely responsive at least partially (although this does not mean that full entrainment can be reached with current therapies). Periods of more than 28h are likely too wide to be responsive enough to current treatments (this does not mean that they are not responsive, but that the phase advanced benefit from currently available therapies are likely not enough to reach any satisfactory reduction of the circadian period, which anecdotally match with the feedback received in private communications). Extreme periods of 30+h are usually associated with intensive past use of benzodiazepines and other strong psychotropic medications, and/or alcohol, and/or RLS/PLMD (which symptoms can be triggered/worsened by melatonin and hence indirectly by bright light through photic history's increase of melatonin). There appears to be a sweet spot for periods of about 25h-26h, as this represents an average daily freerunning delay of 1h to 2h, which means that the individual goes through a complete phase reversal (ie, a 12h shift, which means going from wakefulness during the day to during the night and vice versa) every 1 to 2 weeks, which according to private communications feedbacks and public posts on reddit seem to allow for a better quality of life as they can expect to be able to resume typical daytime activities every 1 to 2 weeks, alternating with 1 to 2 weeks of nocturnal activities. This rapid-but-not-too-rapid cycling allows them to find consistency in the alternance of this schedule. Indeed, slower circadian periods such as 24.5h cycle phase reverse about every 1 month, which means they cannot do typical daytime activities for a full month, and is especially problematic with work (severe chronic sleep deprivation may be powered through for 1 to 2 weeks, but not a full month). Faster circadian periods more than 28h cycle so fast that they complete a phase reversal under less than half a week.
Are circadian rhythm disorders acquired during teenage?
TODO: add refs.
It can be commonly heard from both laymen but also by non specialized clinicians that circadian rhythm sleep-wake disorders such as DSPD or even non-24 can be acquired during adolescence.
This is false, this is a misattribution of data.
What is common at puberty is a limited phase delay (of 1-2h).
DSPD and non-24 appearing around puberty is not supported by any data, this is conflating the 1-2h of normal phase delay at puberty with the severely debilitating circadian sleep disorders.
It's exactly like when someone with insomnia gets told by a typical sleeper "ah but me too I experience bad nights sleeps sometimes and I don't sleep as much as I would like, just get a grip!". So don't fall for this unsupported amalgamation.
There is very strong evidence from twin studies that the circadian rhythm is mostly inherited, so this strongly supports the hypothesis that circadian rhythm disorders are most likely innate. They however often go undiagnosed in children, for two reasons:
- Children tend to have a natural phase advance compared to later ages. Similar to elders. When you look at the curve of an individual circadian rhythm phase, it changes with age in an inverted U shape: phase advanced when a baby, then it goes to phase delayed at adolescence, and then back to phase advance with older age. So this can mask a DSPD disorder, because a baby or young kid with severe DSPD later may instead sleep at midnight when young (when others sleep at 6-7pm), so you can see how later in life at adolescence, when others sleep at midnight-2am, they will be sleeping at 5-6am. For the same reason, there is a misconception that circadian rhythm disorders will self-resorb with age, but that's conflating the slight 1-2h phase advance with older age with circadian rhythm disorders, once again, because DSPD is several hours of phase delays, not just 1-2h.
- DSPD and non-24 tend to be overlooked in children and only considered in adults (not even in teenage - because a lot of clinicians confuse the data on the natural phase delay in adolescence and DSPD and will delay diagnosis unduly, when the magnitude is clearly totally different in both cases). This is partially caused by the point before, but is mostly due to the clinical practice not following the standards of care (for example non-24 is clearly described in children in the ICSD-3, but I heard several sleep clinics and hospitals will refuse to diagnose it in children for no good reason).
Medical misdiagnoses
How to detect and avoid a misdiagnosis?
The greatest early sign of misdiagnosis is the dismissal of sleep diaries by a doctor. If the doctors you met shrugged off the sleep diary, or the sleep clinic where you had your sleep study did not include a 2-weeks sleep diary, then that's a clear sign they are not properly trained to diagnose circadian rhythm disorders, as sleep diaries over at least 2 weeks are the standard method to diagnose insomnia since 2008 and circadian rhythm disorders according to american and british guidelines. Furthermore, the american guidelines on patient-generated health data (PGHD) using consumer-grade sleep technology such as fitbit or sleep diaries state that this data should be used to (at least) open dialogue with the patient, that the clinician should understand the data and that "clinicians should recognize the patient's use of consumer sleep technology as a commitment to focus on sleep wellness". In other words, handing over your sleep diary of at least 2 weeks should be sufficient for any properly trained sleep specialist to diagnose you if you have a circadian rhythm disorder, should be considered an indication of your motivation to get better, and should certainly never be shrugged off (see for example these unacceptable patient experiences, with general practitioners denying specialized testing despite adequate data). If this happens to you, seek counseling from another medical professional.
Although there is an official diagnosis criterion according to the AASM (simply to have a sleep diary over at least 2 weeks showing a freerunning pattern), the author is convinced this criterion is both too vague and too restrictive. Indeed, it is too restrictive as it doesn't account for people who constraint their sleep schedule and hence can't freerun, and too vague because all humans can freerun given an environment devoid of timecues, the specificity of non-24 is that freerunning (and the cyclical inability to sleep and wake up) happens continuously despite environmental timecues (ie, zeitgebers).
Causes of misdiagnoses: psychology misdiagnoses
Circadian rhythm disorders are often misdiagnosed (see also here), which can cascade and leads to unnecessary distress despite being easily diagnosable and may lead to inappropriate prescriptions of psychoactive drugs. Most often, the misdiagnosis is confusing non-24 or other circadian rhythm disorders for DSPD, since this is the most commonly known circadian rhythm disorder in non specialists fields of medicine, but misdiagnoses of inexistent psychiatric disorders is also common. Misdiagnosis and medication errors are frequent and the most common types of medical errors. A psychological misdiagnosis (such as the ill-defined and evidence-lacking psychosomatic disorder, medically unexplained symptoms, or others as seen below) worsen these issues, as this can have dramatically detrimental consequences for the patient with a rare disease such as non-24, as they already wait an average of 4.8 years to be diagnosed, and a psychological misdiagnosis delays 2.5 to 14 times longer the proper diagnosis of their chronic rare disease, according to a survey of 12,000 European patients, with this delay being harmful for a majority of patients. Psychological misdiagnosis does not affect only new and rare diseases but also well-documented physical diseases such as epilepsy. This was sadly illustrated in a horrible case of iatrogenic (medical) mistake from both psychiatry and psychology practitioners on a 14-year-old boy as reported in this study:
> A 14-year-old male was referred for sleep disorder assessment with the complaint of daytime sleepiness and lack of motivation. [...] During the 4 years before referral, the patient suffered from major functioning difficulties including conflicts with teachers, parents, and peers. He was described by a licensed child psychologist as being extremely introverted with severe narcissistic traits, poverty of thought, and disturbed thinking, including thoughts with persecutory content and self-destruction that led to a paralyzing anxiety, anhedonia, social isolation, and withdrawal. [...] Two years before referral, the patient dropped out of school and was sent to an inpatient child psychiatry center. Three months of psychiatric evaluation yielded diagnoses of atypical depressive disorder with possible schizotypal personality disorder. He was described as sleepy and passive, especially in the mornings. The patients psychiatrist suggested further assessment, including assessment of sleep disorders. [...] Failure to make a correct diagnosis led to psychological distress and personal turmoil for a boy whose sleep disorder was easily diagnosable and treatable with melatonin. [...] Greater awareness of sleep disorders may prevent psychiatric misdiagnosis of treatable sleep-wake schedule disorders.
It's worth noting that the misdiagnosis of sleep disorders is much more frequent in children, as historically, sleep disturbances in children have largely been ignored by the psychomedical field.
As demonstrated by this case, misdiagnosis of sleep disorders (here non-24) as a psychological disorder is common, especially of schizotypical, schizoid or schizophrenic disorders. Indeed, two major items of the schizo* spectrum are dissociative symptoms such as depersonalization/derealization (see also here and here), and social isolation, both being also caused by severe chronic sleep deprivation due to sleep disorders. Add on top the specific difficulties of non-24, such as the nightwalking phases during which the individual will be exclusively living at night and sleep during the day for months, which necessarily leads to social isolation due to the mismatch of the individual's sleep-wake schedule with the rest of the world and cause feelings of being "disconnected from reality" which are perfectly normal as they were also experienced by typical sleeper archeologists such as Siffre during their "expériences hors du temps" in caves disconnected from external interactions and zeitgebers for months. Furthermore, episodes of depersonalization and derealization are extremely common in the general population, as 26% to 74% experience them at some points in their lifetime according to a systematic review (see also here). For healthy, typical sleeper participants, 52% experience dissociative symptoms including depersonalization and derealization after 24h to 48h of acute sleep deprivation according to a systematic review. Despite the normalcy of these feelings, and the challenged unproven assumptions that dissociative disorders cause sleep disorders in response to trauma, whereas empirical evidence demonstrate the opposite to be true as shown by the excellent works of Dr. Dalena van der Kloet (see also here), the mere evocation of these feelings by the patient is a recipe for a misdiagnosis of a schizotypical disorder by psy* clinicians, regardless of the chronicity and context of their occurrences.
Unfortunately, although these effects of sleep deprivation are well documented, most psychiatrists and psychologists are unaware of this knowledge about sleep, even when they practice in a sleep clinic. Even in the rare instances they are, they tend to focus on treating the psychological symptoms (assumption of an intrapsychic cause), assuming this is the cause of the sleep disorder. However, the results of a rsystematic review state that sleep disorders often precede psychological disorders symptoms onset, they persist even when psychological disorders are well controlled and hence that "sleep problems require independent attention irrespective of co-morbid conditions". Unfortunately, psy* clinicians are unlikely to consider sleep disorders as anything other than secondary to psychological disorders contrary to the evidence, as otherwise they would be unable to provide any service to their insomniac patients. This kind of misdiagnosis also happens at sleep clinics, since they often have psychiatrists and psychologists in their staff.
These misdiagnoses are unfortunate, as an accurate diagnosis of their medical condition allows circadian rhythm disorders sufferers from being relieved from the "humiliation" and social guilt of their self-perceived "bad behavior", which can dramatically improve their and their family's wellbeing.
These misdiagnoses, especially psychological ones, are often due to a failure in adequately testing the potential hypotheses. For example, studies on "paradoxical insomnia" always fail to test the circadian rhythm, and a reddit member even reported that the sleep clinicians, after misdiagnosing non24 as paradoxical insomnia, argued that testing for a circadian rhythm disorder was unnecessary as it was useless, given there is "no cure anyway" according to them (which fails to consider the possibility for handicap recognition and accommodations). It's surprising some clinicians consider that getting an appropriate diagnosis for a condition that is partially treatable, versus another condition that is not, is useless. A simple sleep diary, or more stringent objective tests such as an actigraph or melatonin sampling, would have elucidated the issue at little to no cost.
The crux of this issue lies in the fact that psychiatrical clinical guidelines (but not other fields of medicine obviously) still consider circadian dysregulation as secondary to a primary psychiatric disorder, as explained by this 2019 systematic review:
> Historically sleep problems have been neglected in groups with neuropsychiatric disorders due to diagnostic overshadowing, and assumptions that sleep problems are purely secondary to psychiatric symptoms. Unfortunately sleep problems often persist even if affective or psychotic symptoms are well-controlled. There is increasing recognition that sleep problems require independent attention irrespective of co-morbid conditions. In accordance with this the ‘primary’/‘secondary’ insomnia distinction was removed from DSM-5 and ICSD-3. Circadian dysregulation disorder definitions have not been similarly modified; the ICSD-3 stipulates for diagnosis of CRSD the sleep disturbance must not be “better explained” by another medical, neurologic or mental disorder. Further, it contains no category for CRSD secondary to another disorder [27]. Studies which examine circadian dysregulation in samples with neuropsychiatric disorders find high prevalence of patterns similar to ASPD, DSPD, ISWD and non-24hr, but usually CRSD terminology is not applied.
This is unfortunately a common pattern, with newly discovered disorders with unknown causes being misdiagnosed as idiopathic (ie, self-caused) instead of cryptogenic (ie, of unknown causes, as defined by Walter Dandy in 1932) until more evidence arise that they are in fact not caused by mental issues, as happened for epilepsy:
> A number of general thoughts arise from this historical survey. First is the importance of societal and nonscientific influences on theories of epilepsy etiology. Examples are numerous. Concerns about degeneration at the end of the nineteenth century for instance were widely discussed in politics, the arts, sociology, and criminality. Eugenic research in epilepsy was primarily driven by economic, political, and social forces. Psychoanalytic thought was found in almost all social discourse, and the similar tendencies are arising now in relation to molecular genetics. It is a delusion of neuroscience that its progress is linear or that contemporary position inevitably is the most scientifically advanced. The awkward reality is that the march of neuroscience has had an erratic course sometimes in a backward direction and veering up many cul-de-sacs. It is partly subjective largely and markedly influenced by fashion and social forces, socioeconomic factors, dominant personalities, and the full gamut of human failings. Science is never neutral or objective, and thus has a social responsibility, a fact often forgotten in the laboratory sometimes with disastrous results as was the case in the 1930s. Second, it should be realized that clinical neurology, being an essentially applied science, is heavily methodology- driven, and methodology in large part sets the agenda. We know only the etiologies we can measure, and what we cannot measure we cannot know. The introduction of clinical chemistry, EEG, neuroimaging, and neurogenetics has each changed our fundamental perception of etiology, and over the last 150 years, the focus has moved from one category to another, often overstating the importance of that in fashion (the switching interest between inherited and symptomatic causes for instance).
Some argue that the first case of diagnosed hysteria, an old form of the somatoform disorder diagnosis, was in fact just a case of misdiagnosed epilepsy. But just like epilepsy now has a well documented wide set of causes, all being of biological or physiological origins (see also here and here), circadian rhythm disorders are also progressing towards a path of being recognized as biophysiological disorders.
In practice, avoid psychiatrists, psychologists and psychotherapists when looking for a clinician to diagnose a sleep disorder, as they will rather favor intrapsychic explanations and hence diagnose and treat psychological disorder (even if there is none) rather than the sleep disorder. For example, epilepsy is still being heavily underdiagnosed (32-38%) in individuals with intellectual disabilities because of "the misinterpretation of behavioural, physiological, syndrome related, medication related or psychological events by parents, paid carers and health professionals". They are in any case not the field of preference to treat these disorders, just like psychiatrists have no pertinent training to treat lung damages, they should not be considered competent to treat sleep disorders, both being biophysiological diseases. Furthermore, avoid any clinician recommending sleep deprivation, chronotherapy or any form of sleep deprivation such as avoiding naps, as sleep restriction is never a treatment for sleep deprivation, just like dietary restriction is never a treatment for malnutrition. Timing therapies to an absolute/fixed target bedtime or wake up time instead of relative to the current circadian phase (eg, natural bedtime and wake up time) is a red flag too, as it is a fundamental error no sleep chronobiology expert would do, it's literally nonsensical given how zeitgebers work (ie, PRC curve).
Causes of misdiagnoses: medical procedure and cognitive errors
All fields of medicine are plagued by systematic procedure and cognitive errors that can lead to misdiagnoses. Although studies on this topic are sparse, especially for mistreatments and prognosis, several authors could identify the most common errors that account for misdiagnoses.
- Premature closure, which is the failure to continue considering reasonable alternatives after an initial diagnosis was reached, was found to be the single most common system-related factor of diagnostic error. System-related factor (involving problems with policies and procedures, inefficient processes, teamwork and communication) contributed to 65% of diagnostic errors.
- Faulty synthesis is the most common cognitive error causing diagnostic errors. Cognitive errors (faulty synthesis, faulty context generation — cherry-picking of contextual information —, misjudging the salience of findings — cherry-picking and hyperfocusing on some clinical signs and ignoring others that do not fit an expected diagnosis —, faulty perception, and errors arising from the use of heuristics aka clinical lores or rules of thumb) contributed to 74% of diagnostic errors.
- Most healthcare professionals are not properly trained to understand research data, and "few healthcare professionals are aware of the pervasiveness of biased and inaccurate medical literature" according to a Ioannidis study. Most do not read systematic reviews such as Cochrane's. In addition, medicine is on average 17 years behind translational research. Hence, the lack of filter allows inaccurate or outdated medical information to unduly guide some of the clinical practice, sometimes in the form of so-called "clinical lore", which is the set of clinical procedures that are done out of experience but is not actually supported or founded in evidence based medicine. A related issue is that medical historians tend, just like physicians, to avoid reporting and documenting medical (iatrogenic) errors.
- Clinicians often suffer from chronic sleep deprivation themselves due to the rotating shift work schedule that is absurdly inadequate for the human physiology and is decried by all shift work societies and sleep research societies. It was demonstrated by several studies that clinicians who suffer from chronic sleep deprivation demonstrate a much more cynical behavioral towards their patients and likely cause them to burnout (see also here and here), hence increasing the likelihood of medical errors, and especially for sleep disorders, the very issue that clinicians widely suffer from too. Indeed, a cross-sectional study of 11 395 physicians controlling for various factors found that, independently from burnout, training status, practice specialty or gender, sleep deprivation increases the rate of medical errors by physicians by 53% to 97% depending on the severity of the sleep deprivation! As the authors note, this "suggests that interventions to mitigate sleep-related impairment in physicians are warranted". Hence, this is a vicious cycle, as sleep deprivation impairs the judgment capacities of clinicians, who in turn misevaluate their patients impairments that are caused by sleep deprivation. Nobody likes to hear someone else complain about something you suffer from. But this is unfortunate, as a more widespread awareness of sleep health and better treatment of sleep disorders would also benefit clinicians from their own burden, by at last being taken seriously themselves (and help abolish the hellish rotating schedules...). Knowing how burnout in the medical personnel has become the norm rather than the exception, including even internal medicine program directors, it is urgent to explore solutions, such as work hours reduction, which was found by a 2017 systematic review to reduce burnout rates without decreasing productivity. Not only would the healthcare staff benefit from such work reorganization to factor in the essential sleep needs of the workers, it would also benefit the patients by increasing the quality of healthcare, and indirectly reduce expenses by reducing errors that leads to delayed treatments or inadequate tests and treatments due to misdiagnosis.
Among systemic errors, the lack of education on the latest findings in sleep medicine is certainly a major factor:
- An important factor to consider is that the clinical practice (medicine) lags on average 17 years behind translational research. Note this is an average! The paper shows the intervals for various domains, and in some cases the clinical practice can be "221 years" behind the current scientific knowledge! This is why the consensus found in scientific papers can seem very remote, even opposite at times, to the clinical practice (eg, insomnia being wrongly assumed to be secondary to psychological disorders).
- In the case of sleep medicine, this lag is verified. Historically, sleep disturbances have been largely ignored by the psychomedical field, missing unique opportunities for improved health outcomes. Even nowadays (as of 2021), "education about sleep and sleep disorders is lacking in medical school curricula, graduate medical education, and education programs for other health professionals" according to the AASM (see also here), which the AASM recommends to change by teaching sleep health as a prominent element since a young age at school and up to medical school curricula. As the authors write: "A multi-nation survey of medical schools found that the average amount of time spent on sleep education is just under 2.5 hours, with 27% responding that their medical school provides no sleep education."
- More generally, there seems to be a lack of adequate training in the medical cursus for the handling of chronic illnesses, and the public is now discovering this at large now that they experience the widespread long covid chronic illness.
- Gender also plays a role, since women are much more likely to be discharged without a physiological illness diagnosis but instead with a "junkbin diagnosis" such as anxiety or stress (TODO: add refs). For example, sleep apnea has historically been underdiagnosed in women.
- "Iatrogenesis is the fifth leading cause of death in the world. There are about 5%–8% of deaths due to adverse drug reactions worldwide. [...] Among the European Union Member states, WHO concluded that the healthcare-related errors occur in 8% to 12% of hospitalizations." https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6060929/
- The medical work culture is not tolerant of chronic illnesses in their workforce, which removes profiles who could provide a interesting transversal contribution to the field. See for example the reddit r/DisabledMedStudents. More specifically, there are lots of complaints from medical students with DSPD, despite DSPD med students being ideal to work night shifts, but the prevalent dogma is that med students should work the day, and those who work the night are expected to be available day and night (see r/NightShift).
Another major factor is burnout (at least) partially caused by chronic sleep deprivation and the "no sleep culture as a badge of honor" in the clinical workplace:
- Medical students' judgment and reaction time are also impaired by sleep deprivation, contradicting past opinion pieces by surgical doctors who claimed that sleep deprivation had no effect on the clinician's work quality. Another study also report significant impairments in healthcare professionals work after sleep deprivation.
- The majority (up to 4/5th) of medical students in their 3rd year experience an increase in cynicism / lack of empathy (see also here), due at least partially if not fully to sleep deprivation caused burnout (see also here and here).
- Burnout is epidemic, it's the norm in the medical field: almost all medical students are at risk, and 30% of medical program directors report having experienced burnout.
- Indeed, while burnout has and is still mostly considered a psychological stress-related issue, there is accumulating empirical evidence that burnout is rather caused by chronic sleep deprivation, and a 2017 systematic review on potential interventions to reduce burnout rates in resident physicians found that a reduction of work hours, potentially mediating a reduction in sleep deprivation, led to a significant reduction of burnout scores and emotional exhaustion, with no impact on performance. There are hence calls to further study this promising lead, about the involvement of sleep deprivation and circadian misalignment in the pathogenesis of burnout, since both burnout and sleep deprivation involve "(1) a chronic depletion of energy stores; or (2) activation of the hypothalamic-pituitary-adrenal axis and increasing levels of bodily stress", and hence share the same pathways of action and similar physiological consequences. This 2019 review further notes that "although sleep deprivation is associated with clinical burnout, direct studies showing that sleep extension can improve burnout recovery are lacking", and that "early detection and early intervention to improve both sleep deprivation and burnout are warranted in health-care professionals", with "interventions [that] should be directed not only at individuals but also at the entire health system".
Given the above, it is understandable that actors of the medical field, which is one of the profession with the most endemic and dire chronic sleep deprivation out of all professions, are reluctant or even feel unempathetic or hateful when patients with disordered sleep come to share their sleep complaints, when they themselves are desperately lacking sleep. Yet, this is obviously inadequate and unacceptable, just like it would be unacceptable to for clinical professionals to be spiteful of diabetics having difficulties with their diet because clinical staff rarely have the time to eat at all during their shifts.
Cause of misdiagnoses: Rare illnesses are poorly considered by modern medicine, leading to high rates of diagnostic wandering
Modern medicine is optimized in an utilitarian way due to economical constraints, by aiming to treat the greatest number of cases, hence the most common diseases, but with fewer or no resources allocated to rare illnesses care.
However, rare diseases are not that rare:
>Although every single rare disease affects only an extremely limited number of patients (defined as incidence <1/2000 in Europe and <1/1250 in the United States), approximately 6000–8000 disorders are classified as rare diseases along with 250–280 new additional ones annually, which accounts for 6%–10% of the global population being affected. Among the numerous and varied problems experienced by rare disease patients and their families, the first and perhaps most significant problem that prevents them from achieving a better quality of life is the difficulty in accessing a definitive diagnosis. A definitive diagnosis does not only mean a possible treatment and relief from pain, but also means release from pressure of not knowing, access to ancillary social welfare or subsidies for special needs, connection with rare disease support groups, and obtaining information for life planning and reproductive decision-making.
An online 2018 survey of people with a circadian rhythm disorder by the Circadian Rhythm Sleep Disorders Network found that "of those formally diagnosed, 22% took 10 years or more to receive an accurate diagnosis, from when they first sought help for their circadian rhythm disorders", and "about half were wrongly diagnosed initially, many with multiple incorrect diagnoses over the years" with the main incorect diagnoses being the usual suspects: depression, insomnia, and no diagnosis ("nothing is wrong").
This is unfortunately unsurprising, and not even specific to circadian rhythm disorders. Indeed, misdiagnosis, diagnosis delay, and a lengthy journey to diagnosis are a common experience for people with a rare disease:
> A 2006 study of eight rare illnesses in 17 European nations revealed that up to 25% of patients had spent 5–30 years to access the correct diagnosis, and 40% of the patients experienced an erroneous diagnosis. [...]
> [...] The impact of the geographic distribution of healthcare and patient mobility should also be taken into consideration. Due to the uneven distribution of healthcare, many patients have to travel across regions to obtain a definitive diagnosis. Eurordis’ survey revealed that 25% of rare disease patients in Europe had to travel to a different region to receive the definitive diagnosis, and 2% even had to travel to a different country. The variance of accessibility to healthcare can also be caused by differences in patient mobility, such as affordability, physical disability, and education level.
> The first national survey on rare diseases in 2016, covering 1771 patients across the country, reported that the various social and economic difficulties faced by these people are “beyond imagination”.
In countries with a less developed healthcare system such as China, the situation seems even more dire:
> (1) Accessibility: 72.97% of patients were misdiagnosed; they waited an average of 4.30 years and visited 2.97 hospitals before the definitive diagnosis; 67.13% were diagnosed outside the home city and traveled an average of 562 km. (2) Interrelationships among accessibility indicators: the experience of misdiagnosis significantly increased diagnosis delay and the number of hospitals visited, but had no significant effect on healthcare utilization across cities. (3) Impact factors: the rarity of disease only increased the number of hospitals visited and residence–hospital distance; high-quality healthcare distribution was key in determining accessibility; the older, disabled, poor, and less-educated individuals, and those in Central/West China were disadvantaged.
Some factors appear to indirectly affect the misdiagnosis rate. A 2020 study of individuals with rare illnesses in China found that misdiagnosis was more likely for those with less accessibility to information on rare diseases (ie, less health literacy), and those with disease multimorbidity, which when the individual reports multiple ailments. These findings were true for both adults and children.
Misdiagnosis of physical illnesses is much more frequent when the patient has a psychological illness, even if properly treated and controlled.
Modern medicine often disregard patient's feedbacks and personal knowledge on their own diseases, which some argue is a loss of opportunity for furthering medical knowledge.
Despite this issue of diagnostic wandering having dire consequences, the solution is apparently simple: physicians should think zebras, not only horse, when they hear hoof beats, as James Fadden wrote. In other words, physicians should be reminded to consider, and to inform the patient to consider, not only common causes for their symptoms, but also possible rare diseases.
It's worth noting that the International Rare Diseases Research Consortium aims in its 2017-2027 agenda to enable all people living with a rare disease to receive a definitive diagnosis within one year of coming to medical attention (see original publication here), but this vision currently falls short of what the current clinical practice can achieve.
Developing health literacy to avoid misdiagnoses and improve health outcomes
Given the findings in the previous subsections, it appears necessary for people with a circadian rhythm disorder to be resilient and develop their health literacy as recommended by medical organizations in order to navigate the information and circumvent the potential misdiagnoses they may encounter during their journey to get medical help for the diagnosis and management of their condition. Indeed, it was demonstrated by several studies across various subpopulations that people that have more understanding of their health and medicine have better health outcomes (see also here). Online forums have plenty of anecdotes of life-saving self-diagnoses. Hence, monitoring of one's own symptoms and vital signs, as well as reading about one's own (suspected) ailment ought to be recommended to the patients, and these patient-generated data should be taken into account by the medical practitioners.
Indeed, excluding patients' personal knowledge from medicine is not scientific but scientism which impairs the medical practice and ethics:
> Medical scientism is the imperative to define and achieve all medical goals through science. This imperative manifests in numerous ways and is particularly evident in the objective−subjective dichotomy, whereby objective knowledge is viewed as superior and subjective knowledge is regarded as inherently suspect. In this paper, we argue that medical scientism is flawed because it only recognizes what we call general and explicit knowledge and excludes what we call tacit and particular knowledge. This exclusion is epistemic, in that tacit and particular knowledge may be implicitly recognized by scientism, though not as genuine knowledge but as some modulating factor in the application of scientific knowledge (from a Kuhnian perspective [Kuhn 1996]; this is because recognizing tacit and particular knowledge as valid knowledge would undermine the foundational assumption of the scientistic paradigm that the quality of knowledge is related to the extent and rigour of its justification). Such factors, which are invoked through terms like clinical expertise or patient preferences, we suggest are more accurately described as valid and necessary forms of medical knowledge, with features that distinguish it from the knowledge science delivers. We argue that the exclusion of tacit and particular knowledge impairs our ability to achieve medicine’s goals, primarily because these knowledge forms are essential to doing medicine and secondarily because the overemphasis of general-explicit knowledge distorts our perceptions of what legitimate medicine should be. These impairments relate to a range of domains of medical practice, from the reasons supporting one treatment over another through to health policy and even to the legitimacy of different kinds of ethical arguments. [...] Scientism, we argue, excludes tacit and particular knowledge and thereby distorts "clinical reality" and impairs medical practice and medical ethics.
Paradoxically, the clinician's personal knowledge is often used to guide diagnostic and theurapeutic courses, regardless of their (lack of) evidence base, which is usually termed "clinical lores".
TODO: also read Michel Foucalt on history of medicine and how it was used to control populations as part of "biopolitics", term that he coined.
Malingering the non24 disorder
WORK-IN-PROGRESS SECTION: need to add links to references (they are already present elsewhere in this doc).
Can the non24 disorder be faked? This is the issue this subsection will explore.
Some clinicians may doubt of the existence of the sighted non24 disorder as a real illness. While it is true that the human circadian rhythm is naturally slightly greater than 24h as was demonstrated by experiments under constant conditions such as the cave experiments "ee dehors du temps", there is no evidence it can be greater than non24 when the individual is exposed daily to indoor or outdoor sunlight (under the same timezone). Indeed, the human circadian rhythm is normally supremely responsive to bright light, hence there is no way for an individual to freerun under normal urban conditions.
Some will argue that the modern urban lifestyle, with most work positions in closed offices, reduce our exposure to natural sunlight and weaken our circadian pacemaker. While this is certainly true, this does not explain why the vast majority of humans are still untrained to a 24h sleep-wake schedule. Furthermore, studies have now demonstrated that the circadian pacemaker uses several tricks to be more robust, such as using color, not just light intensity, hence it is well know that it is extremely difficult for scientists to isolate subjects from natural zeitgebers under lab experiments, and impossible in freeliving conditions. The latter is strongly supported by the experiment on the NASA Mars monitoring crew, who, despite being highly trained and (financially and scientifically) motivated, revolted after just one month of trying to forcefully follow a non24 sleep-wake pattern (see also here). Similarly, numerous studies have observed severe cognitive, physical and mood impairments in highly trained submarine military crews subjected to non-24 sleep-wake cycles. Furthermore, the much more common occurrence of night shift wake disorder, which is essentially when typical sleepers try to forcefully shift their circadian rhythm but fail to do so, show that just phase shifting, not even constant freerunning, is extremely hard to achieve under freeliving conditions.
Anecdotally, the first clinically documented case of sighted non-24 was reported in 1971 along with a "trick" experiment performed on the participant: "He was then confined in an isolation unit, without a timepiece, and his habits were recorded by a remote signalling device; he there followed an activity cycle of 26hr. After 5 days a clock, which he knew could be adjusted to gain or lose several hours a day, was started, and he was asked to try to conform his habits to the time recorded on the clock; unknown to the subject, this clock was running at a normal rate, though its absolute time was in error since it was started at the time which he believed it to be. He was still unable to conform his habits to a 24hr cycle, just as when living in nychthemeral surroundings (Fig.1). Measurements of his plasma 11-hydroxycorticosteroids, body temperature and excretion of sodium, chloride, potassium and steroid indicated that these followed a rhythm in accordance with his activity cycle."
Hence, sighted non24 can NOT be faked, nor simulated, nor forcefully induced, except by complete isolation from zeitgebers especially sunlight. Hence, if a clinician doubts whether a patient really has sighted non24, they only need 2 items: a sleep diary or an actigraph over weebs showing a non24 sleep-wake pattern, and they can ask the patient whethes they are living in a cave or are systematically isolated from sunlight and other bright light sources. If the patient is not, there is no logical reason nor evidence based motivation to deny a non24 diagnosis. The author of the present document challenges any critic of the above statement to demonstrate empirically by forcefully inducing a non24 sleep-wake schedule in a sighted individual or animal under freeliving conditions exposed to daily sunlight at least in indoors. Until such a demonstration can be produced, the possibility of malingering a signted non24 disorder should ve dismissed as nothing more than prejudice based on ignorance of the empirical circadian rhythm science. In summary, sighted non24 is a disorder that cannot be simulated nor faked, except by isolating completely the subject from any zeitgeber.
While an assessed non24 sleep-wake pattern cannot be faked, the assessment can be manipulated depending on the acquisition modality : if a sleep diary is used, it can of course be faked by the patient since this a patient reported metric. The simplest and cost effective solution is to make the patient wear an actigraph on their wrist (adults and older kids) or ankle (for infants), as this will allow an objective monitoring of the sleep-wake pattern, and is very hard or impossible to fake, as it is obviously insufficient to detach the sensor to fake sleeping, since humans do move during their sleep, but with very specific patterns that are different for each sleep stages and along the night. If there still remains some doubts, even more objective metrics such as melatonin sampling in saliva or urine, or the non invasive core body temperature monitoring with zero or dual heat flux sensors can be used to produce internal vitals monitoring that cannot be faked nor manipulated by the patient.
Lastly, it is worth questioning the potential motivation for malingering. Indeed, it is hardly understandable why anyone would fake an obscure disorder that, due to being poorly recognized, only rarely opens any recognition or rights, when easier and more known disorder and diseases can be faked more easily and for more profits.
Why can't people with non-24 simply sleep when needed? Why trying to do so causes a chaotic sleep?
Sleep is not a matter of personal preferences. Humans cannot control the circadian rhythm by will, that is a common misconception and the root cause of ineffective treatments. Otherwise, if sleep control by will was possible, sleep disorders such as insomnia and non24 would not exist. Night shift would not lead to chronic insomnia and major health issues such as cancer. Humans cannot control their circadian rhythm by will, just like they cannot control their insulin levels by will, as they both are biological processes.
However, we can manipulate our insulin levels to some extent by controlling our carbs intake, just like we can manipulate our circadian rhythm to some extent with external tools such as zeitgebers (eg, bright light and melatonin). But not by will. That is a common misconception about sleep and the major cause of improper management and lacking development of proper tools to manage sleep disorders. This major realization underlies the VLiDACMel protocol, after countless of failed attempts with various will-based schemes (eg, chronotherapy), only the use of external tools allowed some degree of manipulation of the circadian rhythm.
The intuition that sleep is controllable is only natural, as the circadian rhythm is deeply hidden and often imperceptible. When everything works, it's only natural to think it's easy. But there are a few cases where anyone can experience circadian disruptions and hence the existence of their own circadian rhythm: jet lag and night shift work. Indeed, anyone travelling between timezones will feel restless for days/weeks, having difficulties falling asleep at the local night time, until magically after a few days/weeks we become accustomed. That's because of the circadian rhythm progressively readjusting, which takes some time. If we could sleep whenever we wanted/needed, jet lag and night shift work disorder wouldn't exist. Now imagine being constantly jet lagged, as if your body never recovers from jet lag after a travel, and you can get an idea of what non-24 and other circadian rhythm disorders (eg, DSPD, night shift work) are like.
Another way to experience the hidden sleep processes such as the circadian rhythm and sleep homeostat is to try to do the opposite: to avoid sleeping altogether for 48h, in an environment with no artificial light. Kleitman, the father of sleep research, describe extensively the difficulties in preventing participants from sleeping, even when they are just allowed to sit for a few minutes every few hours, they still fall asleep, and in the end failed to fully prevent them from sleeping. Nowadays, artificial bright light from screens or home fixtures can alleviate sleep by directly modifying the circadian rhythm, but without, the individual will necessarily fall asleep uncontrollably upon the pressure of their endogenous sleep processes, whether they wish it or not.
The previous two simple experiments that can be done by anyone demonstrate two things: 1) it is almost impossible to stay awake when the biological processes underlying sleep signal that the body should sleep, and 2) it is almost impossible to fall asleep when these biological processes are not signalling it is time to sleep. Hence, sleeping is not a matter of when someone wants to, but when someone can, as regulated by their biology.
When an individual tries to sleep outside of their circadian rhythm to meet social obligations, this means that they then have to rely only on the second sleep process, the sleep pressure (aka sleep homeostat or process S of Borbély's model), to be able to initiate sleep while fighting the natural sleep propensity induced by the circadian rhythm (process C). Since the circadian rhythm continues to periodically cycle in the background, it will periodically enhance the ability to sleep (too early or too late compared to the individual's social constraints), which will compound with the sleep pressure and produce alternating periods of hyposomnia (sleep deprivation because of social constraints preventing sleep initiation when the circadian rhythm allows it) and then hypersomnia (because of the accumulated sleep debt that will magnify the circadian rhythm). Adding in the ultradian gates to sleep, this all combines into producing a chaotic sleep pattern, where the individual with a circadian rhythm disorder or doing night shifts will alternate between sleeping little to none one day, and then crash into bed for a very early and long night of sleep the next day, or even unexpectedly fall asleep suddenly even in an unfit situation such as while driving, because of the huge sleep debt, and this alternating cycle will repeat until the body can't take it anymore.
Case study: the MyNon24Sleep dataset contains several examples of sleeping solely using the sleep homeostat but not the circadian rhythm, for example day 2021-12-19. Indeed, it is possible to build up enough sleep homeostat pressure to sleep even outside of the circadian night, either earlier or later, but at the cost of a shorter sleep duration and less reparative sleep, which happened in this example from 1.35am to 7.33am. In this case, the individual still felt an energy crash during the circadian night as evidenced by the core body temperature minimum for this 24h period, which in this case happened suddenly later that day, starting from 2.00pm and lasting up to 4.30pm, despite the use of bright light therapy and having slept almost 6h, which is sufficient to not feel daytime sleepiness unless if caused by the circadian night. The same scenario got repeated the day after, only for a nap to be done between 1.00pm to 4.30pm. During both days, the individual (the current document's author) felt a confusing mixed signal where both a lack of sleepiness, but some form of sleepiness/brain fog were concomittantly present throughout the days, which only disappeared after sleeping/napping during the circadian night. In other words, even a long sleep session is not fully restorative when it is happening outside of the circadian night, and the body will still be exhausted during the lowest period of the circadian night. The energy loss, extreme coldness (due to very low core body temperature, causing shivering and inability to stay in room in ambient temperature), headaches, mind confusion and motor incoordination during the circadian night are so sudden and extreme that even if the individual can stay awake as in this example, activities such as work and driving are obviously too dangerous to perform in this state. Given the worsening between day 1 and day 2 with this schedule under circadian misalignment, the individual felt that if this schedule was maintained for just a few more days, it would cause major dysfunctions on par with sleep deprivation, which is in line with the results from previous studies.
Hence, an individual with non-24 who tries to maintain a socially acceptable schedule will necessarily restrict their sleep in some way (either a shorter sleep duration, or an unrestorative sleep in circadian misalignment, or both) which will only cause more sleep deprivation and chaoticity in their sleep patterns, which then reduces their productivity and their ability to plan appointments, which makes this whole approach highly counter-productive. As this study explains:
> The pattern of sleep disruption experienced by patients with the disorder does not always present as a shift in sleep timing each day. A majority of individuals will attempt to maintain sleep at a socially normal time. As a result, some individuals will produce a sleep pattern with the nocturnal sleep episode expanding and contracting as they move in and out of phase and with the build up and pay-back of homeostatic sleep pressure. Due to the pleomorphic variation in patient's sleep timing, a review of sleep history may not reveal a clear cyclic pattern to indicate the presence of N24HSWD. These more subtle cyclic changes are termed “relative coordination” and often require an expert to review.
People with no circadian disturbances often advise to "just try to sleep at the time you need". More formally, this is a form of sleep hygiene, the oldest supposed treatment for insomnia. However, AASM guidelines since 2008 and in a 2021 systematic review state that sleep hygiene is not supported as a single therapy, it is not sufficient to improve sleep disturbances, which should not come as a surprise given the above insights on how the circadian rhythm orchestrates sleep.
The use of sleeping pills is also inappropriate for non-24: "The circadian basis of N24HSWD distinguishes it from other sleep-wake disorders, and therefore use of hypnotics and stimulants to address the sleep and sleepiness symptoms, respectively, is not appropriate."
When you sleep outside your circadian phase, it's the sleep pressure that you will then use to sleep. The first day you can't sleep so you'll sleep late, but since your sleep is restricted you'll wake up with some residual sleep pressure, so the next day you'll sleep early because you'll have today's sleep pressure, plus yesterday's residual. This day you'll sleep a long night, maybe even too early and too long. Bu the next day the vicious loop starts again: can't sleep until late because your biological night is delayed compared to the objective night, so you'll keep some residual sleep pressure at wakeup, etc...
If your biological night is just a bit delayed, let's say a few hours at most, you can sustain like that for a long time although it's unhealthy and straining. But if your biological night is a lot later, let's say into the day, then you'll accumuate too much sleep pressure debt to repay in one night, and so you'll keep residual sleep pressure and eventually just collapse with a behavioral sign thad resembles hypersomnia or narcolepsy.
Hence, a pattern of chaotic sleep is likely a sign of oscillation of sleep pressure. This suggests that chaotic sleep can simply be caused by sleeping outside of one's own biological night as defined by the circadian rhythm. This is not specific to individuals with circadian rhythm disorders, but it more often happen to them due to their circadian rhythm being out of phase with social obligations.
TODO: add graphic and sourcecode of model reproducing a chaotic sleep pattern from simply: process C, process S, ultradian cycle, exogenous sleep onset constraint: only outside of process C (can define anytime to see what happens when can sleep in the process C), endogenous sleep onset constraint: addition of all processes must reach a certain threshold for sleep to occur (hyposomnia, can't sleep) + high threshold when sleep onset is irresistible (hypersomnia). Sleep onset is not deterministic but is probabilistic. Generate graphs with various values for these parameters, should reproduce non24 pattern with chaoticity. Can extend the usefulness of the model by learning with viterbi the hidden parameters from real sleep logs to make a predictive model and estimate the parameters on an individual bases (may help in differenciating different subkinds of non24 and adapt therapies accordingly, ie some may have a stronger process S than others compared to process C). ADDENDUM: meanwhile, there was a post on reddit ELI5 that more or less describe the same idea in more layman terms.
Health issues of a circadian rhythm disorder
Keep in mind that ignorance doesn't shield from detrimental health effects. But knowledge may allow to overcome.
Why do we need to sleep?
It's common to hear among insomniacs claims that one sustained a month without sleeping at all, while another didn't sleep in years. This is false. Dangerously false.
The almost mythologic search for a way tfor humans to live without sleeping to free up more time to achieve goals is not new. In fact, a lot of funding has been devoted to this goal by the military, with no significant result.
The world record for the longest sleep deprivation is held by Randy Gardner, who could stay awake for a little more than 11 days straight with the help of friends and families keeping him awake, until he had to stop for health reasons. Indeed, he was on the verge of dying, plainly. After achieving his world record, this "good sleeper" according to his own words became a very severe insomniac ("unbearable insomnia"), being unable to sleep more than a few hours in a single session for decades. His extremely prolonged sleep deprivation streak left his sleep process dysregulated for decades, making him publicly state that he regreted his world record. He is not the only one, before him, Peter Tripp did a similar experiment, which shown that sleep deprivation detrimental effects on health and cognition appear much earlier, as soon as the next day without sleep. Indeed, it's now well established by systematic reviews that sleep deprivation causes hallucinations, depersonalization, derealization and delusions.
Contrary to what was previously assumed by scientists, sleep deprivation kills not because of impairment in the cleanup of brain's generated junk, but instead of the guts' junk: indeed, sleep primary function is to clean up and relieve the oxydative stress from the guts that is produced by ingested food, the greatest source of reactive oxydative species (ROS). These ROS elements accumulate in the guts, and sleep cleans them up. In the absence of sleep (or melatonin), ROS continues to accumulate unchecked and leads up to a swift death. The brain has nothing to do with sleep deprivation induced death, it's irrelevant whether the brain sleeps (or secrete melatonin) or not, what matters is only whether the guts are cleaned up. Interestingly, the effect of antioxydants on the reduction of ROS is in parts because antioxydants hyperactivate the HPA axis.
The AASM (finally) published a position statement in 2021 affirming sleep as an essential need, just like food and water, for all humans and especially children (see also here for a vulgarized article):
> Healthy sleep is as important as proper nutrition and regular exercise for our health and well-being, and sleep is critical for performance and safety. It is the position of the AASM that sleep is essential to health, and we are urging educators, health care professionals, government agencies, and employers to prioritize the promotion of healthy sleep.
They further state four points to implement:
> * Sleep education should have a prominent place in K-12 and college health education, medical school and graduate medical education, and educational programs for other health professionals.
> * During patient visits, clinicians should ask about sleep patterns and symptoms of sleep and circadian rhythm sleep-wake disorders, and hospitals and long-term care institutions should improve sleep environments.
> * To enhance health-related outcomes, public health and workplace interventions should focus on good sleep, and habits that assist people in achieving healthy sleep should be actively supported.
> * To better understand the relevance of sleep for public health and the implications of insufficient sleep to health inequalities, more sleep and circadian research is needed.
Overview of health risks of circadian disruption and sleep deprivation
Why modify your circadian rhythm? What are the health costs of free-running, circadian disruption/misalignment or chronic sleep deprivation?
Sleep is a highly conserved functionality throughout the animal kingdom, despite the dangerosity for animals to sleep and be defenseless against predators. All species also have a "profound drive to maintain a regular sleep-wake cycle" (ie, circadian alignment), with disruptions having wide reaching detrimental effects in performance, safety and health. On a scale of urgency of importance for survival, sleep deprivation kills in a few days, whereas food in a few weeks. Hence, both sleep and circadian alignment must serve at least one or several vital purposes.
Everybody has a set period to sleep, even typical sleepers: those who have a night shift job but can't sleep a full night during the day have shift work disorder. The difference with (endogenous) circadian rhythm disorders as that the individual's circadian rhythm is different from the socially acceptable norm. Those who can't sleep as early as socially acceptable have Delayed Sleep Phase Disorder (DSPD), and those who have a sleep period that changes everyday have non-24. In all of these cases, including shift work disorder, sleeping outside of the biological night leads to detrimental health issues. Indeed, everybody can wake up at any time for some time at the expense of sleep deprivation, but doing so for too long or too regularly will invariably lead to death.
The primary issue with having a circadian rhythm disorder is obviously the huge chronic sleep deprivation that is constantly induced by the social jetlag (ie, the attempts to constraints to social expectations/requirements for work, family, hobbies, etc). Chronic sleep deprivation is a major health issue, not only for circadian rhythm disorders, as most people in modern society are sleep deprived (social jetlag), although usually not to the extent and frequency that individuals with a circadian rhythm disorder or shift workers experience. There are plenty of resources showing how harmful and dangerous sleep deprivation is, and is one of the rare disorders that can directly and swiftly cause death if too prolonged or too chronic. Circadian misalignment, also called chronodisruption, was also shown to lead to a higher mortality in general.
According to the AASM and CDC:
> Chronic inadequate sleep and untreated sleep problems, according to the study’s authors, are associated with an increased risk of cardiovascular disease, diabetes, obesity, occupational accidents, and motor vehicle collisions.
>
> According to the CDC and the Maternal and Child Health Bureau, 34.1 percent of children, 74.6 percent of high school students, and 32.5 percent of adults in the United States do not get enough sleep on a regular basis. As a result, one of the aims of Healthy Individuals 2030, which sets 10-year, quantifiable public health goals for the United States, is to help people get adequate sleep.
A large-scale epidemiological cohort study on the UK BIOBANK found the following factors that increase the risk of heart failure (summary here):
- 34% higher in those reporting daytime sleepiness — in other words chronic sleep deprivation.
- 17% higher in those with frequent insomnia,
- 12% higher in those who had a short sleep (slept less than 7 hours daily),
- 8% higher in later risers — note however that the authors did not control for the individuals' circadian misalignment with their job's hours, which is likely the main factor for health and not the chronotype per se. This result was barely significant when controlling for other factors.
What is impressive about this cohort observational study is that the first 3 effects were highly significant independently of other factors across 3 different models: age, gender, alcohol intake, medication, diabetes, hypertension, even the first 10 genetic principal components, etc. (see Table 1 legend). This shows these effects are very strong, the only exception being the morning lark chronotype with a very weak p-value across models and getting weaker with better controlled models, barely reaching significance on the most controlled Model 3 (p-value = 0.04, whereas for the most lenient Model 1 p-value = 0.002). Another similar study found that "poor sleep efficiency and long wake after sleep onset" increase the risk of cardiovascular diseases. Given that daytime sleepiness and brain fog are very common for people with circadian rhythm disorders, this is a serious risk to consider for this population. Another study on the same dataset but investing healthspan reduction from a variety of mortality causes that are related to sleep found similar results.
This increase in the risk of heart failure, as well as the variety of other diseases caused by sleep deprivation, is likely primarily caused by the increase in oxydative stress, in other words cellular damage, which is the primary purpose of sleep as evidenced by a landmark 2020 study. Indeed, the authors found that prolonged sleep deprivation causes death by the accumulation of reactive oxydative species, in other words a buildup of cellular damage. During sleep, there is a widespread release of strong antioxydative agents such as melatonin which cleans up and keep under control the oxydative damage and inflammation, especially in the digestive system (the primary source of oxydative damage due to food ingestion). Indeed, it is now strongly suspected the core pathway of sleep-deprivation-induced damages is via increased oxidative stress and inflammation. For instance, a study on mice found that continually phase advancing (chronic jet lag) mice by 6h/daily led to a 4.2x increased death rate (89% versus 21% in unshifted mices), which is in practice what individuals with non-24 experience when they restrict their sleep to fit into a typical schedule without entrainment therapies.
> In otherwise healthy adults, short-term consequences of sleep disruption include increased stress responsivity, somatic pain, reduced quality of life, emotional distress and mood disorders, and cognitive, memory, and performance deficits. For adolescents, psychosocial health, school performance, and risk-taking behaviors are impacted by sleep disruption. Behavioral problems and cognitive functioning are associated with sleep disruption in children. Long-term consequences of sleep disruption in otherwise healthy individuals include hypertension, dyslipidemia, cardiovascular disease, weight-related issues, metabolic syndrome, type 2 diabetes mellitus, and colorectal cancer. All-cause mortality is also increased in men with sleep disturbances. For those with underlying medical conditions, sleep disruption may diminish the health-related quality of life of children and adolescents and may worsen the severity of common gastrointestinal disorders. As a result of the potential consequences of sleep disruption, health care professionals should be cognizant of how managing underlying medical conditions may help to optimize sleep continuity and consider prescribing interventions that minimize sleep disruption.
A non-managed circadian rhythm disorder such as non-24 results in all 4 of the issues above, suggesting that unmanaged circadian rhythm disorders such as non-24 likely significantly increases the risk of cardiovascular complications. Indeed, the cardiometabolic system shows a clear circadian component, and the suprachiasmatic nucleus (SCN) "innervates the heart and other organs involved in hemodynamic control, such as kidney, vasculature and adrenal, via a multisynaptic pathway, probably including direct projections form the SCN to the paraventricular nucleus of the hypothalamus (PVN)" (see also here for an updated review).
Although we do not have data for non-24 specifically, there is some data about evening chronotypes, which we can infer to share similar albeit lesser chronic sleep deprivation. Evening chronotypes are 5 times more likely than morning larks and intermediate chronotypes to retire early on a disability pension. It's likely much worse for non-24.
Sleep deprivation, which is likely the root cause of most of the issues above, can either be resolved by free-running or by being entrained, the latter being very difficult if not impossible to reach for most non24 at this stage of scientific knowledge. So is free-running enough to live heathily? Unfortunately, that's unlikely.
Indeed, free-running means that there is an almost constant environmental-circadian misalignment (ie, misalignment between the individual's circadian rhythm and the day/night cycle). But the production of some hormones, such as melatonin, is dependent on the external day/night cycle. Melatonin is produced most at night, as it is inhibited by bright light. Hence, a free-running individual is likely to have reduced levels of melatonin due to unwanted inhibition by light exposure.
What are the consequences of melatonin reduction? Well, melatonin is a strong (maybe the strongest) naturally secreted anti-oxydant in the body. It is also immunomodulatory. Hence, a lack of melatonin is linked in animal models with various diseases and immunodepression.
Furthermore, mistimed eating when melatonin is at high levels in the body has been linked with metabolic dysregulations, and even directly caused diabetes in an animal model, without any other change of any other factor (TODO: add links to refs). Hence, both the lack of melatonin and the mistiming of melatonin with other factors such as food can produce detrimental effects on health, whether or not the individual is sleeping enough by free-running. (But of course these risks are far lower than what chronic sleep deprivation causes, so health-wise it's preferable to freerun rather than suffer from sleep deprivation, but it's also preferable to be entrained than to freerun, if of course an efficient treatment is possible for the individual).
Hence, supplementation of melatonin serves two purposes: to both supplement to overcome the lack of melatonin, and help with entrainment which directly reduces unwanted inhibition of melatonin by light. Would an oral supplementation of melatonin be enough to reduce the rate of diseases due to circadian misalignment and/or sleep deprivation? Likely yes, but it depends on the dosage: a study on animals shown that totally sleep-deprived animals could survive if supplemented orally (or intraveinously) with melatonin, and it's known that blind individuals have a much lower rate of cancers which is hypothesized to be because of higher melatonin levels since their melatonin is never inhibited by light, and since then melatonin was shown to indeed reduce cancer progression. Future trials are needed to know the proper dosage and how much benefits can be expected in humans, see the section on Melatonin below for more infos about the few already conducted trials (such as on sepsis and cancer).
Beyond sleep deprivation and melatonin reduction, circadian misalignment reduces our immunological response. Indeed, the circadian rhythm modulates the immunological response, especially the macrophages, as a 2021 study found. This means that our immunological system works better during our biological day, but worse at night so we are more likely to get ill or more severe diseases at night. For example, an individual with a circadian night happening in the day, as is always the case for DSPD and cyclically for non-24, means that if these individuals try to force themselves to stay awake during the objective day, they will be more prone to getting infections, contrary to typical sleepers who would be more vulnerable during the objective night since their circadian night happens during the objective night. This also means that even without sleep deprivation, sleeping in circadian misalignment increases the risks and severity of illnesses. This finding shows that individuals with circadian rhythm disorders are more prone to infections when they try to follow a typical sleep-wake schedule. The authors also found that the circadian rhythm mechanisms in the cells are much more complex than previously thought, with their study showing one of these new mechanisms, and suggesting that there is likely much more to discover on the ways the circadian rhythm controls the immunological system. Hence, it is crucial to sleep in phase with the circadian rhythm, as "the latest research has demonstrated that life habits coherent with the internal clocks should be adopted, especially during childhood, to prevent metabolic diseases." For instance, it was found that shift workers were 2 to 3 times more likely to get infected by COVID-19, with the highest odd being for those who performed irregular/rotating night shift work.
> Most immunological functions, from leukocyte numbers, activity and cytokine secretion undergo circadian variations, which might affect susceptibility to infections. The intensity of symptoms and disease severity show a 24 h pattern in many immunological and allergic diseases, including rheumatoid arthritis, bronchial asthma, atopic eczema and chronic urticaria. This is accompanied by altered sleep duration and quality, a major determinant of quality of life. Shift work and travel through time zones as well as artificial light pose new health threats by disrupting the circadian rhythms. Finally, the field of chronopharmacology uses these concepts for delivering drugs in synchrony with biological rhythms. https://doi.org/10.1186/s12948-018-0080-0
NB: chronopharmacology dates back to at least 1973 or 1971.
Furthermore, beyond general health damages, chronic sleep deprivation and circadian misalignment drastically increase the risk of transportation and work accidents, with sleepiness being the major cause of preventable accidents in all modes of transport, surpassing alcohol and drugs. Indeed, drowsy driving due to even slight small sleep deprivation, such as staying awake for 1-3h later than usual, is more dangerous than driving drunk.
The potential therapeutic benefits of circadian-based medical interventions, or at least medical interventions integrating the circadian rhythm in their protocol, are such, not only for circadian rhythm disorders but for all pathologies beyond sleep, that well established researchers are confident of the advent of "circadian medicine" as a crucial step forward in future medicine.
Historically, the "father of sleep research" Nathaniel Kleitman published his first research work on the effects of prolonged sleep deprivation in humans, in the 1920s (original paper here). This paper is worth a read, although knowledge has much progressed since then, a lot of what is known nowadays can already be glimpsed at in it, with additional historical context such as the prevalence of psychologists in sleep research being due to the lack of technological tools at the time to study sleep biologically, and also the lack of a robust definition of sleep at the time which brought some authors to even argue that plants could be sleeping.
An excellent military medical review investigated the severe health effects of subjecting military and commercial personnel to non-24 sleep-wake schedules. The same effects are likely to be expected for individuals with the non-24 disorder when subjected to a forced typical sleep-wake schedule.
While the independent but additive effects of sleep deprivation and circadian misalignment adverse effects on health, and the great health improvements that can be obtained by restoring these issues, is well documented in the scientific and medical literature, most people with circadian rhythm disorders still report feeling tired despite sleeping in alignment with their circadian phase. Beyond the possibility of bias, such as misidentifying one's circadian phase due to the lack of wide availability of unattended circadian rhythm monitoring tools such as actigraphy with circadian rhythm detection algorithms or non-invasive core body temperature monitoring, this is actually very likely caused by the almost unavoidable effect of sunlight exposure. Indeed, even though the individual with a circadian rhythm disorder has a shifted circadian night, sunlight will still affect the individual by shifting the circadian phase chaotically around due to uncontrolled exposure, melatonin inhibition, cortisol secretion and a whole lot of other bio-hormonal changes throughout the body due to sunlight exposure. Hence, there are likely only two solutions to ensure a completely healthy lifestyle for people with a circadian rhythm disorder: 1) find a therapy that allows to entrain to a typical schedule, in phase with the day-night cycle and hence aligning sunlight exposure with their circadian day, this is the approach taken by the present document; 2) live in an environment completely isolated from sunlight, but still connected to an electrical network to allow for a dynamically timed artificial light system to reproduce a day-night cycle but aligned with the individual's circadian phase, which is more easily achievable for DSPD since the timing does not change much from day to day, but would be more challenging for non-24. In both cases, the solution is to either change one's phase to align with the environment's zeitgebers, or to exchange the environment's zeitgebers for an artificial replacement that can be aligned with one's circadian phase. Hence, it appears there is no perfectly healthy solution that does not involve alignment between one's circadian phase and environmental zeitgebers, especially sunlight.
That said, the survey reported that some tiredness is still felt even after sleeping in circadian alignment without control of environmental zeitgebers, but it is highly likely this is always a significant improvement in health outcomes over sleeping in circadian misalignment.
Due to the paucity of research, and because sleep and the circadian rhythm are highly conserved vital biological processes throughout evolution and species indicating that the same results should apply to all organisms, other health issues involving circadian misalignment and sleep deprivation were included below, similarly to what was done by other studies. For example, a very close model of non-24 (endogenous disorder) is chronic jet lag disorder (exogenous disorder) as experienced by flight staff, and a similarly analogous model for DSPD (endogenous) would be night shift work disorder (exogenous), we can assume similar health issues although the cause differ for these pairs of circadian rhythm disorders.
A classification of the health risks of circadian rhythm disorders
Unfortunately, the potential health issues of free-running non-24 or DSPD sleeping according to their natural schedule remain unexplored in the medical scientific literature. But we do have indirect evidence from extrinsic circadian rhythm disorders, such as night shift disorder or jet lag disorder, which are much more common. We can define 4 different broad classes of health risks related to circadian rhythm disorders:
Sleep deprivation, usually chronic (ie, regularly experienced)
Sleep deprivation happens when not sleeping enough, and it becomes "chronic" when it happens regularly. Sleep deprivation not only has major impacts on health that majorly increases all-cause mortality, including by cardiovascular diseases and cancer (see also here), and can even lead to sudden death by cardiac arrhythmic arrest through oxidants accumulation in the body, particularly, but not only (see also here and here), for those with obstructive sleep apnea (see also here), and sleep deprivation is now a primary target of treatment for the modern comprehensive approach for cardiac diseases prevention. The influence of subqualitative sleep on cardiovascular risks is so important that the American Heart Association acknowledged the issue since its 2016 guidelines and aims to run public health campaigns on the importance of sleep for cardiac health. The increase in cardiovascular risks also affects children, and it may be dependent on predisposition to metabolic syndromes. It can also curb the benefits of diet or lifestyle changes and it impairs the evaluation of risks by causing an overly optimistic bias. Sleep loss majorly impairs the immune system. Sleep and the immune system are interacting bidirectionally: severe infections can cause sleep deprivation, and a shorter sleep impairs the immune response to infections and inflammations so that the risk of infections is increased and vaccines efficacy is decreased by directly decreasing the number of antibodies produces, since sleep promotes their production. During the COVID-19 pandemic, short sleep was assessed as a risk factor for more severe symptoms, and scientists suggested that requiring longer sleep prior and after vaccination may be an effective and inexpensive way to increase a COVID-19 vaccine's efficacy. Furthermore, again with COVID-19, each 1-hour decrease in sleep was associated with 12% higher odds of infection (see also here). Indeed, sleep deprivation can alter the DNA, RNA and proteins. An informal survey reported that half of COVID-19 long-haulers complained about new sleep disturbances. All these risks also affects children and teenagers ("general pediatric population") and lead to poor academic performance. Since sleep deprivation reduces light therapy effectiveness, a vicious cycle can appear where chronic sleep deprivation impairs the very therapies that could reduce sleep deprivation. Chronic sleep deprivation has a dose-dependent cumulative effect on cognitive impairment: sleeping 4h per night is worse than 6h per night, and the impairment will only increase day by day, even though subjectively the individual doesn't feel more sleepy than on the first day sleep deprivation started. Compared to partial sleep deprivation (eg, 4h), total sleep deprivation (0h for 3 days) results in a "disproportionately large" neurobehavioral impairment. Hence, fixing sleep deprivation by allowing the individual to sleep according to their natural sleep schedule or by napping (or to another schedule with entrainment) should be a primary target for general health improvement. Ironically, chronic sleep deprivation can cause treatment-resistance chronic insomnia as suggested by the Randy Gardner case, or even lifelong psychoses and personality changes even after recovery sleep as shown by the Peter Tripp's case (although he lost his job due to the payola scandal and not due to the lasting effects of sleep deprivation), which shows that all-nighters can only worsen the condition. Personality makes little difference, but if anything and if you believe in the psychological concept of personality, extroverts performed worse after sleep deprivation than introverts. Interestingly, it seems that partial chronic sleep deprivation requires more time for cognitive recovery than total sleep deprivation. A neuroimaging fMRI study found that sleep deprivation affects differentially various brain regions, with decreased activation in the posterior cerebellum, right fusiform gyrus and precuneus, and left lingual and inferior temporal gyri, and increased activation in the bilateral insula, claustrum and right putamen. A neuroimaging review details that the DMN in particular demonstrates significant alterations under sleep deprivation, including decreased connectivity between the anterior and posterior cingulate gyrii of the DMN and a degraded connectivity segregation with the external awareness network, which is the type and areas of connectivity that is impaired in disorders of consciousness. Chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if from a young age, hence the necessity for accomodations of children with sleep disorders as chronic sleep deprivation can not only stunts cognitive performance, but pave the way for the development of neurological disorders. Sleep deprivation impairs auditory attention cognitive performance (see also here and here). Sleep deprivation causes more impairments and damages than just the loss of the benefits of sleeping. Three nights of consecutive, chronic sleep loss of just a few hours per day was sufficient to significantly impair mood and cognitive functions. Multitasking is impaired by sleep deprivation, as well as innovative thinking and adaptation to new situations (flexible decision making). Medical students' judgment and reaction time are also impaired by sleep deprivation. A lot of evidence is emerging that sleep deprivation, whether acute or chronic, drastically increases the risk of cardiac events, including arrythmias, atrial fibrillations and bradyarrhythmia and regardless of other traditional factors (see also here for an overview). Sleep disturbances have also been shown to increase the rate of cardiac arrests and ventricular ectopy.
Unfortunately, chronic sleep deprivation has been much less studied than acute sleep deprivation, and it remains mostly unknown the full extent of sleep-deprivation-induced damages, especially neuronal, and the potential for recovery, if any. The authors also note:
> The basic scientific findings regarding sleep loss have not yet been routinely applied in the clinic. [...] Sleep abnormalities are robustly observed in every major disorder of the brain, both neurological and psychiatric. Sleep disruption merits recognition as a key relevant factor in these disorders at all levels, from diagnosis and underlying aetiology, to therapy and prevention. More collaborative work between basic and clinical scientists in the field will be necessary to accomplish this goal. Notably, the answers to all these questions have perhaps never been more pressing considering the professional, societal and clinical implications that continue to scale in lockstep with the precipitous decline in sleep duration throughout industrialized nations.
Circadian misalignment
This is when you sleep the duration you need, but outside of your biological night. The detrimental health effects is well known and experimented worldwide every year because of daylight saving timezone (DST) changes, causing an increase of 8% in strokes, an increase of depression disorders and of car accidents due to decreased vigilance, and 25% increased rate of cardiac arrests, all due to one single hour of sleep lost after the timezone change. The most common type of circadian misalignment, social jet lag, which happens weekly in the transition between weekends to weekdays, is likely the reason why the vast majority of cardiac arrests happen on Mondays (or in other words, due to the sudden circadian misalignment and slight sleep deprivation after the week-end). Furthermore, it was observed that most adverse cardiovascular events happen between 6AM and noon, although the data is more reliable at 6AM than noon due to a possible under-reporting at night. Sleep-wake cycle is the most robust output rhythm of the circadian system, is significantly affected by neurodegenerative disorders, and may precede them by decades, and hence emerging evidence suggests that circadian disruption (ie, non24, shift work) may be a risk factor for these neurologic disorders. Circadian misalignment alone can lead to life-threatening cardiac arrhythmias (without sleep deprivation) and increases general cardiovascular risks (see also here). Circadian misalignment is associated with increased risk of metabolic syndromes such as diabetes and obesity as shown by epidemiological studies, prompting the renaming of metabolic syndromes as Circadian Syndromes, as circadian misalignment can actually directly cause diabetes in mice by causing a loss of pancreatic beta cells and by impairing the insulin-regulated glucose metabolism in hepatocytes (liver cells) (see the section about Food and metabolic syndromes for more information). Several studies have demonstrated a clear circadian regulation of the cardiometabolic system, with strong evidence suggesting circadian disruptions "may impact cardiometabolic and overall health", with for example a study finding "a 4% increased risk of ischemic stroke for each 5 years of shift work" (see also here and here). There is now a general consensus in the scientific medical community that circadian misalignment is a major risk factor for metabolic disorders, independently from sleep deprivation (see here, here, here). Furthermore, circadian misalignment is the major cause of accidents by far, as "sleepiness surpasses alcohol and drugs as the greatest identifiable and preventable cause of accidents in all modes of transport [...] industrial accidents associated with night work are common, perhaps the most famous being Chernobyl, Three Mile Island, and Bhopal." The IARC, the highest authority on defining probable causes of cancer, recognizes that "circadian disorganization" in night shift work is a "probable carcinogen in humans" (group 2A) (see also here). Circadian misalignment, in particular with aberrant light exposure, can also cause major cognitive, learning and mood impairment. Circadian misalignment affects wounds healing, with wounds happening during the biological night healing more slowly than those during the day. Circadian misalignment in women is associated with disturbances in menstrual function, particularly menstrual irregularity and longer menstrual cycles and may increase the risk of breast cancer. Circadian misalignment is not only observed in non-24 and DSPD but also night shift work disorder and social jet lag. The reduction of light exposure can worsen mood since bright light exposure has antidepressant effects. Sleep apnea is known to be a major cause of cardiovascular death, but it interestingly shows a marked circadian rhythm, with more deaths by sleep apnea during the biological night, whereas for other cardiovascular deaths it's rather during the day. Circadian misalignment also reduces the immunologic system and hence may increase the risk of infections such as COVID-19 and its severity. It was further found that shift workers were 2 to 3 times more likely to get infected by COVID-19, with the highest odd being for those who performed irregular/rotating night shift work. Circadian misalignment due to aberrant light exposure or melatonin inhibition is associated with increased breast cancer rates, and inversely timezones where melatonin is less inhibited by light such as the Arctic zones have lower rates of breast cancers. Circadian misalignment (chronodisruption) caused by chronic melatonin inhibition/deficiency due to urban artificial lighting increases the risk of cancers. Breast cancer survivors have a shorter disease free interval in metastatic breast cancer if bedtimes are misaligned with their circadian rhythm according to a meta-analysis. The disruption of cellular-level functional clocks has been evidenced by biologist as a pathway that can cause cancers, and in practice a 2020 systematic review found moderate grade evidence that shift work and long work hours increase the risk of (breast) cancer and strokes. Circadian dysregulation can cause metabolic disorders including non-alcoholic fatty liver disease (NAFLD - the most common liver disease worldwide) (see also here), and circadian realignment may improve NAFLD. Circadian misalignment is associated with digestive pathologies such as constipation and irritable bowel syndrome. The cognitive impairment induced by circadian misalignment can be objectively observed neurologically with a reduced default mode network's functional connectivity in night owls. Circadian misalignment can also affect proper administration of medication (chronopharmacology), since depending on the time of administration, a drug can have more potent and beneficial effect, whereas at the wrong time (usually during the circadian night), a drug may have more adverse effects (see also this talk). Neurologically, chronic circadian disruption impairs temporal lobe's and especially hippocampal neurogenesis (see also here), which explains the wide range of cognitive funcitons impairements observed in chronic jet lag and circadian rhythm disorders, such as decreased performance, memory, reaction time and depressive symptoms, with an estimated 6.5 years of cognitive decline in long-time shift workers compared to age-related controls, which persists for 5 years after conclusion of shift work. Ample evidence from animal studies strongly suggest that circadian rhythm disorder underlies several psychological disorders, such as major depression, anxiety and schizophrenia. Circadian disruption is common (20%) among survivors of traumatic brain injuries such as car accidents, and is a primary target of treatment as it can "precede, exacerbate or perpetuate many of the other sequelae". A study on mice demonstrated premature aging and prediabetic profiles in circadian misaligned mices (see also here). The circadian rhythm and sleep regulate the blood brain barrier notably by increasing endocytosis and re-equilibrium of metabolites, which may be relevant for brain cleanup and sleep inertia. Bright light exposure during the circadian night can also cause decreased insulin secretion and overall failure of pancreatic islet cells as shown in a rats study, which suggests that bright light exposure in circadian misalignment may increase the risk of metabolic disorders such as diabetes. A rigorous forced desynchrony protocol demonstrated in humans that acute circadian misalignment (here a 12h phase reversal) per se increases postprandial glucose and insulin responses, suggesting increased insulinoresistance, as well as increased blood pressure and decreased sleep efficiency and leptin levels (hunger). In old mice, chronic jet lag drastically increased mortality, especially for mice who were phase advanced by 6h, with a 53% mortality rate, whereas 32% for a 6h phase delay and 17% for unshifted mice, and interestingly it was not caused by chronic stress since daily fecal corticosterone levels were unchanged. Circadian alignment of drug therapies, ie pharmaceutical chronotherapy, is also a "plausible strategy" to optimize the therapeutic effects while reducing adverse effects of medication for gut infections. Circadian misalignment has been demonstrated to hamper development in plants and longevity in insects, especially when the environmental cycle is shorter or longer than the range the individual can sustain (ie, non-24 cycles forced onto typical sleepers). Forcing individuals, even when highly trained such as military personnel of NASA and submarines, to follow a non-24 sleep-wake cycle, cause a wide array of cognitive, physical and mood impairments. A 2019 study found thatsleep deprivation or a non-24-h working schedule independently lead to extensive alterations in physiology and behavior, including changes in insulin levels, significantly delayed reaction time and microbiota bacteria composition and concentration. Evidence is emerging in the 2020s that circadian and sleep disturbances are harbingers of neurodegenerative diseases, with sleep disruptions predicting beta amyloid accumulation and hence Alzheimer disease.
Hormonal suppression (especially melatonin) by unwanted/uncontrolled light exposure
Given the wide range of protective actions of melatonin on all cells in the body, unwanted inhibition of endogenous melatonin by bright light exposure during the biological evening and night is a serious health issue. This can happen without circadian misalignment because of artificial lighting, as it often happens for night shift work. There is some evidence that the lack of light exposure and reduced melatonin secretion may be the cause of the increase in breast cancer due to circadian misalignment. It's difficult to discriminate what is due to melatonin inhibition or what is due to circadian misalignment as this field of medical research is still in its infancy, so apriori the health risks associated with circadian misalignment should be considered to overlap those of hormonal, especially melatonin, suppression.
Cognitive impairments, social isolation and accidents
Sleep deprivation impairs the ability to suppress unwanted thoughts especially when presented with reminders and hence to multitask. Sleep deprivation largely impairs attention, but not reasoning abilities. Drowsy driving is responsible for a million crashes and 500 000 injuries each year in the USA, with "sleepiness surpassing alcohol and drugs as the greatest identifiable and preventable cause of accidents in all modes of transport". Indeed, driving with even the slightest sleep deprivation is akin to driving drunk with alcohol: even slight small sleep deprivation, such as staying awake for 1-3h later than usual, leads to more cognitive disruptions than 0.05% of blood-alcohol level, which is considered a "drunk state" in most European countries (see also here), hence "a drowsy driver may be as dangerous as a drunk driver". A review found an almost linear increase in accident risk in shift workers beyond 8h of work. There is a 15x increase in driving accident risk for truckers after more than 13h awake compared to the first hour. Cognitive performance and memory, including short-term and long-term memory formation and recall, are greatly diminished when occurring out of phase with the natural circadian rhythm, and can even manifest as other cognitive deficits. Catastrophic industrial accidents associated with night work are common, including Chernobyl and the space shuttle Challenger catastrophic accidents were mostly attributed to poor judgment consecutive of chronic sleep deprivation induced by extended night shiftwork (see also this video). Crew accidents due to sleep deprivation is also common in aviation and military and responsible for hundreds of millions of material loss (see also here, here). The Navy started in 2021 to acknowledge sleep deprivation as a major issue and pledged to try to find new solutions to better manage it. Sleeping allows to find innovative mathematical solutions and patterns compared to individuals who do not sleep during the time gap between problem presentation and restitution (see here for a layman presentation). The excellent 1998 review "Black times: temporal determinants of transport safety" by Folkard "confirmed the presence of a clear circadian (ca 24 hour) rhythm in road accident risk with a major peak at ca 03:00" not due to drivers falling asleep at the wheel, but rather because of lowered performance capabilities due to the circadian misalignment.
Sleep fragmentation
Sleep fragmentation, which can be caused by melatonin deficiency or sleep disturbances such as noise and unwanted exposure to bright light, can cause various impairments. Sleep fragmentation impairs motor skills learning. Sleep fragmentation is associated with more periods of unconscious wakefulness, also called cortical arousal, which may increase the risk of premature death and cardiometabolic diseases. Sleep fragmentation is one of the primary factors increasing the likelihood of delirium in hospitals' intensive care units' (ICU) patients, along with aberrant bright light exposure (see also here and here), with some scientists even suggesting that the expression of delirium is very similar to sleep deprivation. Sleep disturbances can have a very strong detrimental effect on sleep efficiency, sleep quality and the circadian rhythm especially when there is unwanted exposure to bright light, that can carry over several days after the disturbances happened.
Social inadaptation
Non-24 inherently disrupts the social network around the individual, which has a direct consequence on both socioprofessional and health. Non-24 was found to be a worsening comorbid condition for blind people compared with a control group of blind people without non-24. For individuals with autism, the often comorbid sleep disorder further impairs the employability. An informal survey of individuals with non-24 suggest a very high rate of unemployment, with a vast majority of respondents feeling that non-24 is fully or partly to blame. A lifetime longitudinal study using biomarkers found that social isolation, which is worsened by sleep deprivation and non-24 (circadian freerunning) independently, was associated with vastly elevated risks of health conditions such as inflammation at all stages of life, and even exceed the clinical risk factors of old age. This association between social isolation and survival is well established in both humans and other social animals, as highlighted by a 2020 Science review.
Note that non24 likely has worse health prospects than DSPD, due to the increased frequency and magnitude of circadian misalignment and potential of chronic sleep deprivation.
TODO: add content from https://www.reddit.com/r/DSPD/comments/h8och2/i_sleep_between_9am_and_16pm_every_day_i_this/fuw6h3k and https://www.reddit.com/r/N24/comments/hqz5ix/does_n24_have_long_term_effects_on_health/fy25ppz
For more detailed information on the detrimental health effects of sleep deprivation and circadian misalignment, these two great reviews are recommended:
> Foster RG. Sleep, circadian rhythms and health. Interface Focus. 2020 Jun 6;10(3):20190098. doi: 10.1098/rsfs.2019.0098. Epub 2020 Apr 17. PMID: 32382406; PMCID: PMC7202392. https://doi.org/10.1098/rsfs.2019.0098
> Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139-154. doi:10.3109/09540261.2014.911149 . URL: https://pubmed.ncbi.nlm.nih.gov/24892891/
Depression, anhedonia, running thoughts and social isolation
Although a lesser known fact, depression, anhedonia (lack of pleasure and will, feelings of being empty) and social isolation are normal part of circadian rhythm disorders, and especially in non24 where they appear cyclically depending on the current phase (nightwalking) and season (winter is worse).
> In anhedonia, both wanting and liking are muted.
Source of the quote.
Although prevalence data is sparse for sighted non-24, depression appears to be a common comorbidity similarly to other sleep disorders, as this 2005 study of 57 sighted non-24 participants cohort found that 34% of them also suffered from depression after the onset of non-24. For DSPD, there is ample evidence that depression is the most common comorbid disorder.
Both a single night of sleep deprivation and lack of bright light exposure or aberrant exposure in the biological night (see also here) independently cause depressive symptoms such as anxiety and moodiness in non depressive, healthy individuals, with the combination likely causing even greater distress. There is a two-fold risk of developing depression when chronically sleep deprived. Severely sleep deprived individuals "manifest an anxious, depressed, negative cognitive-affective set". Plenty of evidence from animal studies strongly suggest that chronic circadian disruption likely cause depressive symptoms such as anhedonia and memory impairements as well as anxiety and psychological disorders such as major depression, regardless of sleep deprivation. A large-scale study using the UK BioBank dataset found a causal link between the genetical chronotype and circadian misalignment on depressive and generalized anxiety disorder symptoms, explaining why morning owls on a day job have higher well-being, whereas intermediate chronotypes and night owls who constantly defy their circadian rhythm (body) clock suffer from lower well-being and increased anxiety and depression, in line with previous studies (see here and here). Sleep disorders during childhood are robustly associated with a modest increase in the risk of early onset of major mental mood and psychotic disorders in teenagers and young adults. Sleep deprivation has been linked to an increased risk of suicide in a systematic review. Although some psychologists claim (REM) sleep deprivation may treat depression, most studies were not properly controlled, and a 2021 systematic review only found 9 controlled studies to review, from which it appears that sleep deprivation only has a temporary and often non-significant effect on depression, but it can increase by 4-fold the risk of transition to mania. Since sleep deprivation and circadian misalignment independently cause depressive symptoms, it has been established that on the first day of the work week, the day with the most social jet lag due to the transition from weekend to work days, is the most likely time for suicidal posts on Reddit, which interestingly enough mirrors the increase of cardiac arrest, both pointing to circadian misalignment as the likely main cause.
In fact, there is a study by Soomi Lee et al that precisely modeled with mathematical equations how mood and cognitive impairments are related to chronic sleep deprivation:
> Daily negative affect increased and positive affect decreased in curvilinear fashion as the number of consecutive sleep loss increased. For example, daily negative affect increased (linear), but the rate of increase decelerated as the number of consecutive sleep loss increased (quadratic). Results were consistent for the number and severity of physical symptoms. For negative affect and the severity of physical symptoms, cubic effect was also significant such that the rate of increase accelerated again in the days most distal to baseline (no sleep loss).
Circadian misalignment also appears to cause depressive-like behaviors, but not in all individuals. In male mice, chronic circadian phase advance induced depressive-like responses, but not anhedonia, and suppressed neuroimmune activation. Interestingly, the finding that circadian misalignment appeared to not cause anhedonia would suggest that anhedonia is rather a consequence of sleep deprivation than circadian misalignment, although more studies are required. Another study on a small number of human participants found that the effect of a circadian misalignment on mood was relatively benign, with most of the mood changes being contributed to by sleep loss. However, given the cyclical nature of depression and disturbed sleep-wake patterns and physiological rhythms, there are strong suspicions that dysfunctions in the circadian rhythm may underlie depression (see also here), and indeed a recent systematic review supports this. This strongly suggests that targeting circadian rhythm may improve depressive symptoms, such as with agomelatine (a melatonin agonist) or bright light therapy (see also here), with light therapy being effective much faster than antidepressant drugs and much fewer side effects especially of suicide.
Fatigue is also a hallmark of circadian rhythm disorders such as DSPD, but not excessive daytime sleepiness. Fatigue is defined as "tiredness and lack of energy without increased sleep propensity", and although it is a common complaint, it is underestimated both in research and clinical work. Sleepiness is defined as "a subjective difficulty in maintaining wakefulness and an increased ease of falling asleep". About 10% of the general population is affected by moderate or severe excessive daytime sleepiness, and is associated with socioeconomics (work, lifestyle, etc.), climate conditions (high temperatures) and is a cardinal symptom of sleep disorders, but not of circadian rhythm disorders, which are rather affected by insomnia and fatigue, and with DSPD individuals for example spending less time in bed, a likely consequence of insomnia and fatigue.
In addition, sleep deprivation impairs the ability to suppress unwanted thoughts (ie, increases racing thoughts, also called cognitive hyperactivity) especially when presented with reminders (see also here and here), which can partially explain the higher propensity of sleep deprived individuals to anxiety. Indeed, neurologically, sleep deprivation impairs sensory gating and reduces P50 suppression, in other words sleep deprivation reduces inhibition of irrelevant information, which leads to racing thoughts and human errors and accidents. Furthermore, a CBT-i study by Harvey et al shown that instructing the insomniac patients to suppress their thoughts before sleep (more technically called "suppression of presleep cognitive activity") led to a worsening of the sleep issues, with an increased sleep latency and reduced sleep duration. Hence, having racing thoughts before sleep is in fact a consequence of sleep deprivation, rather than the cause, and treating by suppression the running thoughts does not help and even worsen the insomnia. Actually, scientists even think sleep deprivation may be the root cause explaining the dissociative symptoms as observed in schizophrenia and schizotypical disorders as well as PTSD, although there is no link with non-24 for the moment, it just further supports that running thoughts are a common symptoms of all diseases causing chronic sleep deprivation. Another study found both running thoughts and dissociative symptoms increased after sleep deprivation. A 2021 systematic review further found that although CBT-I does improve insomnia complaints and sleep metrics, and that it also reduces the occurrences of worries, one type of repetitive negative thoughts (RNTs), this reduction in RNTs was not correlated with reductions in depression or anxiety, so that CBT-I dowas not found to be an effective mean to reduce RNTs, but is more indicated for insomnia. This hence shed some doubts about whether RNTs reare of any importance to treat sleep disorders, since CBT-I is effective without a significant effect on RNTs.
Furthermore, a study demonstrated that sleep deprivation and sleep disorders impair interoception, which is the ability to feel one's own internal body signals including the ability to feel sleep pressure, with impaired introception being associated with major depression according to a 2019 systematic review.
Practical tip: signs of anhedonia include lack of appetite, lack of motivation and lack of pleasure. If this is caused by sleep deprivation or circadian disruption, anhedonia should resorb in the following days. Otherwise, if this persists, this may be a sign of major depression and should be investigated with a clinician.
Not only does sleep deprivation cause depressive symptoms, sleep deprivation is also literally and objectively painful, with modest changes in sleep quality tremendously increasing pain experience. Indeed, this study, focusing on the neurological basis of sleep deprivation magnified pain, found that even small sleep disruptions can cause major increases in pain the subsequent day while also decreasing reactivity and quality of decision-making:
> Sleep loss increases the experience of pain. [...] Here, we demonstrate that acute sleep deprivation amplifies pain reactivity within human (male and female) primary somatosensory cortex yet blunts pain reactivity in higher-order valuation and decision-making regions of the striatum and insula cortex. Consistent with this altered neural signature, we further show that sleep deprivation expands the temperature range for classifying a stimulus as painful, specifically through a lowering of pain thresholds. Moreover, the degree of amplified reactivity within somatosensory cortex following sleep deprivation significantly predicts this expansion of experienced pain across individuals. Finally, outside of the laboratory setting, we similarly show that even modest nightly changes in sleep quality (increases and decreases) within an individual determine consequential day-to-day changes in experienced pain (decreases and increases, respectively). Together, these data provide a novel framework underlying the impact of sleep loss on pain and, furthermore, establish that the association between sleep and pain is expressed in a night-to-day, bidirectional relationship within a sample of the general population. More broadly, our findings highlight sleep as a novel therapeutic target for pain management within and outside the clinic, including circumstances where sleep is frequently short yet pain is abundant (e.g., the hospital setting).
Furthermore, another study demonstrated that pain sensitivity is mostly (~80%) modulated by the circadian rhythm, with only a minor (~20%) contribution of sleep pressure.
This is on top of the objective worsening of comorbid conditions and of general health by sleep deprivation, so that sleep deprivation increases pain both objectively and subjectively. And indeed there is an interaction, as a review found that not only sleep deprivation does increase pain perception (hyperalgesia), it also decreases the effect of pain medication such as opioid and serotoninergic analgesics:
> Chronically painful conditions are frequently associated with sleep disturbances, i.e. changes in sleep continuity and sleep architecture as well as increased sleepiness during daytime. A new hypothesis, which has attracted more and more attention, is that disturbances of sleep cause or modulate acute and chronic pain. Since it is well-known that pain disturbs sleep the relationship between the two has since recently been seen as reciprocal. To fathom the causal direction from sleep to pain we have reviewed experimental human and animal studies on the effects of sleep deprivation on pain processing. According to the majority of the studies, sleep deprivation produces hyperalgesic changes. Furthermore, sleep deprivation can interfere with analgesic treatments involving opioidergic and serotoninergic mechanisms of action. The still existing inconsistency of the human data and the exclusive focus on REM sleep deprivation in animals so far do not allow us to draw firm conclusions as to whether the hyperalgesic effects are due to the deprivation of specific sleep stages or whether they result from a generalized disruption of sleep continuity.
Sleep deprivation also causes social isolation, as individuals who are sleep deprived feel much less comfortable with other people being physically close to them. Chronic insomnia also decreases the ability to feel empathic with others emotions such as sadness and fear and also happiness. Combined with the sleep deprivation induced anhedonia, this results in a decreased engagement and enjoyability of interpersonal relationships, as was already well established for insomniac patients. To its extreme, sleep deprivation also causes paranoia, which drastically impairs communication. Paranoia being likely experienced at least a few times during the lifespan of any individual with untreated non-24. These neurocognitivo-social isolating processes compound with the mistimed wakefulness schedules of non24 individuals which further worsen the issue by making it very difficult to plan and attend to social appointments and events. This effect of sleep deprivation on social isolation seems unidirectional, as social loneliness does not impair sleep, hence treating the social isolation is unlikely to improve sleep.
Sleep deprivation is an underappreciated strong factor of interpersonal conflicts: frequency of interpersonal conflicts in romantic relationships increases with sleep deprivation, and are more often resolved when both partners are well rested, and these effects are not explained by stress, anxiety, depression, lack of relationship satisfaction, or by partners being the cause of poor sleep. According to David Randall's book Dreamland about the "no sleep" culture in military and military studies done on sleep deprivation, which may be inaccurate but there is no other sources to check given the type of sources used, air force pilots had a different vocal pattern when sleep deprived, being often unable to speak loudly to be understood and with a monotone speech that lacked emphasis on key words. Another military study was conducted on the cooperation of military duo's ability to planify and communicate together after sleep deprivation, which shown they most often were unable to succeed, whereas their sleeping counterparts could, which shows that sleep deprivation also affects professional interpersonal communications and collaboration even under highly trained and rigorous environments.
Furthermore, sleep deprivation largely impairs attention, but not reasoning abilities. Sleep deprivation also makes the patient "forget their life" as it impairs autobiographical memories.
Especially during the nightwalking phase, when completely out of phase with the day-night cycle, and the winter season, with longer nights and shorter days, the combination of sleep deprivation and lack of exposure to bright light is a perfect recipe for anhedonia and social isolation. Combined with the necessity to live on eggshells, the individual can justifiably feel like they are encaged.
The author also noticed that these cognitive and mood disruptions can go both ways in practice: often the effect of sleep deprivation is mood depressive, but sometimes there are maniac-like phases, hence chronic sleep deprivation can cause bipolar-like episodes, which may explain why it was hypothesized, but since then refuted, that non-24 was associated with the bipolar disorder.
These effects of sleep deprivation on cognition are in many respects similar to the cognitively disruptive effects of alcohol, with one sleepless night being analogous to 0.10% of blood-alcohol level, much beyond the drunk driving threshold in most European countries, with similar impairments in judgment and other cognitive functions:
> Staying awake for just 17 to 19 hours straight impacts performance more than a blood-alcohol level of .05 percent (the level considered legally drunk in most western European countries). This level of impairment slows an individual's reaction time by about 50 percent compared to someone who is well rested. Twenty-four hours of continuous wakefulness induces impairments in performance equivalent to those induced by a blood-alcohol level of 0.10 percent, beyond the legal limit for alcohol intoxication in the United States. Source (see also here for a similar statement from the Belgian police)
It is hence not surprising that sleepiness is the major cause of preventable accidents by far in all modes of transport, surpassing alcohol and drugs, being responsible for 10-20% of city car accidents and 20-30% of morotway car accidents according to Belgian police, being the first cause of motorway car accidents in several european countries since years including France (see also here and here). A driver who slept 5h on several consecutive nights is 3 to 6 times more likely to be involved in a car accidents compared to a driver who slept 8h. Hence, "a drowsy driver may be as dangerous as a drunk driver". A review found an almost linear increase in accident risk in shift workers beyond 8h of work.
Hence, a circadian rhythm disorder also limits the transportation possibilities, which further increases isolation and impairs the possibilities of getting hired, as most work positions require independent car transportation.
Mood and cognitive dysregulations are not just limited to practical abilities, as one night of sleep deprivation drastically impairs innovative thinking and flexible decision making, in other words, sleep deprivation impairs the ability to "think outside the box" to find new solutions and adapt to new situations (see here for a layman presentation). Likewise, sleep deprivation impairs multitastking ability, in other words, when sleep deprived, there is a tendency to hyperfocalize on one task.
Often, this set of cognitive and mood disturbances are expressed by the patients as "feeling like a zombie". It's crucial not only for the clinical practitioners to recognize these signs associated or caused by sleep deprivation, but also to teach the patient how to recognize them too, in order for them to avoid risky situations (such as driving while sleep deprived, or taking important decisions - delaying to a later time after recovering some sleep is a sound strategy).
More precisely, this systematic review provides prevalence figures of the cognitive impairements due to sleep deprivation:
- Hallucinations (visual, auditory and somatosensory) for nearly all participants in all studies, appearing after 24h to 48h of sleep deprivation.
- Mood changes (including anxiety and irritability) for 76% of the participants in 16 studies, usually appearing under 24h (very fast!). These are followed by "depression, apathy alternating with euphoria, anger, and hostility within 45 h without sleep".
- Disordered (running) thoughts, confusion, and bizarre behavior (14 studies, 66%), usually appearing on the 2nd day of sleep deprivation, and get to their worst from the 5th day on.
- Dissociation including derealization and depersonalization (11 studies, 52%), usually appearing after 24-48h.
- Delusions (9 studies, 42%), usually appearing on the 3rd day of sleep deprivation, and get to their worst from the 5th day on.
- Distortions in the sense of time (4 studies, 20%).
These impairments are gradual, so that paying attention to these symptoms allows to know when it's crucial to get some sleep asap: "Initially, participants tend to question the veracity of the deceptive perceptual phenomena. With the passing of time and persistence of symptoms, there is a gradual acceptance that these events might be real, which precedes the appearance of full-blown delusional explanations."
Furthermore, for some participants, it took days up to weeks for the cognitive impairments to fully resolve, although usually sleeping at least 50% of the total time spent awake was sufficient.
Indeed, recognizing and remedying to sleep deprivations may allow to better manage, or even prevent, co-morbid psychiatric disorders: "given that ‘depression is announced by sleep disturbances’ (van Moffaert, 1994, p. 9), future research should explore whether an early intervention targeting sleep disturbance may prevent the development of depression (Ford & Kamerow, 1989). [...] In addition, future research should explore the utility of advising patients who have had a previous depressive episode to review relapse prevention strategies or to make an appointment with their clinician whenever they experience an ongoing sleep disturbance. In other words, relapse rates may be reduced if patients are taught to view insomnia as a signal to initiate preventative action."
Although the above review on sleep deprivation signs was done on acute, continuous sleep deprivation, the same is likely applicable to chronic sleep deprivation, but it depends on the amount of daily sleep deprivation. Indeed, a study comparing 0h, 4h, 6h and 8h of sleep "doses" demonstrated that the "neurobiological impairment" is proportional to the amount of daily sleep deprivation, so that the shorter the nights, the more frequent the impairments.
Despite a previously long held belief, morning larks do not display higher morality standards than later chronotypes such as night owls. Rather, studies have shown (see a summary here) that individuals are more susceptible to cheating when performing their tasks during their circadian night, in other words when they are in circadian misalignment. Indeed, morning larks were more susceptible to cheat during the objective night, whereas night owls were more susceptible to cheat in the early morning.
All these cognitive impairments are supported by neuroimaging studies, which found impairments in the DMN connectivity both within and in its segregation with the external awareness network, as well as impairments in the functional connectivity of the amygdata (emotions), the thalamus (memories), the dorsolateral prefrontal cortex and anterior cingulate cortex (consciousness and incentives processing), with conversely an increase of connectivity between the amygdala with the posterior cingulate cortex and the precuneus, denoting an increase in emotionally-directed behaviors. The authors also note that not all brain functions changes are maladaptive, some preserve task performance and are hence clearly compensatory and adaptive, but the extent and limitations of these adaptive strategies remain poorly characterized (ie, they are likely often overestimated by those experiencing or professing chronic sleep deprivation).
There is fortunately no quick and easy solution, as nothing can replace sleep, but some strategies may specifically help improve cognitive and mood disturbance in complement to sleep recovery:
- get exposed to bright blue-enriched light, even artificial light, at wake up, even if it means getting exposed during the objective night (but in your biological day). Indeed, light exposure is as effective as antidepressants to treat both seasonal and non-seasonal depression (see also here), with the combination of both being even more effective (see also here), and potentially other psychiatric disorders too such as schizophrenia or bipolar disorder. Bright light exposure's antidepressant effect may be due to the inhibition of melatonin, since melatonin inhibition by propranolol also has an antidepressant effect. Light therapy can further improve mood and learning impairments by correcting a potentially aberrant light exposure and restore endogenous melatonin levels through photic history. Compared to green light, blue light improved the activity of brain areas associated with emotion processing, which demonstrates that blue light is likely more effective to treat depression (eg, SAD) than other colors.
- nap as much as possible if you feel tired (it's not the mood, it's the sleep deprivation that causes you to feel tired). By reducing sleep deprivation and getting exposed to bright light, the combined effect can relieve a lot of the anhedonic effect of prior sleep deprivation. Treating sleep issues and reducing sleep deprivation can reduce the severity of depression, with long naps being the most effective way to reduce sleep deprivation and eliminate detrimental cardiovascular and neurologic health issues due to prior sleep deprivation, whereas short naps or no naps do not. Indeed, this systematic review found that "participants required approximately 50% of the total time they had been awake to recover from the deprivation period [e.g., 50 h of sleep to recover from 100 h of sleep deprivation]", but the residual cognitive impairments could last for days or even weeks for some individuals.
- Optionally: avoid bright light in the biological evening (dark therapy).
How to reduce the health issues of sleep deprivation and circadian misalignment
WIP: TODO: add references
Obviously, an ideal scenario to reduce the health issues due to sleep deprivation and circadian misalignment would be to eliminate them in the first place through robust entrainment. Indeed, some authors suggest it is sensible to treat the circadian misalignment and chronic sleep deprivation potentially underlying some cardiometabolic diseases using zeitgebers therapies such as light therapy and melatonin.
Unfortunately, that is not always possible, as the therapies do not work for everyone or they are under conditions that prevent them from optimally using therapies (eg, they must use an alarm clock to get to work). What can be done then? Here are a few practical tips:
- As advised for night shift work disorder, who experience similar issues and risk factors, the most adequate strategy to reduce the risks involves avoiding multifactorial causes of sleep disruptions including the "suppression of melatonin secretion by ALAN [bright light in the biological evening], sleep deprivation, and circadian disruption".
- Always respect your circadian rhythm as much as possible. This means to strive to sleep during your circadian night, and put aside other matters. This in effect put your sleep first, as anyone should do.
- Avoid at all cost activities, especially those requiring a high focus and are prone to accidents (eg, car driving), during your circadian night. If you really need to move during your biological night, prefer to ask someone else to drive or take public transportation.
- Take medication during your biological day, avoid during biological night as they will be less effective and have more side effects (see chronopharmocology). Similarly, injuries and wounds heal better during the biological day than during the biological night.
- Avoid eating during your circadian night, as melatonin will inhibit insulin which itself will inhibit your body's capacity to process carbohydrates and so you will be in hyperglycemia all night, which is highly suspected to cause metabolic disorders such as diabetes and obesity and fatty liver disease.
- If at wake up you feel your heart pounding in your chest or head, this is tachycardia. If it happens when you get out of bed, this may be postural orthostatic tachycardia. In any case, this is a sign you are at increased risk of cardiovascular issues, so try to sleep more or take a nap as soon as you can. If not possible, avoid activities or situations that can further cause cardiovascular issues, such as exercise or fast movements (and some temperature settings?).
- Supplement in vitamins to reduce deficiencies due to lack of exposure to sunlight and potential deficiencies due to chronic sleep deprivation such as vitamin D and vitamin B12. In the future, high doses of melatonin may be an avenue to drastically reduce the detrimental health issues of sleep deprivation due to its strong antioxydative properties, but it remains unclear what dose in humans would allow to reach the required extracellular concentration of melatonin to benefit from these antioxydative properties.
There are also a few very promising therapeutic avenues that are still experimental and thus not yet available for patients, as we do not even know the adequate dosage to achieve these effects on humans, but still the research is very promising:
- The strongest and most direct evidence that melatonin supplementation can reduce health issues due to circadian misalignment and chronic sleep deprivation independently from its effect on sleep is from a landmark 2020 study (and its awesome video abstract). Indeed, this study is the first to investigate why sleep is necessary and how exactly it can cause death. Contrary to what was assumed before, it's not the brain, but the accumulation of reactive oxidative species (ROS) in the guts that cause death. By supplementing orally with melatonin to flies and rats who were prevented from sleeping, they could live a full life with no behavioral sign of brain injuries. Three compounds shown effectiveness, with melatonin showing the most. If this result translates to humans, this may be a potential way to live without sleeping. But this may have serious cognitive side effects such as a memoryless moody zombie since the offline replaying theory of sleep is confirmed (see also here for original ref) and circadian misalignment causes major mood swings. Melatonin supplementation is also directly investigated for the treatment of coronary heart disease, and was already shown to be effective to reduce the risks of dying from severe inflammation / sepsis and cancers. In fact, the pleiotropic receptor-independent protective actions of melatonin, especially of antioxydation, are well established and measured, as they require much higher doses than the receptor-dependent sleep effects : "these receptor-independent protective actions of melatonin and its metabolites would require high intracellular levels of the molecules, which can only be met by melatonin in situ production in the relevant tissue, since cellular melatonin uptake is very limited because only 0.1% of extracellular melatonin can enter the cell (Fischer et al., 2006a)." In practice, as shown by the usage of melatonin on septic patients, the dosage necessary to produce the cellular protective actions would be about 8mg/kg/day in humans, which is substantially higher than the dosage required to induce sleep effects (0.5-3mg/day). Another 2021 study on rats with type-1 diabetes shown that administering 10mg/kg/day of melatonin in phase with the circdian rhythm prevented neurological damages and reversed cognitive deficit by attenuating oxidative stress and inflammation, which is a strong empirical finding demonstrating that extracellular doses of melatonin can indeed prevent damages of not only sleep deprivation but even neurodegenerative diseases.
- Light therapy and dark therapy can improve mood and learning impairments by correcting a potentially aberrant light exposure and restore endogenous melatonin levels through photic history and by avoiding unwanted exposure to bright light during the biological evening. There is some evidence that the lack of light exposure and reduced melatonin secretion may be the cause of the increase in breast cancer due to circadian misalignment.
Should all individuals with non-24 get entrained to be more healthy, or is freerunning acceptable? Well, freerunning for sure is much better than restricted sleep, as at least the sleep deprivation - the most acute health impairment - is reduced. However, even under freerunning, there will always be some degree of sleep deprivation and circadian misalignment, as it is impossible to completely control all environment factors such as unwanted sunlight exposure during the biological evening when nightwalking, or ambient temperature and ambient noise, all of which contribute to impair sleep. Hence, entrained non-24 likely is a more healthy state than freerunning non-24.
Interestingly, the periodicity of freerunning and restricted non-24 is also reflected in the periodicity of immunodepression, with individuals with non24 falling more often ill during their nightwalking phase than when they are in phase with the day-night cycle. Hence, being often but periodically ill, or regularly feeling daytime drowsiness, may be strong signs of chronic sleep deprivation.
How to monitor my entrainment progress?
One of the most difficult things for someone with a non-24 circadian rhythm disorder is to find when their biological night is, as it changes all the time and with high variability and sometimes chaotically.
To see if your circadian rhythm is stabilizing to a constant time, or just to find where your biological night is when freerunning, it is possible to use your sleep diary. But do not monitor your bedtime nor your falling asleep time (sleep onset), as they are both unreliable markers of the circadian rhythm and the DLMO. In other words, you can sleep more or less late with more or less variation while your circadian rhythm stays stabilized - the opposite is true, your bedtime can be regular but your circadian rhythm can still freeruns.
A much better measure is your wake up time (sleep offset), which is correlated with the DLMOff and is hence a much better marker of the circadian rhythm. This was demonstrated on both typical sleepers and DSPD. In fact, the wake up time is tightly coupled with the core body temperature, which is the core signalling pathway of circadian rhythm synchronizations throughout the body (see the Zeitgebers section for more infos). In other words, if your wake up time stays constant, this is a strong indication that you are entrained.
But the wake up time can sometimes be variable due to the random occurrence of "weird insomnia" (likely biphasic sleep) or external disturbances. An even better circadian rhythm marker is the midpoint of the sleep period(s) (see also here and here). This was also demonstrated in both typical sleepers and DSPD.
Let's say you usually sleep at 1.30am and wake up at 9.30am. The midpoint is the sleep onset time + average of (sleep onset/falling asleep - sleep offset/wake up) divided by 2 = 1.5+((9.5-1.5)/2) = 5.5 = 5.30am .
Let's now say that the next night, you experience a biphasic sleep, so you sleep earlier at midnight but wake up at 5am and then sleep again between 9am and 11am. If we look only at the wake up time, it looks like you woke up 1h30 later than usual, but if we calculate the midpoint, we get: 0 + (11-0)/2 = 5.30 = 5.30am . This is exactly the same midpoint time as usual, so in fact even if the sleep was biphasic, the circadian rhythm did not change, and you will likely be able to sleep at the same time as usual on the next night.
There are also a few other signs you may indicate that you are sleeping under your biological night:
- if your sleep onset latency is reduced, ie, when you get to bed, you fall asleep faster than usual. In my case, I used to never take less than 30 min to fall asleep, usually it took 1 or more hours, but after entrainment it consistently took me less than 15 min, and usually under 5-10 min, to fall asleep from getting to sleep.
- Tip: If it takes longer to fall asleep, either you are not sleeping in your biological night, or you missed the ultradian cycle window and need to wait for the next one (see below the ultradian cycle section). Accumulated sleep deprivation (eg, pulling an all-nighter) can also make it more difficult to sleep because of dopamine build-up.
- if you wake up on your own without an alarm or disturbance but didn't sleep long, then it's likely you are not sleeping in your biological night, but are rather doing a nap. Duration, along with the wake up time, is a very good indicator of sleeping inside one's own biological night.
- How long is a normal night of sleep? What is the ideal sleep duration for a human? The duration changes for each individual and for different ages. Most 30-60 years old adults need on average 6.5h to 10h of sleep, with less sleep needed over time. The average duration of sleep by age is quite well defined thanks to nation-wide epidemiological data.
>
> Conclusions: Sufficient sleep duration requirements vary across the lifespan and from person to person. The recommendations reported here represent guidelines for healthy individuals and those not suffering from a sleep disorder. Sleep durations outside the recommended range may be appropriate, but deviating far from the normal range is rare. Individuals who habitually sleep outside the normal range may be exhibiting signs or symptoms of serious health problems or, if done volitionally, may be compromising their health and well-being.)
- However, note that sleep needs for newborns, infants and children are systematically over-estimated, and that most studies used only parental self reported measures (see here and here).
- if your hunger and stools are more or less at the same time every day, that's a good sign your digestive system adapted to the entrainment, since these metabolic cues are linked to the circadian rhythm and directly to the suprachiasmatic nucleus. Since the digestive system is responsible for most melatonin secretion, this is certainly an important factor.
- Furthermore, if you are entrained, you should not feel hungry late into the night. If you feel hungry so late, it's a sign your digestive system at least is not entrained, since the digestive system also follows a circadian rhythm (but its own) which should "sleep" too at night.
- if you experience brain fog (aka performance reduction or sleep inertia), try to use light therapy, particularly blue light which is more effective at reducing brain fog and increasing vigilance. Bright light in the morning is well known to not only inhibits melatonin but also have antidepressant properties. In practice, this should clear up the brain fog under 30min to 1h of blue light therapy, as brain fog is likely due to melatonin residues in the blood stream, which can be inhibited most effectively by blue light. If the brain fog sustains all day long, then it may be a sign of circadian misalignment (ie, sleeping all your needed hours but outside of your biological circadian night). Note also that even under entrainment, it may take a few weeks before the all day brain fog disappears, the time for the digestive system to adapt (since it is the major producer of melatonin).
- Weight loss without changing your diet or exercise can be an indication you are sleeping in phase (circadian alignment), and on the opposite weight gain a sign of circadian misalignment, since circadian misalignment is strongly associated with metabolic syndromes (see "circadian syndrome").
In the future, it may become possible to more precisely monitor our circadian rhythm with the help of wearable core body temperature sensors (see the section on Core Body Temperature and also the Wearadian project on GitHub).
Circadian rhythm disorders: body, brain or mental disorders?
Although there is a common misconception that circadian rhythm disorders are either neurological or psychological (as reflected in the DSM and ICD-10 classifications TODO: update with precise classif G vs F), hence stemming from dysregulations in the brain or thoughts, this can only be qualified as incorrect.
Summary: There is now considerable evidence that circadian rhythm disorders are associated and worsened by a delayed peripheral circadian clock (ie, the digestive system 's clock including the liver and intestines), so it's much more likely a whole body disorder. It's even now considered to be associated with metabolic syndromes such as diabetes, so much so that some researchers call for a name change to "circadian syndrome". Another evidence is that sleep is more a product of the body than of the brain, as it mainly serves to clean up the oxydants in the intestines. Another hint that circadian rhythm disorders are not psychiatrical nor neurological conditions but rather whole body conditions is the fact that usually very effective psychiatric interventions such as cognitive behavioral therapy (CBT) are ineffective for DSPD and other circadian rhythm disorders.
For a more argumented study of this question, let's tackle it step-by-step.
Can circadian rhythm disorders be psychologically caused or manipulated?
Summary: no, sleep disorders, including circadian rhythm disorders and insomnia, cannot be caused by a psychological disorder. This is an archaic belief that is not supported by evidence nor the current medical guidelines. Sleep disorders require their own treatment, independently from possibly co-occurring psychological disorders. Circadian rhythm disorders can be indirectly manipulated behaviorally if the behavior changes the exposure to bright light and other zeitgebers, but there is no evidence that behavior per se can manipulate the circadian rhythm when zeitgebers are not involved.
For a long time, sleep disorders were considered secondary disorders, which means that they were considered a consequence of another disorder or disease, physiological or psychological, that would be the root cause, the primary cause. This view was shattered when evidence accumulated to demonstrate that sleep disorders are almost certainly generally primary. Indeed, although sleep disorders are often associated with other diseases or disorders and especially a lot of psychological disorders such as depression, increased suicide rates, emotional dysregulations, decision making impairment and ADHD-like symptoms, or even hallucinations and dissociations under extremely prolonged sleep deprivation up to death at the very worse after about a week of complete sleep deprivation, empirical evidence demonstrated that the sleep disorder almost always appeared before these psychological disorders and symptoms. Thus, since the sleep disorder predates psychological disorders and symptoms, it cannot be caused by the psychological disorders. This is why the current, modern view in sleep medicine and psychology is that psychological disorders are often comorbid to sleep disorders without being the cause, with potentially some contribution of the sleep disorder to the onset or worsening of the psychological disorder, and so both require simultaneous and dedicated treatments: one tailored for the psychological disorder, one for the sleep disorder. This was demonstrated for insomnia in a 2001 review by Harvey, and later for circadian rhythm disorders in a systematic review, concluding that "circadian rhythm disorders [...] require independent attention irrespective of co-morbid conditions".
Although almost all humans have a naturally non-24 circadian clock (~24.2h according to the NIH, bigger estimates were found by older improperly designed studies), and hence can freerun in the absence of external time cues, as shown by the "expériences hors du temps" done by several speleologists or its ancestor experiment by Kleitman in the Mammoth Cave or the modern variants of forced desynchrony protocol or nap paradigms, not everyone can follow a non-24h schedule, with morning larks having the most difficulties to adapt to other schedules (see also here and here). And even when individuals can follow a non-24h schedule (aka freerunning), it is a painful experience (see also here) and there is no evidence this can be sustained for a long time, contrary to people with intrinsic non-24 who function best when freerunning.
Work schedule experiments on the NASA crews monitoring Mars mission, where the well qualified and compensated crew of engineers and scientists was tasked with adopting a non-24h martian sleep-wake schedule to better monitor the robotic missions, ended up with the crew rebelling and dropping the schedule as they felt it was unbearable ("broken"), after ... a single month!
As the authors noted:
> The authors attributed this result to the high motivation of the crew, although motivation has limited ability to override circadian and homeostatic regulation of alertness and performance and is, in fact, subject to these influences itself. (...) Based on NASA surveys of 24 Mars Pathfinder veterans, those supporting the Sojourner Rover indicated that fatigue significantly affected their performance at work to the extent that they discontinued work on the Mars day schedule after only one month and described the schedule as “broken.” JPL managers described the scientists' and engineers' discontinuation of the Mars day schedule as a “rebellion.”
Hence, the non24 circadian rhythm sleep wake disorder is characterized not only by a freerunning sleeping pattern, but by the fact that it is unpreventable and incontrollable (ie, no obvious and easy to fix cause). This case shows that high motivation, well compensation and qualifications are not sufficient to modify the circadian rhythm period. For any humans and likely for any animals as well, the circadian rhythm cannot be overriden simply by motivation.
Besides this observational case study, there are more direct, lab controlled studies that objectively measured the circadian rhythm using physiological variables (eg, core body temperature, melatonin levels), and whether various factors can affect it.
One crucial investigated factor was whether the sleep pattern can affect the circadian rhythm, as indeed all psychological therapies rely primarily on modifying the sleep pattern through a behavioral intervention. Past studies found an effect, but recent well designed and controlled studies (see here and here) found that behavioral interventions on the sleep pattern only modify sleep pressure but not the circadian rhythm, proving that past studies were ill-designed for what they were supposed to study (this is the conclusion of the authors).
Additional evidence come from community-wide interventions in delaying college start time to adapt to teenagers. Indeed, during adolescence, the circadian rhythm experiences a transient phase delay that will gradually reduces with age, with a kind of U shape, with childhood and old age being characterized with the greatest phase advance (although it is worse noting that this does not erase interindividual differences: everyone will sleep later during their adolescence compared to their childhood and old age, but at the same age, a morning lark will always sleep earlier than an evening owl, including during adolescence). Studies have since long found that teenagers are experiencing alarming rates of sleep deprivation, sleeping several hours less than they require, because of the biologically induced phase delay. Community studies have been devised to test whether later school start time would allow them to sleep longer, or whether they would just sleep later and thus end up sleeping the same duration as before, but just later. In other words, is this a "lazy" kind of behavior, or is the sleep duration really constrained by the school start time. These community studies found that invariably nearly all teenagers ended up sleeping longer, as they kept sleeping at about the same time as before, but woke up later thanks to the later start time. This later school start time was reproduced all around the world with the same results (TODO: add ref), providing very strong evidence that this is a universal result and not culturally based, which is in line with what was expected if as hypothesized the circadian rhythm was the cause of the teenagers' increased sleep deprivation, and this hence disproves the hypothesis that this was behaviorally caused. In other words, not only is it observed in adults that behavior can not modify the circadian rhythm, but the same is observed in adolescents, which strongly suggests that this holds true for any age.
Psychologically changing one's circadian rhythm is different from forcing oneself to sleep out of phase (ie, restricting the sleep-wake schedule, in other words circadian misalignment). All individuals with non24 do restrict their sleep schedule sometimes or most of the time depending on one's responsibilities, and they hence sleep under a circadian misaligned time. But, without changing the circadian rhythm, this only leads to sleeping outside of one's biological night, which causes a short and subquality sleep accumulating sleep deprivation if reproduced repeatedly, as is shown by the fact that depression was more prevalent with the circadian DSPDs, and other health issues as described in another section above.
If we reduce the assumption of a psychological cause, but simply that psychological therapies may change the circadian rhythm, we end up with the theory of circadian plasticity and chronotherapy, which is defined as the assumption that the circadian rhythm is not only flexible but that this flexibility can be memorized, in other words, that therapies can affect the circadian rhythm beyond therapeutic discontinuation. Unfortunately, there is absolutely no empirical evidence supporting this assumption, and to the contrary, empirical evidence from this document's author and others strongly support the opposite, that the circadian rhythm is not plastic, and any effect obtained by therapies is lost upon discontinuation. This explains why behavioral chronotherapy as well as sleep hygiene have both been empirically proven to be ineffective despite decades of experiments (see the dedicated Chronotherapy section). Furthermore, usually effective therapies for psychiatric conditions, such as the cognitive behavioral therapy (CBT), have shown no efficacy for circadian rhythm disorders (when they are not confounded with zeitgebers exposure change).
To summarize the previous paragraphs:
- There is currently no empirical evidence that an entrained person, without a non-24 circadian rhythm disorder, can become freerunning by psychological intervention only without modifying the exposure to zeitgebers. The NASA Mars crew example is a pertinent demonstration.
- And inversely, no empirical evidence that an individual with non-24 or another circadian rhythm disorder can become entrained or sustain stable phase advances with a psychological intervention only, which is why chronotherapies nor sleep hygiene are not recommended as monotherapies anymore by the AASM guidelines.
Despite what some may claim, especially the proponents of "holistic medicine" pseudosciences, it remains highly controversial whether the mind can modify the biology. An often cited example is the placebo effect, but according to a Cochrane systematic review, placebo only affects subjective feelings, without modifying any objective measure. In other words, placebos (and nocebos) can help see things differently (eg, subjectively feel less pain), but not modify how the body works nor cure a disease (nor cause a disease). The placebo effect being the most studied and best proved psychologically induced effect, it's doubtful whether the circadian rhythm can be influenced by psychological factors at all. Firstly because the core circadian rhythm signalling pathway is body temperature modulation, hence, for psychological factors to modify the circadian rhythm, they would have to modify body temperature, which was never evidenced experimentally so far (whether by motivation, psychological stress or placebo effect). Furthermore, the chronic sleep deprivation induced by circadian rhythm disorders is not a subjective feeling: it is a real and highly dangerous health issue that can be objectively measured and which requires adequate treatments. The concept of psychological stress being a possible cause for most disorders stems from the psychoanalytical field, rooted in hysteria, renamed to conversion syndrome and later to psychosomatic syndrome. The problem with the theory of psychological stress causing other disorders is that this is mostly based on measures of cortisol, which is the hormone of wakefulness and is also and foremost regulated by the circadian rhythm and can also be modified (much more strongly) by sleep deprivation, and hence those studies are often confounded by the fact they often do not account for sleep duration, as sleep disorders and circadian dysregulations have been historically widely ignored by psychological studies. It is worth mentioning that the psychosomatic school of thoughts originates from the concept of Voodoo Death, which purports that humans can die from the sadness of being rejected by their community, which has later been debunked as death by dehydration, and has never been reproduced since then (absence of proof can be evidence of absence after so long without any empirical evidence reproduction).
The circadian rhythm plays an essential biological role: the survival chances of all living creatures are depedent on their synchronization to the external day-night cycle, whether it is to get food and regulate temperature or to flee from predators. The circadian rhythm modulates not only the core body temperature as a way to synchronize clocks throughout all cells of the body, but also regulates most body organs functions and even RNA transcriptions. The circadian rhythm is hence a biological vital function, just like blood glucose levels or insulin. Psychological factors hence likely have as much influence on the circadian rhythm as they have on diabetes, which is little to none. An analogy would be to ask whether a diabetic individual could control their glucose and insulin blood levels simply by motivation and will (or any kind of psychological therapy). The answer would obviously be no. The analogy with diabetes is not fortuitous, there are strong ties that are found between metabolic syndrome disorders such as diabetes and circadian rhythm disorders (see "circadian syndrome" or the food zeitgeber section below).
A variant of the psychological argument for circadian rhythm disorders is to cite the increase in blue light exposure in the evening due to computer and smartphone screens use. While it is true that blue light from screens can cause unwanted circadian delays and should be managed as part of an appropriate dark therapy, this cannot be used as evidence that circadian disorders are *only* due to screens misuse. One simple counter-argument is that if this argument was true, all computer scientists would have a DSPD or non-24 disorder, which is clearly not the case. Furthermore, the non-24 disorder was medically recognized in 1977 and sighted non-24 first clinically documented in 1971, before the advent of ubiquitous screens exposure. There is obviously an intrinsic component, as per the currently accepted medical definition, that makes the circadian rhythm of some people less robust to entrainment than others (or more prone to unwanted circadian shifts by uncontrolled factors), and hence being intrinsic is a hallmark of circadian rhythm disorders. Unwanted circadian shifts are merely a side consequence of the disorder.
Circadian rhythm disorders are sometimes qualified as being "idiopathic" (ie, of spontaneous and unknown cause) is only a temporary placeholder label, as the number of idiopathic diagnoses decreases over time as technology and knowledge progress, and as such some authors recommend to more properly prefer the term of "cryptogenic disorder" (disorder of yet unknown cause) instead of "idiopathic disorder" (spontaneous appearance - which can be misleading as it can suggest that disorders and diseases can have no physiological cause). As this other review for a dermatological condition states: "The more competent we are in etiological identification, the less idiopathic cases are found", which goes on to show this is a generic process happening in all medical domains.
Hence, there is currently no proof that circadian rhythm disorders can be caused or manipulated through psychological factors, and actually there is evidence of the opposite, which is that sleep disorders and circadian dysregulations actually often precede the appearance of other physiological or psychological symptoms, and the complete management of psychological disorders do not improve the sleep disorders, prompting scientists to recommend to always investigate and treat sleep and circadian rhythm disorders independently of any psychological condition. The same statement was also pronounced two decades prior for insomnia (see also here).
Can circadian rhythm disorders be neurological disorders, or a body disorder?
Since circadian rhythm disorders aren't psychologically caused, it must be a neurological disorder, right? Not necessarily, and there is some strong evidence that it's not.
Here are four arguments that contradicts the hypothesis of a brain disorder, and rather suggest that circadian rhythm disorders are body disorders:
- Firstly, the circadian rhythm is not solely defined by the suprachiasmatic nucleus (SCN) neurons in the hypothalamus, since there are actually molecular clocks in every organs and cells, and furthermore astrocytes arguably play an important role (see also here).
- Secondly, the vast majority of melatonin is produced outside the brain. Even if the pineal gland is cyclically secreting melatonin following the SCN inputs and is hence usually qualified as the "master clock", this is a highly arguable statement since the gastrointestinal tract secretes 2 orders of magnitude (at least 400x!) more melatonin in response to food than the SCN. Furthermore, even after pinealectomy, melatonin levels still increase in a dose-dependent manner (ie, proportionally) to oral intakes of melatonin in animals, just like for animals with their pineal gland, and it even restores an entrained circadian rhythm, showing that extrapineal producers of melatonin, likely the digestive tract, are producing and managing most of the circulating melatonin.
- Thirdly, the SCN is unnecessary for circadian shifting. Indeed, the effect of light, the strongest zeitgeber, on shifting the circadian rhythm is not impacted by the destruction of the SCN. Furthermore, a subsequent study shown that the ipRGC cells are sufficient to cause circadian rhythm and body temperature shifts without the need for the SCN, which shows that the non-visual effect of light on the circadian rhythm is independent from the SCN. Hence both the pineal gland and the SCN, the two major brain structures related to circadian rhythm modulation, are not required for circadian rhythm modulation and entrainment.
- Fourthly, chronic sleep deprivation (often caused by circadian rhythm disorders) causes death not through the brain but through the whole body via the accumulation of ROS in the guts (see the great abstract video or also the Inverse vulgarization article). This can be rescued (avoided) using anti-oxydants targeting the guts (through food or gene overexpression), but not when targeting neurons in the brain. It lends further credence to the consideration that the guts is a "2nd brain".
- Fifthly, the circadian clock and the cell cycle are coupled, which means that the circadian clock is a core regulator of all cells cycles throughout the body. By accounting for the other discoveries, this means that body temperature controls the cells cycles through the circadian clock modulation. This is another strong supporting evidence for the hypothesis that circadian rhythm disorders are bodily disorders, not just brain disorders.
- Sixthly, core body temperature modulation is the primary way that circadian rhythm changes are signalled across all cellular clocks throughout the body, with temperature being modulated by bright exposure through the SCN, although another study shown that the SCN is in fact unnecessary as the ipRGC cells are sufficient to control the body temperature changes. Likewise, melatonin causes a reduction in core body temperature reduction proportionally to the dose, and endogenous melatonin levels are inversely coupled with peripheral (limbs) temperature (heat transfer to limbs is a way to reduce core body temperature, so increasing limbs temperature actually decreases core body temperature). Temperature being the primary signalling pathway of circadian clock changes and synchronization especially via melatonin was already suspected since at least 2007 since supraphysiological melatonin doses were known to cause hypothermia, and it was even earlier hypothesized in 2000 that temperature may be a 3rd signalling pathway and potential treatment approach after light and melatonin. Given that humans, like most mammals, are homeothermic, which means that it's crucial for their survival that their core body temperature is always maintained inside a very specific range, and hence that body temperature control is automatic and unconscious (ie, an individual cannot manipulate their temperature by will) and is very robust to ambient temperature changes, this shows that the circadian rhythm is a very deeply ingrained and automatic biological process that is both uncontrollable without external means, and affecting the whole body.
Hence, circadian rhythm disorders should more properly be qualified as whole-body disorders, instead of neurological or psychological, as both of the latter conveys a large understatement and misunderstanding of the whole-system interactions underlying circadian rhythm disorders, and focusing on brain structures such as the SCN that are in fact unnecessary for circadian rhythm modulation.
Accepting circadian rhythm disorders are whole-body disorders does not mean that there is not a neurologic component, since of course there is given that light exposure is the strongest zeitgeber and is mediated from the eyes' ipRGC cells through the brain to then the rest of the body via core body temperature modulation and melatonin levels inhibition. However, this strongly indicate that a neurocentric approach is insufficient to fully understand and treat these disorders. In practice, this means that treating the brain (master clock) without the body (peripheral clock in the digestive system) will be at least suboptimal or even ineffective.
Circadian rhythm disorders bear strong similarities, or even links according to recent findings, with metabolic syndrome disorders such as diabetes. Hence, it is likely safe to think of circadian rhythm disorders in similar terms as diabetes: a body disorder with some neurological component, which is both varying with endogenous but majorly with exogenous (external, environmental) factors.
What causes circadian rhythm disorders such as non-24?
This section explores the potential biological or medical causes of non-24 and other circadian rhythm disorders.
Although the cause of sighted circadian rhythm disorders such as non-24 certainly is not psychological but physiological (either neurological or bodily) as explained above, the causes are currently poorly understood.
For blind individuals, the loss of pathways to conduct the signal from the eyes' ipRGC cells (the ones that are intrinsically photoreceptive and responsible for the shift in our circadian rhythm and the inhibition of melatonin) is the reason most of them suffer from the non24 circadian rhythm disorder. But for sighted individuals (whether DSPD or non-24), it's unlikely these pathways are lost as most studies found these pathways to be intact in sighted individuals.
Some scientists suggest it can be a genetic mutation in clock genes, as indeed the circadian rhythm is mostly inherited through genes. If this is correct, then circadian rhythms should be inherited among siblings and descendents, and hence diagnosis of one member should prompt the testing of close family relatives. This is the case of the author of the present document, as there is a clear lineage at least 2 direct ancestors above (paternal grand-mother -> father -> son), and anecdotal reports from other individuals with non-24 suggest this is not an isolated case (see here , or also here for a mother->daughter transmission, or here for several testimonials). Since it seems to be inheritable regardless of the parents and children sex, it does not appear to be a mutation in X nor Y chromosomes.
There is also an age effect, as some of these disorders can appear later in life, especially DSPD, with a delayed circadian rhythm phase being common at adolescence compared to childhood. However, this does not always resolve as shown by various testimonials from individuals with DSPD. Furthermore, there is no evidence that freerunning, the characteristical circadian pattern of non-24, can appear later in life and resolve on its own with age, contrary to the delayed phase. Indeed, a delayed phase is fundamentally different from an ever shifting phase.
Other scientists suggest there may be a functional or anatomical dysregulation in the brain, particularly the suprachiasmatic nucleus, which neurons orchestrate peripheral clocks throughout the body, or the pineal gland, which secretes melatonin. There is also some anecdotal evidence (see also here) that damages to the pituitary gland may cause a non-24 circadian rhythm pattern rarely. However, it appears clear that the circadian rhythm is regulated not by a single locus but by a network of brain structures, or even body organs, each with their own clocks interacting and contributing to the overall circadian clock. Furthermore, experimental evidence shows that SCN neurons of animals entrained to a non-24 schedule (ie, T-cycles) implanted in other animals will not cause them to display a non-24 sleep-wake schedule, which strongly suggests that there are other structures, either inside the brain or outside, that are critical to allow entrainment to non-24 schedules. There is some evidence from animal studies that disruptions in the BMAL1 gene of astrocytes regulating SCN neurons actually make the animals freerun in a non-24h sleep-wake pattern, and interestingly there is some evidence that astrocytes are disrupted in individuals with autism, a disorder that often has comorbid circadian rhythm disorders. Hence, it seems unlikely these disorders can be caused by one single point of failure, it's more likely they are the result of a complex set of dysregulations, as happens for other complex disorders such as autism and schizophrenia.
Can these disorders be anthropogenic? The major difference nowadays (since the industrial revolution, see also here) is NOT where we sleep, but where we spend our awake time: whereas in 1800s almost everyone (90% of americans) worked out in fields, nowadays they work in offices, with as much as 87% of americans spending their wake time indoors, in places which sometimes do not even have windows and with reduced light exposure both in light intensity, spectral composition and duration (see also here). Hence their circadian rhythm is only entrained by artificial lights. The exact same thing they use in the evening at home, which delays their circadian rhythm.
Hyposensitivity to zeitgebers such as light may be a factor, as indeed the sensitivity to zeitgebers is highly variable for everyone, with a 50-fold variability among typical sleepers. The opposite, hypersensitivity to zeitgebers, may also cause circadian rhythm disorders. However, it remains unclear whether hyposensitivity and hypersensitivity are causes or just consequences of circadian rhythm disorders, since it was demonstrated that non-24h light-dark schedules (T-cycles) cause SCN desynchrony, similarly to long days, and hence reduced responsiveness to phase shifts and that weak entrainment to a zeitgeber, such as by being entrained to a short day of bright light, causes a higher synchrony of SCN neurons and phase shifts of a wider magnitude (see also here and here). Potentially, hypo and hypersensitivity may be reinforcing feedback loops, that once set in motion worsen the symptoms. However, although there certainly are negative feedback loops, it is in the author's experience that the circadian rhythm always reverts back to its original state after just a few weeks of discontinuing any therapy and resuming exposure to environmental light, whatever environment (ie, living in more brightly lit indoor housing and going outdoors more often did not have any long lasting effect). A 2021 study that assessed alterations in ipRGC cells response to bright light using post-illumination pupil response (PIPR) found that individuals with DSPD and sighted non24 both impaire phototransduction, in other words they have reduced response and pupillary diameters compared to controls, with a much more extreme reduction for sighted non24, which strongly supports the hypothesis that hypophotosensitivity is likely a possible etiology for at least some individuals with DSPD and sighted non-24.
A similar hypothesis is that geography may play a role, with some individuals requiring more sunlight exposure than others for their circadian clock to be entrained. Although this hypothesis may have some merit, non-24 and DSPD disorders have been reported all around the world, in both low exposure and high exposure regions (eg, people with sighted non-24 were reported living since a long time in South Africa, Irak, Vietnam, Southern California, New Zealand, Australia, etc - source: private communications - and there is this study showing DSPD is present in Cyprus), which strongly suggests that although geography may be a factor (formal demographic studies are unfortunately lacking at the moment to confirm or infirm this point), it is unlikely to fully explain the existence of these disorders. However, geography does indeed affect the sunlight exposure pattern regardless, and it indeed has an effect on the circadian rhythm, as it was demonstrated that southern countries such as Spain, Cyprus and Portugal, which are exposed to longer durations of sunlight compared to northern countries, have populations that report later bedtimes. Hence, the duration of sunlight exposure, and hence geography, certainly can play a role in worsening a circadian rhythm disorder condition especially in extremums such as too short or too long exposures, and hence some world regions may have a higher prevalence of these disorders, but geography is unlikely to be the sole explanation for the existence of these disorders, given their ubiquitous distribution around the world.
Another possibility that was observed in studies is that exposure to an aberrant pattern of light exposure diminishes its effect on the circadian rhythm. A simple example to illustrate: if a human is placed in a room exposed 24/7 to bright light, with no variation whatsoever in the light intensity or color, light exposure will have less and less effect on their sleep over time (except on melatonin which will still be inhibited - melatonin inhibition is decoupled from circadian rhythm shifting). Hence that's why for light therapy to be effective, it needs to be combined with dark therapy, ie, the avoidance of bright light (especially blue and green) in the biological evening. While this hypothesis may apply to some individuals, it certainly is not a satisfactory explanation for individuals who have rigorously followed therapy or even changed their environment to maximize timely exposure to zeitgebers and who still observe their circadian rhythm reverting to the original state after artificial light therapy discontinuation, such as the author of this document.
Finally, sleep deprivation is known to reduce the effect of zeitgebers such as light on our circadian rhythm, so if an individual is chronically sleep deprivated, zeitgebers (and light therapy) won't work, hence why it's important to feel rested before starting to use them and to continue from benefitting from their use. A related hypothesis is that non-24 may be caused in parts by an abnormal buildup rate of adenosine (sleep pressure), which was shown to modulate/inhibit the response to bright light, and hence may indirectly contribute to the shifted circadian rhythm phase. However, even slight adenosine imbalances are now suspected to be a key factor of severe neurodegenerative diseases such as schizophrenia and Parkinson, so it is arguable whether non-24 could be caused by an adenosine imbalance without any other sign of a neurodegenerative disease..
Behavioral causes, although historically strongly favored by psychological theories, have never been supported empirically, with empirical observations often showing the contrary, such as a lack of effect of sleep hygiene as a standalone therapy, or the finding that individuals with DSPD actually spend less time in bed than typical sleepers. In addition, they do not require more extra sleep hours during the weekend than weekdays compared to the general population, meaning that they suffer as much social jet lag as anyone else, but not more, which means that their insomnia difficulties during the workweek are not caused by a hypothetically too different sleep pattern during the weekends. Sleep patterns have no effect whatsoever on the circadian rhythm (see also here). Sleep nor sleep pressure have no effect on the core body temperature and hence the circadian rhythm. Likewise, sleep deprivation has no incidence on the core body temperature.
Hence, there are both intrinsic (genetical hyper/hyposensitivity) and extrinsic (environmental) factors that can be at play in causing circadian rhythm disorders, and furthermore these factors are not exclusive, so that it's likely that multiple factors are at play (complex disorder) and with different causes producing the same phenotype (polygenic disease).
However, it appears that there is no single nor set of hypotheses that can satisfactorily explain how circadian rhythm are caused. It is the author's conviction that, at the most fundamental level, circadian rhythm disorders are in fact disorders of body temperature homeostatic regulation, and that future studies should explore this lead as a potentially very promising etiological explanation for these disorders, that may open new therapeutic avenues.
Why do circadian rhythm disorders exist? Do they serve a purpose?
This section is different from the last one: whereas the previous one tackles potential biological/medical explanation, here we explore the potential evolutionary reasons for a population to display a percentage of individuals with circadian rhythm disorders, ie, their potential utility for the specie.
Anthropology of sleep dates circadian rhythm diversity back to the hunter-gatherers tribes, where it is hypothesized that the various chronotypes allowed to always have someone on guard at any time. This "sentinel hypothesis" seems verified with another study on modern tribes, showing that even nowadays their members have a wide variety of chronotypes, so that the tribe has someone awake at nearly all times to stand guard (only 18 min was left unguarded over 24h). Although this study did not observe the circadian rhythm at the individual level, one interpretation is that humans have a natural genetic variability in circadian rhythm phase distributions to increase the odds of always having someone in the community awake to defend against predators and enemies at any time of day and night.
Chronotype repartition among the general population is indeed known to follow a bell shaped curve, in other words a gaussian distribution, which strongly suggests a random and natural variability. In other words, the repartition is normal (in the mathematical sense), no two individuals have the exact same chronotype/circadian rhythm, and it's ingrained in our biological, genetic code. Indeed, it is estimated that ~40% of sleep disorders are inherited, and 46% to 70% of the circadian rhythm is genetically inherited, with minor influence from environmental factors, with similar heritability for the propensity to regularly do a siesta. In fact, heritability of the circadian rhythm is so strong that it was shown that rats kept in the dark for generations maintained the same circadian rhythm, which strongly suggests that behavioral exposure to light patterns cannot affect the circadian rhythm between generations (neither negatively - by bad habits - nor positively - by following an entrainment therapy, children likely won't benefit from genetic improvements). The repartition of chronotypes in the general population is about 30% of morning larks, 40% of night owls and the rest in-between (see also this informal review). Circadian rhythm disorders such as DSPD or non-24 are not accounted in these statistics, and are likely on the tails of the bell-shaped curve ("extreme" chronotypes). This biological diversity is further supported by some evidence that prehistoric mammals were likely all nocturnal to avoid the oversized reptilian predators that were the dinosaurs, and only later some mammals, including humans, switched into bright light vision (see also here for a layman summary). Furthermore, DNA microarrays demonstrated that the central clock's circadian rhythm itself controls an estimated 8-10% of all transcriptomes in any tissue.
Although very few studies investigated this factor, the few that did observed unexpected ethnic differences in the circadian period, with european-american having longer circadian periods than african-american, which may suggest an evolutionary genetical adaptation to geographical location, with shorter intrinsic periods genetically encoded in populations living in places with longer days.
Occupation does not seem to play a role, since there are several counter-examples: 1) most computer scientists, nor professional gamers and computer graphists, do not have the non-24 disorder despite extensive or even excessive use of screens, 2) according to a November 2023 survey of the AASM on ~2000 americans, 45% use their smartphone and 50% their TV when having troubles sleeping, yet 45-50% of the population does not have circadian rhythm disorders, even if the prevalence is underestimated, this is certainly way above the pathological prevalence, and 3) 75% of the total workforce is estimated to have been involved in shift work and night work in industrialized countries. Furthermore, the first clinical documentation of a sighted non-24 disorder case was published in 1971, predating the advent of ubiquitous screen use and LEDs, with the most common type of at-home lighting at the time, incandescent bulbs, having negligible effects on the circadian rhythm. However, there is one study on animals which suggest that exposure to even very dim light during the circadian night can significantly lengthen the circadian period to an extreme non-24 pattern, although this would need to be reproduced to be confirmed.
Preliminary evidence from migrating birds suggests that the lessening of the robustness of circadian rhythm entrainment may be a natural way to allow some individuals to be more sensitive to external zeitgebers and hence more easily adapt to new environments. If this hypothesis is correct, this would mean that individuals with a circadian rhythm disorder may in fact more easily adapt to different timezones and length of day/night when the zeitgebers (particularly artificial light and food) are controlled, and play a crucial role in the survival of the species. This hypothesis is further strenghtened by findings from the "expériences hors du temps" during which humans live in an environment completely isolated from environmental timecues such as in a cave. During one of these experiments involving two individuals for about one month, one could readily adapt to a 28h/day sleep-wake pattern and core body temperature profile, demonstrating that their circadian rhythm also adapted, whereas the other one could not and stayed entrained to a 24h/day schedule as demonstrated by the core body temperature profile. Of note, after the end of the experiment, the individual who entrained to a 28h/day schedule had additional difficulties to re-entrain to a 24h/day schedule, which extent is left unreported unfortunately.
> The ease with which some people can adjust their habits to an alteration of phase or cycle length is thus no evidence against an endogenous rhythm, and the difficulty experienced by others is in favor. Perhaps the most striking of such evidence is that obtained by Kleitman (131) in the Mammoth Cave of Kentucky with 2 subjects living there on (‘days” of 28 hr. The temperature and wakefulness rhythms of one adapted readily but those of the other did not, so that at the midpoint of every week they were exactly out of phase; the poor adaptor was not constrained into feeling sleepy even by the presence of a soundly sleeping companion, nor into wakefulness by a wakeful companion. All influences, social and environmental, were working one way, but he maintained his 24-hr rhythmicity for the whole 32 days. Perhaps even more striking, the other subject, who adapted well, showed some persistence of a 28-hr sleep-wakefulness rhythm after emerging. A similar persistence of a 24-hr sleepiness rhythm is recorded in some, but not all, subjects living on a 220hr day (26, I 50). A phase shift, a common experience for travelers, is more easily accomplished: Sharp (226), studying a community of six men who altered their phase by I2 hr under almost ideal conditions, records a minimum of 2 and a maximum of 9 days before they were sleeping satisfactorily again. The regularity of sleep in Arctic communities was much the same during the continuous darkness of winter, the alternation of light and darkness around the equinoxes, and the continuous daylight of summer (220), both in the working population and in convalescent patients in a hospital.
A related hypothesis is related to the new finding that the Earth's rotation, and hence day-night cycle, has not always been of 24 hours (see also here and here and here), as they rather were 23.5h and progressively increased to 24h by 2.3ms each century. With cataclysmic environmental changes, it may also happen that the rotation duration may lengthen further in the future too. Hence, having in the genetic pool a diversity of circadian rhythm lengths different to 24h (ie, non-24) can increase the survivability of a specie in case of such a catastrophic cataclysmic event.
Hence, it's becoming increasingly apparent that endogenous circadian rhythm disorders are due to natural diversity with the purpose of increasing the survival chances of a community. Unfortunately, with the modern society expectations rooted in agriculture, chronotypes other than morning larks are at a disadvantage and suffer from social jet lag, despite the majority having an intermediate or night owl chronotype (see also here).
See also: Anthropology of sleep: Worthman, C. M. (2008). After dark: the evolutionary ecology of human sleep. In Evolutionary medicine and health (pp. 291-313). Oxford University Press.
Prevalence and demographics of sighted non-24
Let's first study the prevalence of circadian rhythm disorders in general:
The most common circadian rhythm misalignment is an exogenous one: social jet lag, which is affecting two thirds of the population worldwide, with only one third of the global population of adults achieving the recommended 7-8h of sleep. This is however not commonly considered a circadian rhythm disorder, although it could be argued that social jet lag is a lessened version of shift work disorder.
Let's first examine the prevalence of circadian rhythm disorders in the population, regardless of insomnia. Few studies have investigated the prevalence of circadian rhythm disorders in the general population, some of them estimating the prevalence between 0.13% and 0.17% in the general population. However, almost all these old studies were methodologically flawed, such as a study in an extreme north region with very short days lasting only a few hours during winter, or others using the morningness-eveningness questionnaire as a circadian disorder screening tool when it is stated it should not be used this way. A more recent 2021 study found a prevalence of 12.8% had a circadian rhythm sleep-wake disorder among a representative sample of the general population of Cyprus. The most prevalent circadian rhythm disorders were shift work disorder (6.7%) and DSPD (5.1%). The sample consisted of 195 enrolled participants out of a survey of 4118 of adults in Cyprus, a south-eastern europe region, which is exposed to plenty of sunlight. Although non-24 was included in the criteria, the diagnostic tools used (questionnaires) included no criteria that could be used to diagnose non-24, hence none were detected in the sample, and likely some were misdetected as DSPD or typical sleepers depending on their current circadian phases. The authors defined DSPD as individuals falling asleep between midnight and 3am, which is reasonable given the social expectations in the region. The key characteristic for many patients was chronically inadequate sleep, and the most common complaints were insomnia and fatigue and sometimes hypersomnia for intrinsic circadian rhythm disorders but not excessive daytime sleepiness, contrary to other sleep disorders where excessive daytime sleepiness is a key symptom. Although it was published in 2021, the study was conducted before the COVID-19 pandemic. Depression is the most common comorbid disorder with DSPD.
Let's now examine the prevalence of circadian rhythm disorders by considering them a subtype of insomnia. Sleep disorders are highly prevalent, as it is estimated that the "worldwide prevalence of sleep disorders is about 50% with even higher occurrence in psychiatric population", and is increasing over time. Other studies find a prevalence of insomnia in about one third of the general population. The most common type of intrinsic circadian rhythm disorder, Delayed Sleep Phase Disorder (DSPD), is much more prevalent than previously thought, accounting for 10% of all sleep disorders and is often misdiagnosed as sleep-onset insomnia, hence about 5% of the worldwide population by combining with the previous figure, which is exactly what the 2021 Cyprus study found. Relatively to the global population, a genetics study by Dr. Alina Patke estimated that potentially 0.6% of the population is carrying a gene mutation CRY1 that may cause Famillial DSPD (ie, inheritable DSPD). However, sighted non-24 is undoubtedly much rarer than DSPD, although it is not uncommon for non-24 to be misdiagnosed with its better known cousin disorder DSPD.
Hence, whether we examine the prevalence of circadian rhythm disorders as a subtype of insomnia, or as a standalone class of disorders, we end up with similar prevalence figures, which strengthen the case that circadian rhythm disorders are highly prevalent in the modern society.
Now, onto the prevalence of the non-24 disorder:
Non24 is very common in blind individuals, with an estimated 2/3rd of blind people having the non24 disorder. Other estimates such as in the visually impaired Japanese population found that 33% had a circadian rhythm disorder, with the most common being the non-24 disorder affecting 26.8%, followed by ASPD (3.8%) and DSPD (2.5%).
Given the rarity - or wide misdiagnosis rate - of non24 in sighted individuals (ie, sighted non-24), there is as of 2021 currently no reliable estimate of its prevalence. This may partially be caused by the fact that the same billing and diagnostic codes are assigned to both blind and sighted non-24, despite having different etiologies, which prevents researchers from querying their databases to isolate sighted non-24 from blind non-24. However, there is evidence according to the french sleep medicine institution SFRMS that circadian rhythm disorders are underdiagnosed, and some indirect evidence can allow to produce a vague estimation.
The most direct evidence, but non-statistical (because the sample was not random), of the prevalence of sighted non-24 comes from a survey done by the Circadian Rhythm Disorders Network association (mirror, and more results here). They found that out of all the respondents who declared to have a circadian rhythm disorder, between 29% to 33% declared to have sighted non-24, and at least 19% had a medical diagnosis of sighted non24, which is much higher than any previous estimate. However there are some limitations. To quote them:
> With careful questioning we could also get a rough estimate of Non-24 prevalence in sighted people, which we suspect (from our survey—see next paragraph) is considerably higher than generally believed. Currently there is almost no evidence of the prevalence of sighted Non-24 and limited evidence for the prevalence of ISWD, mostly based on its occurrence in the context of other conditions such as head injury. (We do have good estimates of the number of Non-24 cases in the totally blind population.)
> In our survey, 29% of respondents with CRDs believe they have Non-24. Some may have misunderstood the definition of Non-24. But of the people diagnosed with a CRD by a medical professional, 19% were diagnosed with Non-24. Our sample is biased by self-selection—people with Non-24 are more likely to have major problems with their lives and be more likely to participate in our survey. Still, the results suggest that the prevalence of Non-24 is much higher than is generally recognized.
Hence, if the results of this survey are valid, which needs to be verified by future controlled studies, sighted non-24 may account for at least 1/4th of all the circadian rhythm disorders (accounting only the medical diagnoses) and up to 1/3rd of all circadian rhythm disorders if we account for the self-diagnoses. But it is worth noting that, similarly to what was observed in the above mentioned survey, online communities of non-24 are overwhelmingly mostly filled with individuals with sighted non-24, not blind non-24, which suggests, but does not prove, that sighted non-24 may be much more prevalent than blind non-24 in absolute terms. Furthermore, even though sighted non-24 may be much rarer in the sighted population than blind non-24 in the blind population, given the sighted population is much bigger, it's possible sighted non-24 still represents a sizable population in absolute numbers (ie, base rate fallacy), according to James Fadden.
Sighted non-24 seem to often first appear, or at least gets noticed, at teenage: "The onset of non-24-hour sleep-wake syndrome had occurred during the teenage years in 63% of the cohort, and the mean ( +/-SD) period of the sleep-wake cycle was 24.9 +/- 0.4 hours (range 24.4-26.5 hours). The mean sleep length of the patients was 9.3 +/- 1.3 hours, and 44% of them had a sleep length of between 9 and 10 hours. Psychiatric disorders had preceded the onset of non-24-hour sleep-wake syndrome in 16 patients (28%); of the remaining 41 patients, 14 (34%) developed major depression after the onset of non-24-hour sleep-wake syndrome." From this 2005 study of 57 participants cohort. There is indeed evidence for neuroendocrine changes during adolescence that can affect the circadian rhythm.
There is a misquoted figure of a 0.03% prevalence of sighted non-24 in the general population, originating in the Sleep Misfits book, which finds its root in an estimation by the Circadian Sleep Disorders Network of 0.03% prevalence of all forms of non-24, including both blind and sighted, as per a clarification statement by the Network. However, even this figure of 0.03% prevalence among the general population is not plausible: according to the International Agency for the Prevention of Blindness’s Vision Atlas 2021 report, there is an estimated 43 millions people worldwide living with blindness, out of a global population of about 8 billions people. Given a presumed prevalence of 0.03%, this would make for an absolute prevalence of 8 billions * 0.0003 = 2.4 millions blind people with non-24 with this estimation method. However, if we estimate given the prevalence of 55-70% prevalence among blind individuals, we would get between 24 and 30 millions blind people with non-24, hence a 10x bigger order of magnitude. Likewise, using the results of the visually impaired Japanese population study, 26.8% of prevalence of non-24 among the 295 millions of people living with a moderate-to-severe visual handicap according to the IAPBVA 2021 report, we get an absolute prevalence of 79 millions blind people with non-24 globally. Hence, the 0.03% prevalence figure has currently no evidence based foundation (could not find any empirical study that produced this estimate), and it is anyway underestimate the known prevalence of non-24 in blind people, hence it also likely underestimates the prevalence in sighted individuals.
Do circadian rhythm disorders such as DSPD and non24 disappear with age?
TODO: add refs on longitudinal and between-groups differences of chronotype vs age (diu).
This is a common misconception that is prevalent in medicine, that cryptogenic diseases magically fade away with age (eg, adhd, autism, epilepsy, etc all are ailments that were supposed to disappear during adulthood) . While it's true that a sizeable proportion of adolescents experience a temporary phase delay in their sleep-wake patterns, this phase delay is much smaller in magnitude than the phase shifts observed in pathological circadian rhythm disorders such as DSPD or ASPD. And of course continuous daily phase shifts as observed in non24 are an extremely abnormal sleep pattern that is not observed at any age in individuals without this disorder, and there is absolutely no evidence this pattern can change with age as it likely has a neurobiological cause. Actually, non-24 in children is described in the ICD-10, so its existence in childhood is known and is diagnosable.
Hence, while a teenager waking up around noon may expect to wake up naturally around 10am or forcefully around 8am after reaching adulthood, children and adolescents with DSPD waking up past noon and those with non24 who wake up at later hours every days have no reason to expect an improvement with age without any therapeutic intervention.
Indeed, there is ample evidence that the circadian rhythm is not only inherited, but is mostly stable during the whole life of an individual unless there is some severe neurobiological lesions, strongly supporting the notion that the circadian rhythm is an intrinsic feature. Indeed, longitudinal studies shown that although there is a slight phase delay often happen during teenage - which is not solely attributable to lifestyle (eg, sleeping late to party) or screens exposure but is intrinsic to this age, given the teenage phase delay is present across species - and is more pronounced in boys than in girls, although both do get this phase delay. Hence, the circadian phase shows an inverse U curve, with the individual waking up earlier when younger or older, and the latest around their adolescence.
However, when comparing longitudinally and between subjects, studies show that the chronotype is a much bigger difference than the evolution of the circadian phase with age: if we have two individuals of the same age, one being a morning lark, the other an evening owl, the morning lark will always wake up much earlier than then evening owl at every age. At adolescence, the morning lark will wake up later than they did when younger or will when older, but so will the evening owl.
There are some rare exceptions of a drastic change in the circadian rhythm, but they were so far only documented by rigorous studies in either brain lesioned patients (eg, people in a disorder of consciousness after a concussive trauma or suffocation - the latter causing worse brain damages because of the lack of oxygen) or a neurodegenerescence (eg, Alzheimer) or iatrogenic and drug abuse (eg, alcohol). In other words, the circadian rhythm seems to only be affected durably by extensive, severe, permanent brain damages.
In summary, the circadian rhythm is a strong intrinsic characteristic of each individual, and although there is a slight phase delay at adolescence, the chronotype continues to affect the sleep-wake timings much more than age, and stays the same throughout life unless there are neurobiological lesions. This appears to be the current medical and scientific consensus as of 2024.
How to explain non-24 to others?
Explaining what is non-24 to other people who don't have it, whether they are relatives or acquaintances, can be quite difficult as circadian rhythm disturbances are usually not comprehended by typical sleepers.
The best way to explain is your way to explain, and it's your choice whether you want to explain or not.
However, keep in mind that most people won't be able to understand, as sleep is such a covert process that most do not realize how much sleep and the circadian rhythm regulate their daily life, and thus, sleep disorders are beyond their comprehension. This is well evidenced by the experience of night shift workers, who, despite being typical sleepers required to modify their sleep-wake schedule to accomodate their work needs, experience the same difficulties in explaining their situation and their issues being recognized by their day-time colleagues and hierarchy. This is not helped, and even partially caused, by the prevalent no-sleep culture, a modern view with sleep being disregarded as a convenience, when it is an essential need.
Nevertheless, here is a list of suggestions to build your own explanation if you want to:
- Having non-24 is like having to wake up 1h earlier everyday for the rest of your life, just to get to work on time and do your groceries, otherwise the world leaves you behind. This means that regularly you'll wake up and go through your day without any sunlight at all, for weeks at a time, until eventually you see sunlight again. You have to plan all your activities with relative time. Also regularly you'll wake up 3h earlier, sometimes 1h later, there's a lot of randomness between days.
- To drive the point home for the recalcitrants, and if you're brave, you can call your boss/colleagues/relatives at the same shifted time relative to your biological night. For example if they can't stop calling you when you just went to sleep 2h earlier, you can call them at 2am and go over the points they wished to discuss. This method is plebiscited by r/nightshift workers on reddit.
- Here is a variation of this approach including a figure, courtesy of u/sprawn :
>
> Imagine this is your work schedule. At first they are excited. Oh Great! Noon to 8pm! That's easy. Even the first week seems do-able to them. After the first weekend, they begin to understand. Wait! I can't get to work at 5AM! And then 4AM the next day. No one could do that! And that's when I say, "This is what a 'normal' work or school schedule is for me." What I want to say is, "Oh, it's okay. Take some melatonin, and you'll be fine…" By week three, hopefully they can imagine how impossible it would be to live like this. Forcing yourself to sleep earlier and earlier.
>
> If they are imaginative, they can come to an understanding of just how difficult that life would be. They think, "Maybe I could handle the first three weeks. Maybe I could use coffee and naps to make this work for a little while." But they will eventually see that sustaining an insane schedule like this (for years) is impossible.
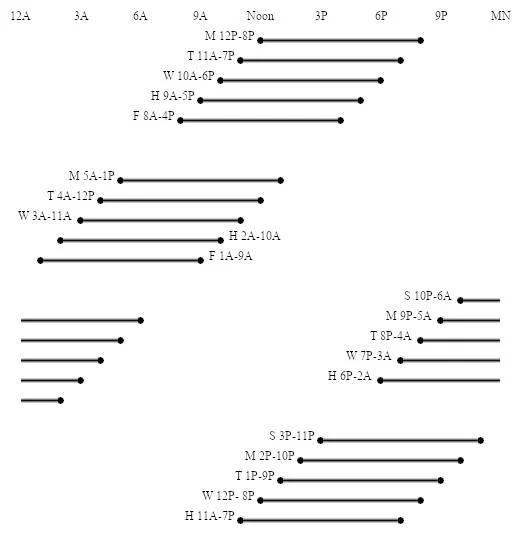
- Non-24 is like being permanently jet-lagged. My internal timezone changes everyday to an unknown timezone. The change is not constant but chaotic, so it is impossible to accurately predict when to sleep. It affects all aspects of general health and can cause cardiovascular diseases and diabetes, especially when chronically sleep deprived since this disease prevents from sleeping. For some it causes throwing up, dizziness, random fainting, car accidents, sudden cardiac arrest and strokes, and a lot of other very not fun ailments. Just like jetlag, but all the time, everyday of your life.
- Non-24 is like diabetes before the discovery of insulin: there is no monitoring tool for the circadian rhythm, and we are not sure what causes these disorders nor how to improve them. Using light therapy and melatonin is like taking insulin regulating drugs for diabetics, but here it's regulating the circadian rhythm since our body can't do it properly. Health consequences of unmanaged non-24 are similar to diabetes, leading to cardiovascular diseases such as arrhythmias and even to diabetes and obesity.
- Even with a working treatment, social and unexpected events makes it difficult to follow the therapy. Just like for diabetics, it's hard to go out while not being able to eat or drink anything because it can kill you with a stroke. Similarly, even when entrained with a working therapy, we would likely have to forego evening and night social events. That's unfortunately the price we have to pay for a stably managed sleep.
- Non-24 causes chaotic wake up and drowsiness, I cannot plan when I will be awake or asleep.
- I have a chronic illness that affects my ability to sleep, and for which no cure is known yet.
- Everybody has a set period to sleep, even typical sleepers: those who can't sleep during the day have shift work disorder, those who can't sleep as early as socially acceptable have DSPD, and those who have a defined sleep period that changes everyday have non-24.
- For work: I cannot know beforehand when I will sleep and wake up. On top of that, I don't know when I will be productive, as every other day the illness causes a zombie-like state between wakefulness and sleep.
- If an alarm clock could cure an illness, it wouldn't be an illness.
- Living with non-24 is living a life in alternance: one month you are good, one month you hibernate and get ill and are fatigued and late and depressed all the time.
- Living with non-24 is like having a rotating day and night shift work but with no off days whatsoever, including on week-ends.
- Living with non-24 is a very solitary life, it's like COVID-19 quarantines but for the whole life instead of just a few months. Everything, every errands, every appointments need to be carefully planned and weighted given the health risk.
- Non-24 is like working a rotating night-and-day schedule that changes all the time. No week-end, no vacations, it's all the time. (And I'm not even paid for that.)
- An excellent accurate depiction of DSPD was written in this couple's story in the Modern Love column of the New York Times, which was later accurately adapted in the TV series Modern Love S2.2 on Amazon Prime. The story mentions the fairy tale called The Day Boy and the Night Girl, which can also be seen as a depiction of DSPD. This isn't a depiction of non24, but it is very rare to find accurate depictions of circadian rhythm disorders in video media, this can provide a valuable and easy to share and watch medium to family and friends for them to understand what a circadian rhythm disorder is.
- Here are more suggestions (mirror).
What are effective treatments for circadian rhythm disorders? How to know an effective from an ineffective treatment regardless of claims?
Circadian rhythm science is still in its infancy, and hence there are a lot of unknowns and gaps in the scientific knowledge. Therefore, patients are often left to their own device, and not understanding exactly how their disorder works, they try various treatments and approaches that can illusorily appear effective in the short-term but in fact lose any positive effect pretty fast, and can even cause more harm in the mid to long term. Clinicians also often verse into the same pitfalls, with the hope of helping their patients, but given the lack of practical guidelines, they are also left just like their patients to process by trial-and-error.
There is theoretically a simple way to determine if a therapy is effective to manage circadian rhythm disorders: if a treatment can directly shift the circadian rhythm phase, it's effective. Since the circadian rhythm cannot be measured, core body temperature profile over 24h can be used as a proxy since it is strongly coupled with the circadian rhythm. If a treatment cannot shift the circadian rhythm phase nor modify the core body temperature profile, it's ineffective and useless for circadian rhythm disorders (although these treatments can still be helpful for other comorbid issues or disorders).
Hence, if you would like to try other therapies not described in the present document, you can first check the academic literature to know if these interventions change the core body temperature. If they do, it's likely the intervention can shift the circadian rhythm, but if it can't change the core body temperature, it's almost certain it can't shift the circadian rhythm either.
For example:
- Light therapy, melatonin, and any zeitgeber, can demonstrably and objectively shift the circadian rhythm phase and change the core body temperature profile, hence they can be used as effective management therapies for circadian rhythm disorders, including non-24. See the next section for more infos on zeitgebers.
- Sleep hygiene, chronotherapy and CBT-i have never been demonstrated to shift the circadian rhythm phase nor to change the core body temperature. They are ineffective to treat circadian rhythm disorders, and should never be attempted as a first-line treatment. Although sleep hygiene can obviously help in other areas, using it as a first-line treatment for circadian rhythm disorders is like advising an alcoholic to eat healthily. Yes it can be a more healthy lifestyle, but this procedure is irrelevant in the context of this clinical entity.
Logically, in accordance with a recently growing body of scientific evidence, another alternative are drugs that increase photosensitivity. Since light therapy affects directly the circadian rhythm and core body temperature, anything that increases the sensitivity to light will also indirectly affect the circadian rhythm, but only when combined with light therapy.
Hence, to summarize, the only drugs that help are those which either directly affect the core body temperature eg melatonin, or sensitivity to light such as antidepressives and ADHD medications. Marijuana and psychedelics cannot manipulate the circadian rhythm. Our body needs a very stable core body temperature to ensure survival, so it's extremely difficult to affect it (humans are endotherm and homeotherm). Manipulating core body temperature is the key to manipulate the cercadian rhythm, but the very safety focused homeostatic processes that safeguard the stability of our core body temperature and hence our survival is also what hinders our ability to manipulate the circadian rhythm.
In practice, this becomes a bit more difficult: how can we know whether a procedure can shift the circadian rhythm? There are mostly two ways:
- Either look at past academic literature, although there are conflicting results and often false positives, so it requires some training to discern what is viable and what is not.
- Either test on yourself and measure the effect on your own individual case. There are multiple avenues:
- The measurement can simply be a sleep diary, but this medium can suffer from high variability so it should be considered as partially unreliable. One way to overcome this is to maintain the sleep diary for a longer period of time (eg, months), to ensure the procedure is consistently effective (and it's not a coincidence with relative coordination - see the section below).
- A more experimental but more reliable and objective method is to record the core body temperature or wrist skin temperature. See the wearables section for more information.
Accommodations for circadian rhythm disorders including non-24
What it feels like to live with a disability
Living with a chronic illness entails a maelstrom of changes to adapt one's life and envionment to the individual's specificities.
The biggest difference between living with a sickness and without is that people without a chronic illness do not need to be mindful about their activities and how they spend their energies, whereas people with a chronic illness do have to be mindful of everything they do and always plan for potential contingencies in case of unexpected things that can wreck havoc when compounded with the chronic illness. This is especially well illustrated by the Spoon Theory by Christine Miserandino, which is a highly recommended read for anyone who would like to better understand, or explain to others, what living with a chronic illness is really like.
Disability rights and disability recognition
The very first step to get accommodations with non-24 is to get to learn about your disability rights in your country.
Disabilities recognition and accommodations should be available in most countries in the world, as 164 countries ratified the United Nations' Convention on the Rights of Persons with Disabilities (CRPD), easy read versions are available here and here, and the list of countries that ratified the convention is here. You may choose to use your rights or not, but it is crucial you do the formalities to get acknowledged and to get offered these possibilities if you need them. Do them while you are still able to, because when you will need them, you may be in the incapacity to fulfill the steps or may be in a dire financial situation.
In practice, to get disability recognition is not easy, the eligibility criteria are usually quite harsh, so that only the most debilitating illnesses are eligible (contrary to what the convention states...), and although non24 usually meet them, insurances and clinicians will often fail to recognize that.
First you need to estimate how many days you can (not) work per week, and check your country's eligibility threshold for disability. Usually it's around 2/3rd of disability required at minimum for recognition. There are also othes eligibility criteria you need to check, but the productivity handicap threshold is nearly always the most important. Then you need a medical report / accommodation letter from your sleep specialist describing your difficulties with the illness and how it affect your ability to work, and most important of all, write their (or your) estimation of your productivity handicap, eg, can't work more than 2 days per 7 days due to the illness without accommodations. This doesn't mean that you can't work more with accommodations, in fact the accommodations are there to allow you to be more productive, with an adapted work environment to your condition. Of course, it's necessary first to be medically diagnosed, so if that's not the case yet, check the informations in the Diagnosis section in this document.
Now with the medical accommodation letter, you can check how is the administrative process and the forms needed in your country to ask for a disability recognition. You can also ask for financial help if you need it, but even if you just ask for a disability recognition, this should at least allow you to ask for work accommodations or even financial access to work help (eg, tax reductions for your employer) which would already be tremendously helpful. For insurances benefits, you can remind the ICD code (ICD-10 code is G47.24), which is the basis used in most countries in the world to bill illnesses costs. Note that although non-24 (and other circadian rhythm disorders such as DSPD) are perfectly billable and fit all the criteria of a disability, it may be easier to highlight a comorbidity such as ADHD or autism to open these disabilities rights.
The other sections below describe specific practical accommodations that either you (the patient/employee) or the employer can be reasonably expected to implement. Indeed, the disability rights only guarantee "reasonable accommodations", which is subject to interpretation. It's hence a good idea to check what previous accommodations were implemented for other cases, as this sets a precedent for what is considered reasonable.
Why are Accommodations Necessary and Fair for People with Disabilities?
A common question that is being asked by people without disabilities is why are accommodations necessary? When digging, this question is often underlying the implicit assumption that an environment that is equal for everyone should be sufficient for accommodations. After all, why would people with disabilities get special privileges compared to others, if everyone is treated the same regardless of their differences? This is because equality is fundamentally different from equity.
To understand the difference between equality and equity, consider the following story:
Imagine you're excited about your new job as a secretary. You've aced the interviews, matched all the job criteria, and you're ready to start. On your first day, you put on your sharpest business attire and head to the office. However, when you arrive, you're confronted with an unexpected obstacle: the entrance is not just small; it's designed in a way that only someone with a very specific body type can enter. Confused, you ask your new colleagues about an alternative entrance, but they all confirm that this is the only one available.
You call your boss, hoping for a solution, like another office location or the possibility to work remotely, as your tasks don't require physical presence. But the answer is a resolute no; that's just the way things have always been. If you can't fit through the door, you can't work there. So, you're left without a job, not due to a lack of skills or motivation, but solely because of a physical characteristic that has no bearing on your ability to perform your job.
You may be thinking that this is unfair, or even stupid, but that you can always get another job somewhere else. But now imagine that it's the same EVERYWHERE, all jobs require to fit in these strange, small, specific-body-shaped entrances, so the same issue pops up time and again for every jobs you try to apply to. You cannot get any job, not because you lack skills, not because you are unmotivated, nor because of any behavioral issue, but only because of your body shape, an intrinsic difference that you cannot modify and that is irrelevant to your job.
This is the difference between equality and equity. In this tale, everyone gets equal treatment, since everyone can access these jobs if they can pass the same entrance, there is no difference in the entrance for anybody. But because we are all born with differences, not everyone can pass through this small entrance. To be truly fair, to truly offer equal opportunities to everyone regardless of their differences, we must account for their differences. This is equity. While equality aims for fairness through uniform treatment, equity strives for fairness by addressing and accommodating individual differences to ensure equal access and opportunities for all.
Now, think about how this scenario would play out if it involved not just physical barriers, but also sensory or cognitive ones. For instance, a visually impaired person might be unable to use a computer without screen-reading software, or someone with ADHD might struggle in a traditional, rigidly structured work environment without some flexibility in scheduling.
Equity doesn't mean treating everyone exactly the same, as this can result in unfair outcomes; it means providing specific accommodations based on individual needs so that everyone has a fair chance to succeed. For example:
- For a visually impaired employee, providing software that reads text aloud allows them to use computers and access information just like their sighted coworkers.
- For an employee who uses a wheelchair,** ensuring that all entrances and facilities are accessible means they can move around and use the office space as freely as anyone else.
- For someone with ADHD, offering flexible working hours or the option to work in a quieter environment can drastically improve their productivity and job satisfaction.
To make these accommodations effective, companies can adopt universal design principles, which create environments that meet the diverse needs of all people, not just those with disabilities. Additionally, technology plays a crucial role, offering innovative solutions that enhance accessibility, such as voice-activated systems, adaptive hardware, and customizable software interfaces.
By understanding and implementing these accommodations, businesses not only comply with legal requirements but also enrich their workforce, tapping into a wider pool of talent and perspectives. This not only benefits employees with disabilities but enhances the work environment for everyone.
In practice, disabilities may impact some abilities that can reduce performance on some, though not all, work-related or study-related tasks (for school students). But excluding people with poorer performance because of their disabilities is not only immoral and against international conventions on disabilities and discriminations that state that everyone have a right to equal opportunities and to be happy, but also is objectively an inoptimal choice as this would exclude unique minds (and everyone has a unique mind) who can contribute unique views that cannot be accounted for in performance metrics. Nature is extraordinarily resilient and evolving not because it has the most quantity of things, but because it has the most diverse set of things.
To illustrate this, let's explore some rethorical questions:
- Is being a woman a weakness and a liability? They are weaker than men, and statistically they work less than men because of maternity and taking care of kids and household chores much more than men. But of course not: physical strength is not the only metric of performance, most modern day tasks do not even require physical strength at all ; and the fact they take care more often of kids and household chores is a byproduct of societal expectations and organization, not a biological hard limit.
- Is being black a weakness and a liability? They are statistically much more likely to die in police encounters, to have lower wages, to end up in prison, and to die by illnesses. Of course not: all of these effects are provable byproducts of direct or indirect racism. For example, while it is true they are more likely to die by illnesses, this is because 1) they are often less wealthy than other communities, and hence have access to less resources, 2) recent studies demonstrated that they benefit from worse standards of care than what they ought to expect, and this is particularly caused by the fact that medical guidelines and most of medical research is done on white or non black people and skins, so that a lot of tests and treatments were not properly tested to ensure they work on black people. There are indeed several tests that do not even work or at least not the same when done on a darker skin.
- Is having a disability a weakness and a liability? As we saw from the two previous examples, statistics do not represent everything. While of course being disabled is a disadvantage compared to not being disabled, the limitations are primarily caused by an inadapted environment, hence factors that can be modified.
In conclusion, accommodations are not about granting special privileges; they are about removing barriers and ensuring that everyone, regardless of their physical, sensory, or cognitive differences, can contribute their skills and talents fully, and this increased diversity is objectively a net benefit for any system especially in resiliency and innovation. Accommodations are essential because they ensure that everyone, regardless of their abilities or disabilities, has equal opportunities to participate and succeed in various aspects of life. This is the essence of equity—recognizing and addressing differences to achieve true fairness.
Advocacy for awareness and accessibility for people with disabilities
Advocacy, especially for oneself, can sometimes be seen pejoratively, and hence people who could benefit from it, and require it, may avoid doing it. But advocacy is not necessarily selfish, it can be very altruistic, and necessary.
Indeed, people with disabilities have rights in the countries that ratified the UN convention for disabilities rights as explained in the previous section. However, a lot of these people with disabilities are unable to advocate for themselves, for their rights to access facilities and get equal chances than non disabled individuals. Hence, advocating for accessibility of the people with disabilities must necessarily a collective endeavor of the whole society. This includes when you advocate for your own disabilities rights: this also improves the access for others with disabilities who cannot advocate for themselves.
This advocacy culture is currently lacking in most disabilities community, partly because of the self-guilt due to internalized ableism. But inspiration could be taken from the neurodivergent community, especially the autism spectrum community, who have a very developed advocacy culture and numerous publications on the topic.
Furthermore, advocating for disabilities access can also highlight the convergence of other causes, such as costs and ecology footprint reductions.
Indeed, circadian rhythm disorders including non24 and DSPD require accesibility accommodation solutions that actually reduce ecological footprint, such as the direct delivery in a collection point instead of at home which has been made into an official policy the year after, and online meetings (eg, free tools such as Jitsi Meet exist) or even asynchronous communication instead of physical events, which eliminates transportation and hence reduces both the ecological footprint, cost for the attendants, and cost for the hosting institution since they do not have to book a big venue nor food nor hotel. This also reduces viral transmissions risks, another ecological risk. And these accommodations usually require no cost (original survey here), as the american JAN shown.
There is an International Non-24 Awareness Day on November 24th (N24) since at least 2015.
In summary: We need to take inspiration from autism community's advocacy culture. We need to advocate for oneself's disabilities rights and remind that this also helps for general disabilities inclusion, as solutions for non24 also usually help to include people with other unrelated disabilities. Furthermore, we need to remind that there are also non disabilities related arguments to implementing these accommodations, such as reduced costs and ecological footprints. In other words: nearly all the time, disabilities inclusions involve no cost but actually reduced costs, and so refusing them is often just ideologically motivated but not economically nor ecologically rational. A common example is the mandatory requirement to go back to work physically after covid-19 induced work from home ideological shift. (NB: But in some domains such as research, there are fundings that are given ONLY if physical events are organized - so overall this is still not economically rational, but locally at the scale of the organizers it makes economical sense because otherwise they would not get funding that covers not only the event but also their research - there needs to be a global mindset shift, especially at supranational organizations such as European Union to NOT require ecologically unsustainable practices).
In practice: do not just ask for accommodations for your disability, you may get replies such as "ah yes we would very much like to do so, but we cannot, we will think about it for next time" (even if a simple phone or installing softwares on the presenting computer is enough to make a physical event into a hybrid one...). Instead, ask for your own accommodation, and remind that 1) it also benefits the accessibility for people with other disabilities, illnesses and even pregnant women, 2) it is more ecological, 3) if applicable, that this causes no or negligible cost, or even saves costs.
The book Sleep Misfits: The reality of Delayed Sleep Phase Syndrome & Non-24 by Sally Cat also gives a few other ideas applied by others with circadian rhythm disorders to convince to get accommodations and advocate, such as:
> Nancy Tinney: I once told a medical staffer that I needed an afternoon appointment because I have a circadian rhythm disorder and can't get up before noon. She commented "wow, I wish I could sleep till noon every day!" I told her that saying that to me is like saying to someone in a wheelchair "wow, I wish I could ride everywhere so I didn't have to walk!" she got the point.
Keep in mind that humans cannot comprehend what they did not experience themselves: they can emphathize however, but various levels of explanations are required depending on the recipient's age, background, mindset, mood, etc.
School accommodations for students with the non-24 disorder
Sleep is an essential need for all humans, and is especially crucial for kids, as sleep loss can significantly impair not only their health but also their grades and hence their future. For them to have a fair, equal chance to education and to future work, they are elligible to reasonable accommodations. Reasonable accommodations are accommodations that can be reasonably expected to be implemented by the school, without much cost nor impact on their organization, which is arguably a significant restriction on disabilities rights but nevertheless allows for at least some partial accommodations.
Here are some suggestions of accommodations specifically for students with non-24 that was written by the Sleep Foundation, so you can use that as an official resource to present to the school:
> Students With Non-24-Hour Sleep-Wake Disorder
> As outlined under Section 504 of the 2008 Disabilities Act Amendment Act9, students with disabilities also have the right to an education that meets their needs, whether in elementary, secondary, or post-secondary school. Examples of reasonable accommodation for students might include taking classes online, allowing students to miss some classes and make up the work at another time, or taking a lighter course load.
Source: https://www.sleepfoundation.org/non-24-sleep-wake-disorder/living-managing
In the current document's author's opinion (based on his experience without accommodations), two reasonable accommodations that can significantly improve the fairness of academic training and evaluation of students with non-24 are:
- Allowing the student to get access to the course content that they not be able to attend, for example by having other students share their course notes, or having handouts of the course by the professor, or online recordings accessible by internet.
- Not sanctioning the student for their absenteeism, as it is unwillingly caused by their chronic illness. Attendance should not be a limiting factor, the students should be graded based on their academic merit, not their presence. This point is not for comfort, it is a crucial specific accommodation for non-24, since one characteristical effect of this disorder is a chaotic sleep-wake schedule and health status that cannot be planned. A child with non-24 who is sanctioned for missed classes will face the unfair dilemma of either damaging their health and grades by pulling all-nighters repeatedly, or they will face sanctions and potential school exclusion regardless of their academic merit just because of missed attendance. This would be as unacceptable as sanctioning and excluding motor disabled children because they can't participate in most physical exercise classes.
Both of these accommodations cost little to nothing in terms of effort or time (especially the avoiding of sanction of absenteeism) and hence can be considered reasonable accommodations.
The holy grail is asynchronous remote classes, that can be done at home, just as what was organized during the COVID-19 pandemic. But unless such a system is already in place at the school, it can be difficult to consider it a reasonable accommodation if it needs to be completely setup just for one or a few children.
Note that you can also come up with other suggestions, it's not limited to this list. The important thing is that you discuss with the school to find a common ground between your kid's needs and a reasonably implementable accommodation that the school can do without much cost.
See also the rights provided by the United Nations' Convention on the Rights of Persons with Disabilities (CRPD), especially the section "24. Education" in this easy read version.
More ideas of school accommodations: https://web.archive.org/web/20210927021722/https://old.reddit.com/r/N24/comments/ozzqkd/school_accommodations_with_non24/
Hopefully, with increased awareness about (sighted) non-24 in the future, next generation children with non24 will get proper accommodations and won't get unfairly penalized for their unavoidable and unwilling periodic absenteism.
For extreme cases of non-24, parents may consider remote schooling, some countries such as France (CNED) provide official remote schooling curriculum, which would allow the child to sleep and learn according to their biological schedule, but at the expense of reduced sociability and increased time expenditure by the parents as they need to supervise the child.
Note for DSPD: the accommodations are very simple, allow for classes to start later in the day, preferably no earlier than noon. There are plenty of chronobiology researchers and clinicians in several papers already advocating for such a change with later school start times, as it was repeatedly shown to significantly increase the students academic performance.
Example letters of accommodations are available in the members section of the Circadian Sleep Disorders Network website (for those in hardship, membership only costs $5).
Work accommodations with the non-24 disorder
Accomodating the non-24 circadian disorder and its highly variable and mostly unpredictable sleep-wake schedule with a stable work schedule is very challenging, with arguably most individuals with non-24 remaining unemployed most of their lives, regardless of their skillsets.
Indeed, even despite the "Great Resignation" in 2021 following the COVID-19 pandemic's lockdowns and the more widespread use of remote work and teleservices such as telehealth, a job position is still widely seen as a simple equation: presence during clearly delimited hours at work = salary. Even in job positions that are "tasks oriented" such as software development, being absent at the regular office hours is inconducive to developing good relationships with superiors and getting a promotion. Given that individuals with non-24 are fundamentally, technically, biologically incapable of fitting into such constrained work hours for any meaningfully long period of time (ie, less than 6 months), they are fatally predestined to be repeatedly fired and to jump from one job to another, with long periods of unemployment in-between, until at some point most lose any hope or become too old or their skillset too out of fashion to be employable anymore. Anecdotally, this is what happened to the present document's author's father, and despite the current document's author having two masters in profitable industries (artificial intelligence and machine learning ; neurobiology and neuropathology) and running a small company in the past, the same pattern of job jumping and periods of unemployment was experienced, showing how an invasive handicap dwarfs competence.
An often proposed simplistic solution would be to ditch job positions, and to make your own company, or work as a freelance, in other words: to be your own boss. Although this is certainly a more promising path, as there is more latitude to define your own workflow, you are still restricted to delimited working hours that are those of your clients. One solution is to found a company that can provide services or products to international customers, with an online front, so that you can work mostly asynchronously with clients. In other words, to ensure financial safety and independance, an individual with non-24 needs to seek an independent, asynchronous, online and international workflow.
Nevertheless, this path is hard and not achievable without having funds first, so that a standard job position is still required at least at first. Although no definitive solution exists, here are some ideas for accomodations for a standard job position, to optimize the chances of a stable employment and productivity with the non-24 disorder:
- Get your disorder recognized medically (see the section on diagnosis), then as a disability. This will allow to get "access to employment" helps, including accommodations from your employer and financial help (eg, tax reductions) for your employer or yourself if needed.
- In terms of practical work scheduling, foremost, seek to strive in an asynchronous workflow. Working remotely is not sufficient: because of the variable sleep-wake schedule, remote appointments at set times will also be as impossible to regularly achieve as in-person meetings. Likewise, flexible hours jobs aren't adequate either, since the variable sleep-wake schedule is unpredictable and makes planning "awake hours" impossible from one day to the next. Hence, the job needs to be achievable with no set hours, it's necessary to be able to accomplish work tasks at anytime of the day and night, whenever you are awake. Hence, prefer e-mails to chats (such as Slack) and (rare) videoconferences. If meetings are necessary, prefer videoconferences rather than in-person meetings, as these will allow you to get back to sleep just after in case they are scheduled in the middle of your circadian night (which is challenging to predict beforehand since the circadian rhythm is always moving with non-24, so usually it's impossible to predict exactly beyond a week before the scheduled date of the meeting). However, keep in mind that any event disrupting your circadian night will affect your sleep and work over the next days and hence your productivity, hence they should be avoided as much as possible when the circadian rhythm is not in phase with the day-night cycle. If possible, ask for recording all videoconferencing sessions so that you can watch them at anytime later at your convenience, and it can be useful for your team for archival purposes.
- According to a GitLab report "Killing Time At Work" (see also here), remote work should always involve a shift to an asynchronous workflow to be efficient, otherwise a majority of employees will tend to behave with digital presenteeism, ie, being present online at the usual 9-5 office hours even if this is not required by their tasks. They also are dithyrambic in their opinion that the COVID-19 pandemy should have been used more effectively and aggresssively as an opportunity to transition from old less effective productivity theater oriented workflows to more asynchronous and humane workflows. They offer a short cheatsheet of guidance to transition to an asynchronous workflow.
- Since you should seek asynchronous workflows, it's necessary to train a unique skillset for a career that can be done asynchronously, such as computer programming or art. Both the asynchronousness and uniqueness are important factors for a successful career with a chronic disorder such as non-24. One way to achieve uniqueness is to train to become an expert in some field, so as to reduce your replaceability. But keep in mind that even with a good skillset and a successful career, trying to work without accommodations is bound to cause health issues and ultimately lead to unemployment at some point.
- Seek a non-scheduled work position with minimal time-fixed events and planning, because: 1) due to non-24, it's often impossible to ascertain whether a time fixed event will happen during a sleep session, 2) it's also often impossible to determine how long and good a sleep session will be, hence it's necessary to take into account that several, if not most (depending on responsiveness to entrainment therapy), days will be spent exhausted, with very little productivity. These two points aren't exclusive, as interrupting a sleep session to meet a time fixed event such as an appointment will cause more exhaustion the next days due to the increased sleep deprivation that will take more time to clear up. The major source of time fixed events is business interpersonal relationships (ie, meetings), hence avoid any work that primarily involves client relationships or interactions with other humans, such as sales. Prefer works that can be done independently from other humans. Freelancing can be a partial solution but unfortunately incomplete as it still requires business client relationships, but it can be managed to your convenience as to reduce (but not eliminate) the impact on your sleep. Only non-scheduled jobs can fully accommodate non24, but flexible schedules are potentially partially possible with non24 although at the cost of some amount of chronic sleep deprivation, so the practical possibility depends on the exact terms of the flexible schedule position.
- Adopt a task-based agenda instead of date-based or deadlines agenda. As with many chronic illnesses, energy levels can fluctuate a lot and imprevisibly from one day to the next with non-24. Hence, a usual date-based agenda (ie, "Do errands on Tuesday") is likely going to fail and lead to more self-shaming. Instead, adopt a task-based agenda, based on priorities: do first what matters you most, whenever you feel energized enough to do so. If you can't today, try tomorrow, and don't beat yourself meanwhile, allow yourself to rest whenever you need. It's necessary to regularly re-evaluate priorities as time passes. However, avoid a deadline approach, as any fixed date is likely going to be missed, even if it's "only" a personal deadline. Just plan tasks you want to achieve and rank their priorities according to what matters to you most. What matters is that you do what matters to you, not that you meet a specific deadline. It is unfortunately illusory to hope to meet deadlines when you have a chronic illness, especially a chronic illness such as non-24 that specifically impairs our temporal capacities.
- Learn to rush/botch your work, especially if you are a perfectionnist. As the saying goes: done is better than perfect. With a chronic illness such as non-24, it's necessary to plan to have at least half as less time, and often much less, for any activity than non afflicted individuals. How much can you complete in half the time someone else has? This is what you need to aim for. If then you have time left, you can always further your work, but at least get the most important parts done, the crucial ones, and work on finition much later on if you have time left for that. This doesn't mean you should do work nor good quality work, but you need to significantly lower the bar and focus on fewer items, focus on the primary set of things that needs to be done for the thing to at least be functional/useful/interesting, the rest comes later.
- The working memory (both verbal and visuospatial) are drastically impaired under sleep deprivation. Although sleep deprivation can be reduced with proper management of non-24, it's unlikely to be completely avoided and will still be a regular occurrence. It's necessary to take into account this impairment to reduce the potential for mistakes or accidents. A workaround is to prefer writing for communication and workflows, rather than oral communications, as the written supports will reduce the need to use your working memory.
- It's crucial to account for a very prevalent cognitive bias: "out of sight, out of mind". This means that it's necessary to show up to meet your boss and colleagues from time to time to assert your presence and position in the team, otherwise you will be forgotten. Indeed, work quality and completion is not sufficient, humans forget what they do not see, and start acting regardless of the "missing" elements. This is not even specific to circadian rhythm disorders, as this phenomenon can also be observed with night shift workers, whether in the professional or private sphere. Since the workflow of a non-24 is necessarily asynchronous to be healthy and sustainable, this means there will be fewer opportunities to meet and assert one's own presence and position in the team. To optimize, the meetings can be selected: annual meetings, key projects deadline meetings and other key meetings should be attended, as long as it does not impact health too much (ie, these meetings need to be interspesed and rare, if they are weekly occurrences this is unsustainable, max is bimonthly). Any unnecessary meeting that can be done by an asynchronous mean such as e-mail should be done this way. Do not make the usual mistake of considering that work quality and productivity is sufficient: they are not. Even if you are an over-achiever and finish all tasks beyond expectations, you and your achievements will be quickly forgotten or not even accounted for if you do not show up from time to time.
- Always take into account that you will always have far less opportunities than someone else with same or less abilities or qualifications, just because of the mismatch between your circadian rhythm and the rest of the world. This is a mechanical consequence of the non-24 disorder, as most opportunities are related to the actions of other humans and business networking relationships, so that if you are asleep when they are doing these actions, you miss most of the events, and hence the opportunities. There is no way around this "bad luck". The only thing that can be done is to be aware of this limitation so that to put safeguards in place for when you are asleep, and hence to maximally exploit the few opportunities you get access to.
- Put your safety first. Do not drive if you are sleep deprived, you will have worse decisional capabilities and slower reaction time, just like as if you were drunk. In fact, sleepiness is the greatest cause of accidents in all modes of transport, far surpassing alcohol. Instead, prefer to plan ahead and use other means of transportation. If you are not sleep deprived but your circadian night will happen during the time you plan to drive, avoid driving as you are more likely to doze off on the wheel, especially for long driving sessions (eg, cross-country). Although not studied, circadian misalignment is very likely to contribute to dozing off while driving, whereas sleep deprivation is a well established factor.
- To increase your productivity, energy levels and mood/motivation, manipulate light and your sleep pattern to your advantage:
- Always favor naps/siesta when you can. In terms of productivity, several studies of "assessments of vigilance in monophasic versus polyphasic sleep schedules indicate that performance is comparable given equivalent time in bed (Nicholson et al., 1985; Mollicone et al., 2007; Mollicone, 2008)", which means that what matters is indeed the cumulated total sleep duration of all the sleep periods under a circadian period, including naps, to assess sleep deprivation which is in fine what determines decreases of productivity. In other words, you will be more likely to be productive after napping if you were sleep deprived than by trying to forcefully work instead, as the cognitive fog due to sleep deprivation will drastically decrease productivity (mood and cognitive impairments are curvilinearly correlated with the amount of chronic sleep deprivation). Better to have a few hours of productivity than a day of unproductivity.
- use light therapy for several hours in the circadian morning and/or day, this greatly increases energy levels and reduces negative mood. This effect of bright light therapy is as beneficial and crucial as its circadian rhythm shifting effect.
- avoid dark therapy when you are still in your circadian day. Indeed, humans are diurnal animals, so that their behaviors and activities will become more sluggish under darkness even if they are not sleeping. Hence, although dark therapy is a crucial component of circadian rhythm disorders therapies, it is also important to not use it when it is unnecessary. Hence, even if it's night, if your body is still under its circadian day, keeping the room's lighting reasonably bright will significantly bolster productivity. In case of doubt, using a configurable LED bulb such as Yeelight S1 and setting it at a warm yellow or rosé (between yellow and red) with a medium intensity offers a middle ground between bright enough intensity to not hinder activities if it's your circadian day while also being unlikely to significantly suppress melatonin nor delay the circadian phase if it's your circadian night.
- For individuals with a circadian rhythm disorder such as non-24, having a bifurcated circadian rhythm (achievable using light-dark therapy, see the related section elsewhere in this document) can have significant advantages, such as allowing 2 windows of activity, one during the objective daytime and one during the objective night (whereas nightwalkers such as inversely phased non-24 or extreme DSPD will not have activity opportunities during daytime). For those suffering from chronic fatigue due to chronic circadian misalignment, LDLD allows to multiply by 2 the number of activity periods and rest periods, so this probabilistically increases tremendously the likelihood of experiencing productive activity periods, whereas with a typical schedule the next opportunity is only in 24h, here it can be 12h or even earlier depending on the LDLD scheme used.
- Keep in mind that sleep deprivation and circadian misalignment will cause increased focus difficulties, more precisely your brain wil prioritize external stimulations over internal actions, and with severe sleep deprivation, only external stimuli will keep you awake. This can sometimes be used to your advantage, by being constantly listening to music (in headphones to not bother your surroundings), although of course use your judgment, as music may not be fit for some situations where it can put you at additional risk. Another trick is to do physical tasks when sleep deprived, as although it will be more painful to you, they do not require much focus (depending on the physical task of course). In summary, anything that can provide external stimuli will keep you awake when sleep deprived, whereas self-generated thoughts are much more difficult to focus on (such as reading).
- Setup and furnish your work environment to allow for both working and sleeping, as sleep disorders makes wakefulness very fleeting and varying, which makes it necessary to work in an environment accomodating both wakefulness and sleep and everything in-between. For example, having a couch in addition to an ergonomic chair, and a mobile table to move the workstation to either. Such an environment will allow you to nap or to work in a laid down, more comfortable position when you are tired. Although it is usually disadvised to work in a laid down position, this recommendation is made by people who do not have energy issues due to a sleep disorder, for whom it is regularly impossible to work in a usual sitting position. Working in a laid down position allows to reduce physical energy expenditure and hence allow the body to allocate more ressources to cognitive functions. Ergonomic chairs make a real difference, especially when energy levels are low, as they reduce the tiredness from lombar support by transferring the weight onto the chair. Inexpensive ergonomic chairs are available from some manufacturers such as Sihoo or mFavour.
- The only perennial workspace for non-24 is at home. Due to non-24, the workspace needs to be accessible to work at any time of day and night, to be comfortable and safe for sleeping at any time of day and night, and to be accessible without driving to eliminate the very real risk of car accident due to drowsiness. Realistically, only a home workspace fits with all of these criteria. Dedicate a room for work, separate from the sleep space. Furbish it adequately for both work and impromptu naps/sleep, you can't expect to work to professional standards under an unprofessional environment and with no professional furniture.
- Expect to work at any time of day and night. If you can't sleep after 30min of laying down, get out of bed and get back to activities, including work if you want. You can't force yourself to sleep, closing your eyes and wishes aren't going to make you sleep. But when you'll feel ready to sleep, go back to take another try, but expect this won't happen before several hours so you have time to work or do other activities meanwhile.
- Work whenever you have enough energy on the most important/urgent tasks, as you never know when you will get enough energy again (it can be days or weeks from now), and do not wait for full energy level to work, as this will fluctuate a lot from day to day and during the day. Keep a set of tasks you can do with lower energy levels, and try to do what you can. Accept that you may not be able to achieve the tasks, aim to work and progress, not to complete. Far more will be accomplished by working on minor tasks at a slow pace and reduced capacity than not working at all. There are days when cognitive dysfunctions will be so great that you cannot achieve anything (ie, impossible to focus and think), accept these bad days as part of the illness.
- Find smartphone apps that can be used for your work, so that you can work everywhere at anytime. The lack of a routine in non24 makes it hard to have a defined period at the desk, hence smartphone apps can circumvent partially this lack of physical routine. However be careful not to be overloaded, make sure to disable your smartphone's notifications when you need to sleep.
- A few tips to organize in practice for appointments:
- use a chronobiological alarm clock (also called "smart alarm clock"), which monitors the user's sleep stages via actigraphy and vibrate when it detects the user is in a light sleep stage, such as Sleeptracker Pro (discontinued), Fitbit or Sleep as Android (but apps are much less effective than wearables worn on the wrist since it requires actigraphy). Make sure to select the model that offers both a smart alarm and vibration, it's much more effective and with vibration than an auditive signal.
- for appointments very far in the future, too far for you to predict your circadian phase, try to ask to schedule the same appointment but twice in the same day: one in the morning, and one towards the end of office hours, and that you will call the day before to cancel one or the other. If the office seems reluctant, explain that you have a severe disability, so you cannot know at what time you will be able to go, so having two drastically help improve the odds. This way, by the time the appointment comes, whether you are night walking or day walking, one of the two appointments will likely be during your circadian day.
- try to stack as many appointments in one day, and leave at least one but preferably "free days" with no appointment in-between days with appointments. This way, you can have some time to recover between days when you have appointments and have to cut on your sleep. The worst is to have appointments at specific times every days, as this will force you to suffer sleep deprivation consecutively with no recovery time, this is very dangerous for health and drastically decrease your productivity. Try to interspece "appointments days" as far as possible, the more recovery/free days the better for your health and productivity. When you have one appointment, it's highly likely you will have to be sleep deprived as it is unlikely that it will be during your circadian day, hence you can as well stack as many appointments during this day so that you can plan to be able to recover the next day (by sleeping/napping whenever needed). Note that one or two days are just barely enough to recover from one day of sleep deprivation, but an all-nighter OR suffering from sleep deprivation over several days/weeks will require many more recovery days, there is some proportionality.
Furthermore, the data on insomnia likely also applies to unmanaged non-24:
> With respect to vocational performance, several studies have found that sleep disturbance and/or chronic insomnia are associated with less job satisfaction, lower performance scores, less productivity, and higher rates of absenteeism.13,14 A study by Leger et al15 found that those with insomnia had more absenteeism compared to good sleepers (31% vs 19%) and made more errors at work in the previous month (15% vs 6%) and that 18% of those with insomnia, versus 8% of good sleepers, reported poor work efficiency in the past month. [...] Individuals with a variety of sleep disorders are thought to be at increased risk for motor vehicle accidents.18 Patients with insomnia in particular have been found to be 2½ times more likely to report car crashes because of feeling tired as compared to those who do not report insomnia.
A 2020 systematic review on sleep disturbances and risk of sick leave found the following:
> Sleep disturbances are risk factors for sick leave. Sleep problems can lead to various health problems that affect the amount of sick leave. Improving sleep quality can have a decisive impact on job performance.
There are several resources to help you navigate how to request work accommodations:
- JAN, the US Job Accommodation Network, offers several video tutorials on how to request work accommodations under the ADA. in their Workplace Accommodation Toolkit youtube playlist. In particular, the videos on Performance Management and Telework as an accommodation can be pertinent for non-24. For employers, The Employer’s Guide to Reasonable Accommodation by the Southeast ADA office is an helpful concise tutorial, showing that most accommodations cost nothing to employers.
- In some countries, big companies need to have an employee who can help with negociating reasonable accommodations, such as in France ("la « mission handicap » dans les grandes entreprises ou le « référent handicap » dans les entreprises employant au moins 250 salariés (Art. L.5213-6-1 du Code du travail) ;"). Otherwise, the Human Resources department may help.
- All countries that ratified the UN convention on disabilities should provide informational leaflets on what help and infrastructures dedicated to disabilities are available to you. For example in France, there is the Cahier Orphanet - Vivre avec une maladie rare en France - Aides et prestations, Décembre 2022.
Non-exhaustive list of potential jobs for non24, that would fit the independent asynchronous remote workflow (and online for some):
- content creator for youtube or other on-demand content platforms
- taxi driver (but need enough cath to buy a car)
- artistic works (drawing, photography, literacy, voice acting, etc) with platforms like fiverr to get an async job.
- forex and crypto trading (because these markets are always open, especially crypto, although stock market does influence both and has set hours)
- any work where sales of virtual goods/services are asynchronous and hence can be done while you sleep. Eg, for an artist, an online gallery shop of images you make that visitors can buy online. Or a pro subscription to a software package you develop vs a free one accessible to all but with less features, this example is actually a business i successfully ran for a few years, clients contacted me by e-mail for informations or feature requests, that I then developed at my own pace, and all pro users got notifications and access to the regularly updated software. Can also be an automated web app that provides a service, such as any website with interesting content and ads to generate revenue on web 1.0, platforms that allow users to submit content and others to buy and your platform gets a cut on web 2.0, or crypto defi platforms on web 3.0. In both cases, the platforms offer a service asynchronously even when you are not there. It's a lot if work and stress to maintain, but it can be very prolific. To make sync sales, either need to sell artistic virtual goods, or softwares for professionals, ie, that save them either time or help them gain money.
Some tools may help with organizing setting up and monitoring your own work schedules tailored for non-24:
- Rotime is a web app available for desktop (Windows, Linux, MacOSX) and mobile (Android, iOS) specifically tailored for people with non-24 and other circadian rhythm disorders. The main demographic is workers with circadian rhythm disorders or shift work, but it can be used by anyone who want to schedule their days on a dynamic non-24 timeframe. You can enter your tasks, their durations, and then the app allows to easily rotate the time you start these tasks. It's a great app for designing a work schedule in general, but not for specific tasks. There is a free version for one day, or an older free version all the time in WebArchive, or a paid version with more features and to support further development.
- For specific tasks, open-source softwares such as Super Productivity (cross-platform) or OneList/1List (for Android) are great.
- WarpClock is an open-source alarm clock for Android that calculates when to ring based on how long you want to sleep, so that you don't have to do any math before sleeping. This can also be used during the circadian day to time activities, such as work tasks billing, or set to the individual's non24 period to remind them where they are in their circadian day.
- FairEmail is an open-source Android e-mail client app that works with most e-mail providers including GMail and provides advanced features such as delayed sending.
- For programmers, git-privacy is a potentially useful tool to hide commit datetime in git history, a similar idea to delayed sending of e-mails.
- Try to find mobile apps for most of the tasks you are used to do on computers, so that you are more mobile and can work anywhere. For example, for note-taking, Markor is an awesome open-source Android app, compatible with desktop note-taking apps such as MarkText and Zim Desktop Wiki, and automatic synchronization can be done with open-source tools such as SyncThing.
Additional unorganized information:
- Expect to often be late if you have appointments. The non-24 makes it difficult to plan ahead of time at what time you will be awake, and so any planning becomes more difficult and productive time during office hours becomes compressed, so that you often end up trying to frantically complete as many tasks as possible under a very tight schedule, which is often impossible. Lower your expectations accordingly, and try to avoid appointments as much as possible, flexible schedules are necessary, so prefer deadline and task based schedules which do not require a completion at a specific time during the day.
- Sleep deprivation impairs temporal preparation, so this also participates in the higher likelihood of being late as it makes it difficult to adequately prepare on time, every act becomes delayed and rely on automaticity.
- Since the non-24 disorder makes it hard to maintain a stable employment status, it's necessary to plan to invest part of the earned money during the employed periods to build a safety fund, as is common practice for independent contractors. The first step is to learn how to be more financially literate. The videos of Mark Tilbury are a great start point.
- When sleep deprived, impairments in thoughts inhibition (ie, running thoughts) can be expected, hence on those days avoid interruptions especially social, as they will be much more disruptive to the workflow than on days with less sleep deprivation.
- The home environment is also important for work, especially since with non-24 you will live and work mostly at home. It's necessary to choose a home environment that is well exposed to sunlight, especially in the morning, which will reduce the need or duration for bright light therapy, but also can be equipped with blackout opaque curtains to allow you to fully control your exposure to bright light (and avoid it when necessary). Thus, avoid living at the ground/first floor of a building, the higher is better.
- At school, ask to favor mid term and end of term exams for kids with a circadian rhythm disorder, avoid "continuous exams" (ie, frequent exams done during classes) as these require the students to always be present in class, which is fine and can fit better for some kids, but certainly not for those with circadian rhythm disorders such as DSPD or especially non-24.
- There are a few initiatives to help signal hidden disabilities and get discreet help or accommodations, such as the Hidden Disabilities Sunflower which is implemented since 2016 by several traveling entities in UK such as airports, railway, supermarkets, etc.
- When presenting your non24 disorder to work colleagues and superiors, call it a "handicap", not a "disorder". Because what matters work-wise is not what it is, but how it affects your productivity.
- Here is a list of complaints and tips by individuals suffering from circadian rhythm disorders, demonstrating these issues are common for them, and these resources can provide additional practical advices and ideas for accommodations:
- Non-24:
- https://archive.is/oowqn
- https://archive.is/IALzl
- https://archive.is/7m59Y and https://archive.is/uWwtb
- Even when other health issues including psychological disorders are treated, non24 still remains a hard cap for employment: https://web.archive.org/web/20210922180241/https://old.reddit.com/r/N24/comments/psw8od/overcame_severe_and_persistent_problems_lasting/
- DSPD:
- An interesting post about a business owner, showing it may be potentially even more difficult for them to maintain their job than employees, because they necessarily have to manage customer relationships and have employees dependent on them for their livelihood: https://archive.is/NN4CO
- Accommodations wishlist for DSPD: https://archive.is/zTdtf
- Not all nightshift schedules can fit with DSPD, contrary to what could be assumed. Here is a great tip by u/Gilsworth: "don't let the job intersect with your natural bedtime".
- https://archive.is/vpQl7
- https://archive.is/HTFtu
- https://archive.is/n3kNd
- https://archive.is/PBJXe
- https://archive.is/3ak6f
- https://archive.is/LEwzw
- Non-24:
TODO: Add infos about chronic diseases and unemployment:
- https://pubmed.ncbi.nlm.nih.gov/26567288/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5923637/
- https://doi.org/10.1093/eurpub/ckz185.031
- https://academic.oup.com/occmed/article/66/2/143/2750607
- https://onlinelibrary.wiley.com/doi/pdf/10.1111/1467-9566.ep11340114
- https://www.forbes.com/sites/manondefelice/2019/09/04/the-surprising-truth-about-chronic-illness-and-the-future-of-work/
- One of the sources above show that it is expected by 2025 that half of the american population will have at least one chronic illness.
- Non-24 was found to be a worsening comorbid disorder for blind people compared with a control group of blind people without non-24: https://doi.org/10.3389/fneur.2020.605240
- International Labour Organization: Non-Standard Employment Around The World, 2016 https://web.archive.org/web/20210717065612/https://www.ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/---publ/documents/publication/wcms_534326.pdf
- Even for autism, the often comorbid sleep disorder is what impairs most the employability, more than the neurodivergence:
- Employment status is related to sleep problems in adults with autism spectrum disorder and no comorbid intellectual impairment, 2019 https://doi.org/10.1177%2F1362361317745857
- "Adults with autism spectrum disorder and an International Classification of Sleep Disorders–Third Edition sleep disorder had higher scores on the Pittsburgh Sleep Quality Index and were more likely to be unemployed compared to adults with autism spectrum disorder and no sleep disorder. The findings demonstrate, for the first time, that sleep problems are associated with unemployment in adults with autism spectrum disorder. Further research exploring the direction of this effect is required; sleep problems that have developed during adolescence make attainment of employment for those with autism spectrum disorder difficult, or unemployment results in less restrictions required for optimal and appropriate sleep timing."
- Employment status is related to sleep problems in adults with autism spectrum disorder and no comorbid intellectual impairment, 2019 https://doi.org/10.1177%2F1362361317745857
- A long-term follow-up case study of 10 adolescents with sleep-wake disorders who received intensive treatment found that the illness severity did not improve much, but the social adaptation improved significantly. In other words, these individuals learnt how to manage and adapt their life around their disorder: https://doi.org/10.1111/j.1440-1819.1994.tb02994.x
- An informal survey conducted by the present document's author about non-24 and employment status: https://docs.google.com/forms/d/e/1FAIpQLSccIXjEVIL2bdvBcIG0lpJZl86fbf1KGxsu5GXYeo5KM-tZwQ/viewform
- Dynamically updated results: https://docs.google.com/forms/d/11IuQUonL9L8a_NFSzQZPV_xQxvFjps32w2gT2gHB8kk/viewanalytics
- A previous similar survey (but much more exhaustive) was conducted by the Circadian Sleep Disorders Network, with the list of questions being openly published here: https://www.circadiansleepdisorders.org/registry/survey_questions.php?print — and some preliminary results (but without the responses to employment status) here: https://www.circadiansleepdisorders.org/registry/survey_results_prelim2.php
- Improvements suggested by the community: https://www.reddit.com/r/N24/comments/osdfhv/are_you_employed/ (mirror)
- Idea: make a scale from 1 to 10: How much do you think the non-24 disorder affects your employment status? (ie, makes it hard to find or maintain a position)
- For french readers, a very interesting, although dismaying, book to read on the current status (as of 2021) of disabilities rights and institutional obligations (hint: there are none): « Handicap : l'amnésie collective: La France est-elle encore le pays des droits de l'homme ? », Bachir Kerroumi, Stéphane Forgeron, Dunod, 8 sept. 2021, ISBN: 2100832387, 9782100832385.
- The easiest and most common form of employment is the simple exchange of chronic time expenditure for payment. However, this time expenditure is always depending on the needs of the employer, and in general dependent on commercial aspects such as when clients are mostly present, and hence always entails a predefined schedule (whether fixed or dynamic, it still disregards the individual's sleep needs, especially if out of normal ranges).
- When presented with the almost insurmountable barriers to employment the disorder entails, interlocutors of individuals with non-24 often suggest to simply run your own company, as the panacea to all scheduling issues. While this is certainly a potentially more successful alternative to employment, this is far from the panacea, as it suffers from a similar issue: an entrepreneur's work schedule is also at least partially if not fully defined by social constraints, such as commercial relationships with clients who will present themselves during regular office hours and rarely at night and even more rarely at a rotating schedule, and the management of employees, who need oversight especially during the starting years of a new company. Some interlocutors will interject that some famous CEOs such as Steve Jobs or Mark Zuckerberg are known to be almost always out of their office, they can work (or not) at anytime and whenever they want. While this is true, this only works for companies that are already very successful and well established, which is a low likelihood, since most companies end in failure.
- Some interlocuters will suggest to "just grind it out" until you can afford to be such a CEO, and meanwhile just work despite no to little sleep. The only response that can be given is: "would you also be able to grind it out without food / under malnourishment for years before getting paid adequately?", since, just like food, sleep is a necessary and unavoidable need. Planning to forego sleep for years, as is required to grind a company out of the startup phase, is just like trying to grind it out while being malnourished, this drastically reduces performance and the chances of success, over the already low chances of making a new business a viable long-term prospect. Hence, the non-24 is always a severe handicap to employment and entrepreneurship, as it bars from employment and drastically reduces the already low odds of successful entrepreneurship. A practical example: Google products free certifications + fiverr pro, see tutorial: https://youtu.be/Dy1gz4We3Yc
Here are a few ideas of potential jobs for non24 (non-exhaustive list!):
- content creator for youtube or other on-demand content platforms
- taxi driver (but need enough cath to buy a car)
- artistic works (drawing, photography, literacy, voice acting, etc) with platforms like fiverr to get an async job.
- forex and crypto trading (because markets are always open, especially crypto, although stock market does influence both)
- any work where sales of virtual goods/services are asynchronous and hence can be done while you sleep. Eg, for an artist, an online gallery shop of images you make that visitors can buy online. Or a pro subscription to a software package you develop vs a free one accessible to all but with less features, this example is actually a business i successfully ran for a few years, clients contacted me by e-mail for informations or feature requests, that I then developed at my own pace, and all pro users got notifications and access to the regularly updated software. Can also be an automated web app that provides a service, such as any website with interesting content and ads to generate revenue on web 1.0, platforms that allow users to submit content and others to buy and your platform gets a cut on web 2.0, or crypto defi platforms on web 3.0. In both cases, the platforms offer a service asynchronously even when you are not there. It's a lot if work and stress to maintain, but it can be very prolific. To make sync sales, either need to sell artistic virtual goods, or softwares for professionals, ie, that save them either time or help them gain money.
- Online consulting jobs can be a middle ground solution: they aren't fully asynchronous, but you can schedule your clients appointments when you want and potentially around the clock if the job can be offered internationally, and most of the work can be done asynchronously. The big advantage is that there are online consulting jobs in pretty much all segments of the work landscape, eg, typically computer science is the most indicated and it's possible to get free education such as by getting a (free) Google certification for SEO optimization or ads optimization using Google systems, but there are also online consulting jobs in law (eg, legal tech), or even just educating parents to manage their newborn's sleep, there are lots of consultants who do that. All that is required is an expertise in skillset that is often needed and that can be done remotely.
Hence, diagnosing non-24 early in life, especially in childhood, can arguably help the individual pursue early on a career that can lead to a more adequate job for someone with a non-24 disorder. Indeed, when the diagnosis is made later at adulthood, it can be very difficult or impossible to redirect one's career so late in life.
For DSPD, an example letter of accommodations are available in the members section of the Circadian Sleep Disorders Network website (for those in hardship, membership only costs $5).
Home accommodations
Living in an adapted environment is crucial for any individual with a chronic illness, even more for those living in couples or with their family.
Here are some ideas for a home environment adapted to non-24 and other circadian rhythm disorders:
- Move/choose a home with a separated livingroom, and where each room (including kitchen and sanitary) can be accessed without passing through or close to the bedroom. This is the most important tip, as this allows to make the livingroom the literal living room, where you or your partner/family can wake up and go to to do their activities without bothering those who are asleep. The idea is not to make big noises, but to be able to do the necessary activities without bothering others, such as cooking (carefully to lower noise), going to sanitary, and doing any kind of activity in the livingroom as long as it's not too noisy. For example, talking at a reasonable sound level should be possible at any time of day and night in the livingroom. Anyone should be able to go to the bedroom to sleep at any time, whether day or night, without impacting the activities of others in other rooms. Hence, the best are homes where each room is connected through a corridor, not through other rooms. This tip works for both non-24 and DSPD and is the single biggest improvement that can be made, see for example this New York Times story that was adapted in the Amazon Prime's Modern Love S2.2 episode. This reduces the "walking on eggshells" issue but does not eliminate it, especially not for the individual with the circadian rhythm disorder since they will still have to mind neighbors when they are awake at night (ie, nightwalking).
- Reduce noise when you sleep, and take it into account as a primary factor when moving to another home. This is crucially important, as noise during sleep, even if heard only unconsciously (ie, no memories of interruptions due to noise at wake-up), it will still greatly impair sleep quality, to the point of feeling permanently exhausted. For example: building works that are common in cities especially in September-November ("back to work" season) and which usually start everyday at 8am and hence can overlap pretty much with a delayed circadian night (DSPD or non-24), relatives or neighbors in a too small flat, etc. Hence, living in cities is likely not a good idea, especially around the arteries or big roads.
- Given the two points above, the ideal home environment for someone with a circadian rhythm disorder is hence an independent house. Otherwise, there is no other way than to permanently live on eggshells, which is very strenuous and limiting. But this is unfortunately a catch-22, as it is difficult to acquire the financial stability necessary to access the housing market when hindered by a disability.
- Procure and use as many silent tools as you can find, they can be crucially helpful to improve quality of life and health, by allowing to do activities during night time without bothering neighbors:
- Buy bluetooth bone conduction headphones. This will allow to use TV and computer at night, without bothering anyone. Usually, multiple headphones can be paired to the same device, so that for example if you have non24 and your kids have too, they can wake up at night, go to the livingroom (see the previous point), and watch TV or use a computer to watch films and anime, and you can even watch with them if you both have headphones! The advantage with bone conduction headphones is that they are very silent to others and also that they do not make the ears become itchy, so they are very comfortable and they do not damage the ear canals. This allows to freeroam and do activities just as if it was day and the speakers were used, but it's a wireless transmission instead that is silent to the environment. You can share some piece of musics and movies with your kids this way, just like you would in the day.
- Regular physical exercise is necessary for optimal cognitive performance and to improve endurance and hence energy levels. But it can be challenging with circadian rhythm disorders, especially non-24, to go to the gym because of the mismatch with the opening hours or the risk of exercising outside at night, especially for women. An at-home inexpensive but effective alternative is to use resistance bands. Indeed, resistance bands traning was shown by a systematic review to be as effective as conventional resistance training including free weights for muscular and strength gain. Resistance bands allow to do all exercises achievable using free weights (see James Grage tutorials including this excellent beginners tutorial). They can also be used to train cardio in combination with a HIIT or TABATA routine. These can be bought for 30 euros for cheap ones (eg, Decathlon) to 100 euros for the top quality (Undersun Fitness). Prefer to use loop type resistance bands rather than cord-like bands, as the former allow for a broader set of exercises and are more robust over time. Another great advantage is that they are silent, which allows individuals with circadian rhythm disorders the opportunity to train at night without bothering neighbors nor risk going to the gym by night.
A practical guide to getting disabilities benefits (for non-24 and other conditions)
WIP: this guide was originally meant to be posted on reddit, but reddit being reddit and refusing to post high quality long answers, the guide is now written here. This explains the more informal tone employed. Hence this section needs rewriting in a more academic form, but it is valuable enough as-is that it is included in its original form for now.
There has been multiple questions about this issue on social networks, often seemingly resulting in failure:
- https://www.reddit.com/r/N24/comments/1fn38gt/does_n24_count_as_a_form_of_disability_in_the_us/
- https://www.reddit.com/r/N24/comments/1aklke7/any_luck_with_disability_benefits_and_n24_usa/
- (Successful approval apparently) https://www.reddit.com/r/N24/comments/sy75n2/has_anyone_successfully_been_approved_for/
- https://www.reddit.com/r/N24/comments/1gz74c1/us_users_have_you_been_approved_for_disability/
What almost everybody gets wrong, including physicians and people in the administration, is that disabilities benefits recognition != is your disorder/disease in a list of disabilities. That's not at all how it works.
Anything can be a disability. There is no list of illnesses that are disabilities.
Remember these two sentences as they are key to understand how the disabilities benefits system works in countries who are signatories of the UN convention on the rights of the people with disabilities.
Most people fail to get their right acknowledged, because, just like any social benefits, governments are very stingy to produce them. They are made very hard to get on purpose. The UN regularly decries this situation, but it is not getting better, especially with the looming shadow of a possible worldwide, global recession. So there is no guarantee that you can get them even if you should and even if you do everything right, there is a big part of luck. But if you don't understand how it works, you are certainly not getting anywhere.
So how does it work? Let's explain step-by-step with subquestions:
- How are disabilities benefits attributed? They are attributed to people with disabilities.
- So what are considered disabilities? Disabilities are simply things you cannot do in your life because of one or several health conditions you have.
Hence, disabilities can be caused by illnesses, but you can also be born with them without an illness, or have an accident and lose a limb or get brain lesions for example (this is not an illness). Also, you can have multiple causes that combine to cause your disabilities. Eg, being blind is problematic, but being blind and having lost your arms is a whole other kind of disability severity.
Thus, the cause(s) of your disability is irrelevant for the attribution of your disabilities benefits.
The causes are only required to be communicated so they can be used in practice to: 1) double check very superficially with their own physicians that your application is not bogus (but these social service physicians are not expert of your condition(s), so they cannot assess the severity of your condition, they do not have the skills to check anything but superficially, so they cannot go counter to a specialized physician's diagnosis and accommodation letter for example), and 2) to fill databases to compile nationwide stats to see which causes are the most frequent, so that politicians can know where the money goes to most and where to fund research or who to criticize in their blame game...
What does that mean to you in practice? This means that requesting disabilities benefits requires one thing, and only three things: a medical assessment of which of your daily activities and abilities are limited because of your condition(s), and with what severity, and if this is permanent or not. Again, the cause is irrelevant, it's only an additional information but is not necessary for the recognition of a disability. The disability is present when you cannot do what others can because of a physical (or psychological) limitation.
In practice, the administrative process of getting disabilities benefits look like this:
- Proof of condition: Get your condition(s) medically diagnosed (by physicians, not by neuropsys for example, they need a medical degree).
- Proof of disabilities = description of impacts on life: Get a letter of accommodation by your physicians, or an assessments scales report depending on the condition (see below). In some countries (eg, France), you may be required to ask your physician to fill a specific form.
- Request disabilities recognition: Submit your application to your disabilities benefits office, usually involving filling out forms that describe which kinds of sensory or ability limitations you have with your conditions (so they can standardize how much "points" or percentage of disability you get). Contrary to common preconception, this is NOT just about work, but about your daily life activities and abilities! A blind person with a remote work is still disabled when he goes outside for the groceries! Also note that the disabilities benefits are not just financial ones and hence not just the government: you can also get disabilities benefits from your employer, from the university, from the gym you go to, etc. So there is not just one single disabilities benefits actor, there are multiple, so it's very worthwhile to improve your disabilities documentation as they are very reusable with multiple disabilities benefits actors.
- Request disabilities benefits: Once your disabilities are recognized by the disabilities benefits office, then you can request various helps depending on 1) the grading of your disability they did (which depends on a- your medical assessment and especially the quantitative assessment, b- their own criteria and whether the medical assessments fill those boxes), 2) your current financial situation (eg, an unemployed person with autism will likely get monthly financial allowance, whereas Elon Musk is not entitled to such help - but both can get their disabilities recognized, assuming both have a similarly impacting condition). Note that disabilities recognition and benefits may be bundled in the same application in some countries, but internally they remain separate steps with separate outcomes: the administration can always grant you disabilities recognition without necessarily the benefits (but you cannot get benefits without recognition).
As you can see, the disabilities benefits office is NOT the ones who decide if you have a disability, it's the physicians. The disabilities benefits officers only decide whether to grant you or not the social benefits based on the medical information. So I will describe below some common key information your physician need to write in your application/letter of accommodations to increase the likelihood of getting the disabilities benefits, but the exact criteria change for each country and sometimes even for cities, so you should read the documentation and try the simulations of your local disabilities benefits office to know very precisely the criteria for how they assess disabilities, as this is key to success.
So in practice, this means that the first and most crucial steps you have to do are to get medically diagnosed, and get a letter of accommodation, ie the medical assessment, which involves you telling your (openly listening) physician what daily activities are impacted by your condition(s). In some cases, you may be required by your physician to first try treatments, because disabilities account for that (ie, is the disability permanent?). Eg, if you are diabetic, but with diabetic treatments all your symptoms are eliminated, and the treatment has 0 side effect, then you cannot get a disabilities benefits, because what matters is the practical real life impacts of your condition(s), not the theoretical ones. Of course, this never happens in practice, there is no treatment with 0 side effects, and usually they never totally cure all the symptoms... But treatments can reduce the severity, and hence your benefits.
KEY INFORMATION: In addition to describing what abilities/activities are impacted in your daily life (ie, qualitative description), at some point try to also describe how much your daily life is affected in terms of percentage (ie, quantitative description). For example, how many days per work week can you truly work with your current condition(s)? If 1/5 days, this means you are handicapped at 80%. It can also be about your daily life, but it needs to represent a percentage. If this information is not present, written in your letter of accommodation by your physician, the disabilities benefits officers will calculate by default based on other information in your application, and usually they will calculate at the lowest possible of course. This is undoubtedly the reason why most applications fail.
For some conditions, there are standardized assessments, such as autism or ADHD, for which there are well established assessment tests/scales, often combined with other tests such as IQ and others to test deficiencies. In this case, providing the assessment scales results should be sufficient. Unfortunately there is no such widely recognized standardized assessment test for circadian rhythm disorders, hence the necessity of a letter of accommodation, which is a freeform way to describe the impacts of your condition(s) on your life.
If you have multiple conditions causing your disabilities, you should obviously get letter of accommodations/assessment scales results for all your conditions that impact your life if you have multiple ones, and provide them all in your disabilities benefits application. In an ideal world, the impact of non-24 should be better recognized, but for now it is often easier to get the severity of the often comorbid conditions better acknowledged.
In practice, some countries only require a medical letter of accommodations (eg, Belgium), while others also require a specific form to be filled (eg, France). I recommend to always ask for a letter of accommodations, because this is more versatile/reusable (ie, can also be sent to employers, school, gym, etc.), whereas the administrative form is very specific and not very reusable. Also, physicians are less likely to be terse and stingy when it's about letters of accommodations (you don't need to tell them it's for a disabilities benefits application), whereas when it's an application form they can be wary of the government (even if they aren't telling you)...
(NB: Most governments are quite hostile with doctors because they are the ones who can produce medical assessments opening lots of social benefits rights, so the governments often try to pressure doctors into not giving such assessments, eg, in France they investigate doctors who produce "too many" medical leaves, whatever "too many" means, so please keep this in mind, it's a very complex interplay of a blame game that physicians don't want to play but are required to...).
Once you have the medical assessments of your disabilities in written form, you then have to deal with your application. Try to meticulously read the criteria of the disabilities benefits office you want to apply to, understand their logic, and try to fill their application very exhaustively. The goal is not to recount your whole life, but to check boxes, and to check as many as possible. Also, they will assess how severe your disabilities are, ie, how much you are reduced qualitatively (what abilities) and quantitatively (what percent of the job market you can't access - basically how many days you can work fulltime or how many jobs in jobs listing you could access in your field of skills). This is why the written medical assessments are key, because you need to prove everything you declare in the application with the medical assessments, otherwise it's moot.
If it's written in the medical assessments, then they cannot do anything but accept. This does not necessarily means that you will get all the benefits you hope for, but they cannot contradict your medical assessments, so that's why, I say it again, your efforts should be put towards improving your medical documents, not the administrative application forms.
Finally, note that there are lots of disabilities benefits you can get. Most people think about the monthly financial allowance, but this requires a very high severity of disability usually, which is possible with non-24 but it depends on your medical assessments. There are also lots of other benefits you can get that require much less severity, such as disabled worker, reduced taxes, reduced fares for transpotation and museums, reduced prices for electricity, and all the benefits you can get at specific places that you usually interact with, such as your employer, school, gym, etc.
That's why when people say they are not getting any disability benefits at all with non-24, it is almost definitely because they do not know how it works, and unfortunately just are not getting the rights they should be getting. I guarantee it is impossible that you do not get any disabilities benefits at all if you have an adequately written medical assessment.
Also, it is irrelevant for disabilities recognition whether or not you work. As the example I used above already, an employed blind individual is still very disabled when they go to do any daily activities outdoors such as the groceries, so they need of course to be recognized as disabled and to get accommodations wherever they go, not just at work but also at the supermarket. Whether you work or not is only relevant for the monthly allowance, because this is intended to replace your loss of income. It is also worth noting that if you are paid with low wages, you may get a partial monthly allowance to compensate or to help with buying the necessary therapeutic tools or work adaptations. So it's not black or white, do you get disabilities money or not, it's more gradual than that, and the first step, which is not connected at all to money, is to get your disabilities recognized. Then, depending on the number of points/severity they grade/acknowledge, and your financial situation, you may get various financial helps. But that's a different step.
Whether you can get monthly allowances is not something I can guarantee because it depends on your specific situation (ie, how much your life is impacted) and how complete is your medical assessement (eg, what percentage of disability was estimated by you and the physician) and how lucky you are (in finding a listening and competent physician and interacting with competent disabilities benefits administrative officers), but you can certainly get a lot of disabilities benefits that will already make living with your disabilities less of a pain. Also, in a lot of countries, getting the monthly allowance depends on your current income, but you can first apply for disabilities recognition nevertheless, so you will get an assessment of your "disabilities points", and later when you lose your income, you can request your monthly allowances.
For example in Belgium I got the disabilities recognition. I got a lot of benefits (including reduced personal but also professional taxes if I start a company - this is extremely helpful for people with non-24 I think), but no monthly allowance because I am employed, but I got enough disabilities points that I can get the monthly allowance if I lose employment, I do not need to redo the whole process of disabilities recognition, just of requesting the monthly allowance. This may be different in other countries though.
-- Key Takeaways: Disability Benefits and Non-24
(Sub-section generated with ChatGPT-4o on 2024-11-25)
- Disabilities are not tied to specific conditions:
- A disability is defined by how your health condition limits your daily activities and abilities, not by the diagnosis itself.
- How the system works:
- The process involves proving:
2. Your disabilities: A clear medical assessment detailing what activities and abilities are affected, to what degree, and whether the impact is permanent.
- Importance of medical documentation:
- Quantifies the severity of the impact in percentages (e.g., % of workdays lost or % of life functions impaired).
- Administrative process and strategy:
- Understand the specific criteria and logic of your local disability benefits office. Fill out applications thoroughly, checking as many "disability boxes" as possible.
- Use simulations or guidelines from your local office to tailor your application to their assessment framework.
- Challenges in recognition of non-24:
- Co-occurring conditions often receive better recognition, so leverage those when applicable.
- Diverse benefits beyond monthly allowances:
- Discounts on transportation, utilities, or access to cultural sites.
- Workplace or educational accommodations.
- Strategies for long-term success:
- Critical advice for success:
- Understand the system: Research your local disability office’s criteria and processes.
- Apply for all applicable benefits: Even small benefits can improve quality of life.
— Conclusion
The primary reason people fail to secure disability benefits is failing to understand how the system works, especially how disabilities are recognized, and failing to provide adequate medical documentation. By focusing on how your condition impacts your daily life and ensuring your physician quantifies these impacts, you greatly improve your chances of success. Even if monthly allowances aren’t guaranteed, other benefits can significantly reduce the challenges of living with non-24.
Hopefully this will clear up the common misconceptions and help get the best chances for your disabilities benefits application.
Coping and accepting a chronic illness and invisible handicap
Certainly, one of the greatest challenges of acquiring, or discovering, a chronic illness such as non-24 and other circadian rhythm disorders is the necessity to learn how to cope and accept the disease and its chronicity (ie, that the disease is not curable and can only be partially managed with great efforts) and the invisible handicap this causes. Disease acceptance is a lengthy and grueling process spanning years if not decades. Acceptance is more formally called "normalizing" in the academic litterature.
The denial phase is very common and arguably the longest phase. Ignoring the limitations that are inherent to a disability is very different from acknowledging and working around the disability. The former, which is denial, is a sure way to crash into the wall at some point, whereas the latter, acceptance and understanding of the limitations, is the only viable long term solution that can allow to recover a better quality of life to some degree. One very important step to go out of the denial phase is to suppress wishful thinking, which is to believe that the illness can be overcome by willpower, which is a hard fallacy that bit everyone during the COVID-19 pandemic, and is a necessary step to pass through for all individuals with a chronic illness.
It is hence crucial to accept circadian rhythm disorders, and especially non-24, as a debilitating, severe invisible disability. Indeed, they bar from accessing virtually every work positions that exist, whether as employee or as a business owner, due to the severe disruptions they cause in social, cognitive and physical functions. The social disruptions are especially critical, as they cause a physical, logical incompatibility between the person with non24's sleep-wake schedule and the social expectations. Statistics estimate show that contrary to the naive assumptions, 80% of individuals with handicaps have an invisible handicap, whereas only 2% are in a wheelchair. Stigmatisation, as per the works of Erving Goffman, is the major problem individuals with handicaps, invisible or visible, face from society. The stigmatisation of being perceived as lazy is not specific to sleep disorders, but is a common societal mischaracterization of individuals with invisible handicaps, as they are peirceved as lacking the effort to simply willfully fix their handicap that causes them to be incomprehensibly unable to perform simple actions, in this case the ability to sleep at socially acceptable hours at night.
It is important to note that acceptance is here not meant in the usual, idealized form of accepting means that the individual is not affected by the disease anymore and everything gets better and positive. This is unfortunately a widespread ableist view of what acceptance is. Because the disease stays there, it is chronic, it likely does not ever get better for most. There is nothing that can make this soul crushing experience positive. Instead, realistic acceptance consists in accounting for the chronic illness, to acknowledge it really exists and that the individual has it, will likely keep it the rest of their lives, and to plan around it, instead of wishfully thinking it can be powered through via will, as the latter is a sure fire way to head straight into the wall. Acceptance really means to learn how to live with the chronic illness, not despite it, not via its strengths (as usually they provide none), but simply with it. It does not mean to be happy about it, just like accepting the death of a cherished one does not mean it will ever become a positive experience.
Rather than paraphrasing, we will extensively cite extracts from this impressively accurate and fairly exhaustive academic work: The Handbook of Social Studies in Health and Medicine, 2003, chapter Experiencing Chronic Illness, pp 277-292, ISBN: 0761942726, 9780761942726 :
> Chronically ill people seldom want to be invalids; they wish to be accepted as valid adults. Their self-doubts rise if other people imply that they wanted to get sick or harbor questionable motives for seeking care and claiming special needs: 'Are my symptoms real or all in my head?'. Their symptoms may be intermittent or gradually increase until they interfere with everyday life. The person cannot meet obligations, keep up with coworkers, maintain their households, or handle daily child care. Esoteric and invisible illnesses often prove elusive. Then, symptoms may become pronounced before they are recognized as such. Yet, ill people do delay seeking help if it poses risk of further loss. Social purposes rather than health needs take priority. People delay seeking help when they risk losing valued roles, responsibilities, and images of self. For example, a parent who resists relinquishing child-care duties may defer seeking help.
> Recognition of diminished function or inexplicable symptoms spurs a diagnostic search. Stewart and Sullivan (1982) found that patients with multiple sclerosis began their diagnostic search when they could no longer explain their symptoms. However, physicians and relatives typically did not affirm their symptoms as real until after diagnosis more than 2 years later. During this time, ill people live in 'diagnostic limbo' suspended in time. These patients often seek multiple physicians when their complains are discounted and dismissed. Discounting and dismissal also may occur after a problem has been defined as chronic but practitioners cannot ameliorate it, such as chronic back pain.
> Diagnostic shock follows an announcement of serious illness that shows up in testing -- cancer, multiple sclerosis, and diabetes -- before patients either note symptoms or grant them any significance. From the patient's viewpoint, diagnostic shock occurs without warning, such as during a routine physical. Part of the shock means having reality discomfirmed. Not only are the person's suppositions about his body shaken, but also to the extent that a diagnosis has foreboding meaning, prior reality is disconfirmed as this diagnosis is confirmed. Subsequently, prior identities are also disconfirmed. When people do not anticipate bad news, have little knowledge and few symptoms of their confirmed diagnosis, the disparity between diagnosis and self-concept is greatest. Then the person needs time, bodily experiences, social encounters, and self-definitions to redefine self and identity. Meanwhile, the diagnosis confirms being catapulted into a patient role. A new label, a new identity has been applied and given. Yet even the most dreaded and seemingly known diseases such as AIDS, leprosy, and cancer require learning what being ill means.
>
> Learning what Illness means
> In order to be ill, someone has to feel sick. Merely being informed that one has a disease seldom suffices. Until a person defines changes in bodily feeling or function, she may postpone dealing with a diagnosis, even a serious one, and subsequently ignore medical advice and regimen. Illness does not seem real. Then the person may cleam that the diagnosis is wrong, secondary, or inconsequential, and relations with practitioners suffer accordingly.
> People learn what illness is through their experience of it. Lessons in chronicity come in small everyday experiences such as difficulty in opening a can, bending over to pick up a newspaper, folding bedsheets, and weeding the garden. Comparisons with past effortless performance can be shocking. Such jolts later become measures explicitly sought and then assessed. A man with heart disease who used to stride across a golf course now shuffles half way across the company parking lot. A present reality jolt can be reinvoked as a future measure. Measures include time -- the person can only get through part of the work day, rest requirements become apparent to coworkers, fulfilling work standards takes hours or days longer. Indicators become measures when they are impossible to gloss over or to have someone else camouflage. A person may invoke measures, or other people may supply them. These measures can multiply and form a general standard against which to judge self.
> Historical, cultural, social, and situational contexts influence meanings of illness. Waxler (1981) argues that in every society, the sick person learns to take a role that society expects. [...]
Aparté: this progressive learning through experience of what a chronic illness is like is perfectly illustrated by the "spoon theory" by Christine Miserandino (although this is not a theory at all). This can also be used to explain chronic illnesses to external observers and relatives.
> Normalizing Illness and Regimen
> Normalizing illness and regimen means making them routine, and treating whatever changes and improvisations are created as ordinary. For some people, normalizing means letting past plans and projects go and scaling life down. For others, it means struggling with illness and regimen to make life manageable so a valued future is possible. In both cases, normalizing means adapting to the situation at hand. It also means proceeding with activities 'as if normal'. Normalizing means finding ways to minimize the impact of illness, disability, and regimen on daily life, including their visibility. It constitutes an attempt to contain illness to personal experience and not intrude upon interaction. Thus, chronically ill people cover up limitations and keep up normal appearances and activities. They normalize a certain amount of discomfort when they can still function in ordinary ways. Such strategies become hazardous if a person overextends his capacities and perhabs harms an already compromised body.
> However, when ill people normalize symptom control and regimen, they may increase their capacities and maintain their health. This kind of normalizing means making new routines the norm and the normal. What earlier seemed bizarre becomes customary and comfortable. [...] As innovations and changes become routine and accepted, they feel normal and allow the ill person to view the self as normal and they way of living now as natural.
> Normalization reduces disruption. It softens the impact of frailty and disability. Through normalizing, ill people take their way of being and the changes they have endured for granted. As their lives become more restricted, their world shrivels, frame of reference shrinks, and self contracts.
>
> Illness Management Strategies
> Chronically ill people learn ways to handle their physical symptoms through various strategies ranging from withdrawal to innovation. Strategies for managing illness also require strategies for effective negotiations. People in lengthy marriages make managing illness a coupled affair. Visible disability drives other adult relatives away. What people need to manage depends on their illness, its progression, and its meaning to them, as well as their situation and their responsibilities.
> [...] Younger and middle-aged people often make concerted efforts to manage their illness. They maintain hopes and plans, reasons, and responsibilities. They have not given up or given in. They become innovators. To do so they listen to their bodies and stay in tune with them in ways that they had not and in ways that Western culture discourages. They make use of indigenous support groups, newsletters, and computer networks independent of professionals. The groups and methods provide collective information and shared community. They may constitute the only community for people who have become isolated in their homes. Members compare stories, gain information, learn about treatment successes and failures, and offer encouragement to continue to struggle with illness and not to sink into invalidism. They may keep daily logs to refine and extend data for working with their professionals.
> Shared comparison give support group members measures of where and who they are now. Certain chronic conditions such as kidney failure and treatment programs such as cardiac rehabilitation bring people into sustained contact with others with similar problems. A collective spirit may develop in these situations that either supports patients remaining involved in prior pursuits, or confirms that the world of illness now dominates their lives.
> Some chronically ill people become so adept at monitoring and managing their illness that they break through textbook definitions, create individualized regimens, and construct new ways of living with their illness; but medical professionals may not welcome their innovations. Alonzo Plough (1986) argues that patients who know too much use medical terms and request specific treatments that anger their practitioners. Practitioners sometimes push these patients back into the sick role when challenged by their growing expertise. Chronically ill patients sometimes find that their practitioners hold an ambivalent stance toward them. Their practitioners want them to take responsibility for themselves but on professionals' terms, not on their own. When these ill people step outside or beyond medical authority, their practitioners resort to medical paternalism and authoritarian demands. Consequently, ill people's strategies for managing illness can require strategies for effective negotiations with professionals to minimize conflict. [...]
>
> Stigma and Stigma Control
> Experiencing stigma is a common consequence of chronic illness and a constant threat in some ill individuals' view. If so, stigma makes a person vulnerable to negative social identifications and self-definitions. Stigma results from being identified as flowed, discredited, or spoiled. A defined difference from ordinary peers separates a person and confers an actual or potentially devalued identity. That difference often becomes a master status, such as 'disabled person,' 'leper', or 'AIDS victim,' that floods all statuses and identities. The stigmatizing label defines the person and every other defining characteristic she possesses. Thus, a woman who uses a wheelchair because of multiple sclerosis becomes a disabled mother, handicapped driver, disabled worker, and wheelchair dancer. [...] Often other people dissociate the 'understandable' reason for an ill person's difference from his behavior eliciting the stigmatized response. Then blame is turned back upon this person, who is made morally culpable for the stigmatized response itself. In essence, the individual is blamed for the behavior and blamed again for being stigmatized for it. [...]
> Any illness that sets a person apart as different and diminished has stigma potential and thus can affect interaction. The following characteristics increase stigma potential: a high incidence within disparaged groups, compromised adult status, loss of bodily control, sexual transmission, possible pollution, odor, and uncleanliness. [...]
> Davis (1963) argues that efforts toward prior identity preservation fail in direct proportion to the degree and extent of visible disability. Both enacted and felt stigma contribute to difficulties in preserving prior identity. The disability rights movement has made significant recent changes in the lives of its proponents. However, many ill people still find themselves responsible for preserving or reconstructing their identity after losses -- whether their disability is visible or invisible. Concealment of an invisible but potentially stigmatizing mark of difference allows the person to preserve prior identities for a time and under specific conditions. Many disabilities do not remain completely invisible to a discerning observer. Partners or parents may perceive cues more readily than a professional who does not have steady contact. Fatigue, flare-ups, or distress may render symptoms visible. [...]
> When invisible disability undermines fundamental ways of defining self, the person is isolated, and social comparisons are not possible. Then coherence and stability of self-concept is at risk.
>
> Self and Social Identity
> Stigma can wreak havoc upon the self for it forces unwelcome new ways of conceiving self and situation. Still, serious chronic illness alone can render social identity and self problematic. For months and years, people may try to forestall illness from touching the self. Valued roles and pursuits preserve continuity and coherence of self. People may acknowledge that illness affects their lives but resist its effects upon the self. They conceptualize it as a 'condition, not an illness,' 'just aging,' or 'a spell' and therefore maintain a sense of continuity and coherence of self. They put it into the past by saying they 'had cancer' or 'had lupus' and decree that it will remain in the past. [...]
> People with serious chronic illnesses must repeatedly rethink how they live and who they are becoming. Self and social identities are intertwined in daily actions and endeavors. Chronically ill people seek to reestablish their legitimacy after disruption and devaluation makes them vulnerable. However, they may not go about it in ways of which their practitioners and families approve. As life narrows, the ingredients shrivel for constructing a valued self and legitimate social identity. Their quality of life becomes problematic. Social, economic and psychological resources expand possibilities and options rapidly contract. Using available resources may be fraught with risks and increase vulnerability. Taking sick leave can result in increased scrutiny of an employee's performance. Filing an insurance claim might contribute to raising the business's group insurance rates. Sociale resources mean that commitments, assistance, and back-up are available -- as long as caregivers do not wear out. Concrete assistance smoothes problems and reduces anxieties. Commitments keep the ill person within a web of relationships -- from commitments that permit returning to work to commitments to visit or to run errands. Economic resources allow an individual to purchase objects and services that make life easier -- a car with an automatic shift, a one-story home, household help. The more resources available, the more latitude the person has to take time-outs for illness and then return to earlier pursuits. Identity questions and change of self are muted or occur over long periods of time. As resources dwindle, identity questions and changes of self may be forced much earlier.
Aparté: According to studies, disabled americans need to spend on average 30% more than their non disabled counterparts. This represents about $17K/year.
> Experiencing chronic illness can mean embarking on an odyssey apart from the busyness of other adults' lives. Chronic illness separates the person from the social body, but also gives rise to a story that brings this individual back to reintegrate self on a different level. Someone may leave old identities behind but gain deeper meanings. Long stretches of time allow the person to reflect upon jarring images of self and to make sense of loss. Loss of self and social identity do comprise a fundamental form of suffering among chronically ill people. Still, they may come to believe that facing such losses moves them toward trascending loss. Earlier vulnerability becomes a source of strength as they redefine illness as a time for reflection, reassessment, and redirection.
The reading of the rest of this chapter is highly recommended.
Modernity brought forth a generalized rejection of deities, replacing it with a pervasive and even insidious belief of a meritocracy: those who work hard will reap the fruits of their efforts. But this belief gets logically paired with its inverse: those who struggle or fail are responsible for their own demise. Luck, or the lack of, is no longer considered a factor. Pushed to the extremes, this forms the foundation of the "Just World" hypothesis, whereby this assumption is generalized to be a rule of Nature, and hence applying universally, whereas the universe is fundamentally unjust, unfair and uncaring of humans affairs. For more thoughts on this topic, this excellent talk by Alain de Botton is highly recommended.
A few additional practical tips by the present document's author:
- Learn to recognize the effects of sleep deprivation and circadian misalignment, and discriminate what is caused by them (and hence by the non24 disorder) from the rest. By recognizing these effects on your health, it's possible to 1) better manage them (either by waiting out or by doing tasks that do not require high functioning and avoid affecting others with one's mood dysregulations — but it's not always possible to manage), 2) not feel guilty or shame for simply being ill, which can significantly decrease the risks of depression, as the illness's "episodes" just become a hassle rather than a pit of despair. If you don't know what are the symptoms, look up jetlag which is the most accurate model, or alcohol hang over since sleep deprivation is similar to alcohol intoxication.
- Accept that it's likely not possible to fully control the effect of non24 on one's health. Even with the most effective therapies currently available, unproductive days with low mood due to sleep loss for various reasons will still happen regularly. They are part of the disease. Therapies can reduce their frequency, but they will still be relatively frequent (more for non24ers with an extremely long circadian period).
- Accept that non24, just like any other disability, requires accommodations and prevents from doing some types of jobs. For example, it's unrealistic with non24 to pursue a commercial work position with clients contact, as a strict 9-5 work schedule is required. There are only very few jobs that are adequate for non24, but there are jobs that are sure ways to fail and damage health.
- Although you may improve your condition a lot with adequate treatments and tools, you must understand and accept that there is no way back to your idealized future. It is impossible to be permanently cured from this chronic illness, and you will have to learn with it, and prioritize what matters to you and what you can do versus what you wished you could do.
Addendum: at some point, you may have a period when you identify yourself with your illness, that it defines you. Although such a chronic illness can certainly explain a lot of previously misunderstood "habits" that were mere consequences of the illness, it does not define you. Your abilities, your passions, your goals do not revolve around the illness, although it is certainly a limiting factor.
Addendum2: it's ludicrous to claim that acceptance of the non24 disorder and follow the natural sleep-wake pattern is acceptable. It drastically impairs quality of life, it is a very invasive disease that disables from doing everuday activies and essential life stages such as raising kids. Of course, without an effective entrainment therapy, there is no acceptable alternative to just following it, and acceptance involves accepting that the disabilities created by the non24 disorder. But chronic disorders induced disabilities do not get any better by acceptance. Hence, "accepting your disorder" is a fallacious mantra. Accepting the limitations and accommodations necessary around the disability is a necessary step, but the idea that accepting the disability will make everything work somehow is ludicrous. Without a stable entrainment, at least either social life, family care or your health will have to be compromised, often all will be in an attempt to juggle between them, and that's considering the best case scenario where your work position is not impacted. But since robustly stable entrainment is not currently achievable, only imperfect solutions are currently available, and hence sleeping in circadian alignment remains a much healthier and viable solution to working and sleeping in circadian misalignment and chronic sleep deprivation.
Additional reading materials on this topic of normalizing are available here, here, here, here and here. Also, the book Sleep Misfits: The reality of Delayed Sleep Phase Syndrome & Non-24 by Sally Cat is highly recommended, being the only book currently written compiling the experience specific to patients living with non-24 and DSPD handicaps.
For relatives and loved ones of people with a chronic illness such as non-24, here are some helpful resources:
- https://www.urevolution.com/loving-someone-with-a-disability/
- https://www.healthline.com/health/chronic-illness-relationships
- Family planning decisions for parents of children with a rare genetic condition: A scoping review, 2017 https://doi.org/10.1016/j.srhc.2017.08.001
Zeitgebers - The circadian rhythm manipulators
Introduction to zeitgebers
What is the circadian rhythm(s)?
The circadian rhythm is an essential biological process that not only regulates sleep but also wakefulness, its purpose is to be in phase with the environment to improve the chances of survival.
Before the circadian rhythm appears, fetuses display an innate, pre-existing ultradian cycle, which alternates wakefulness phases with sleep phases about every 45min, but there is some evidence that fetuses start developing the groundwork for an immature circadian rhythm based on their mother's rythmic secretion of hormones such as melatonin. The pupillary light reflex, which is controlled by the melanopsin photopigment in the eyes' ipRGC cells, the same pigment that controls circadian rhythm shifting (see also here), appears between 6-7 months (30-34 weeks) postmenstrual age (ie, from pregnancy onset, hence age includes fetuses or in preterm infants). The appearance of this reflex demonstrates that fetuses and preterm infants already have a functioning photic non-visual system, ie, that their circadian rhythm and hormonal system can be affected by bright light zeitgeber exposure. Indeed, genetically muting the melanopsin pigment severely impairs both the pupillary light reflex and circadian alignment, and pupil area is proportional to melatonin suppression. Hence, contrary to common assumptions even among sleep specialists, current evidence support the notion that newborns are already responsive to bright light exposure, but not necessarily like adults, since they do not yet have a circadian rhythm.
Newborns continue to sleep exclusively on their ultradian rhythm according to current findings, despite the pupillary light reflex being established, arguably because of light being red-pass filtered by eyelids since 30-98% of environmental light was incident on the eyelids (ie, newborns often have their eyes closed), until they transition into a circadian rhythm very early in the life, the maturation, stabilization and synchronization of the circadian rhythm to the objective day-night cycle happening around 3 months after birth in most babies, although some newborns can have a mature circadian rhythm earlier at 1.5 months especially with early exposure to a rigorous day-night pattern of sunlight and limited to no artificial bright light at night (dark therapy), while others may need a few more weeks (and bear in mind that most babies don't sleep the whole night like adults at this age, they just sleep more often at night than during the day, but still wake up very often according to the ultradian cycles transitions), potentially helped by maternal milk's hormones such as melatonin (night milk) and cortisol (day milk), although the vast majority of artificial milk fed babies also develop a circadian rhythm around the same time as breastfed babies, if not earlier. A fantastic case study of a newborn from the 2nd week of age up to 6 months old elucidated the timeline of appearance of the various components of the circadian rhythm:
> The circadian rhythm of temperature appeared first, soon after birth, and became statistically significant within one week. The wake circadian rhythm appeared second, attaining significance at day 45; approximately the same time that increased melatonin concentration began to occur at sunset. The sleep circadian rhythm appeared last, attaining significance after day 56. Ninety to 120 minute zones of sustained wakefulness first appeared in the second month of life subsequent to awakening and prior to sleep onset. The infant's nocturnal sleep-onset was coupled to sunset before day 60 and subsequently to family bedtime, giving evidence of initial photic entrainment followed by social entrainment. Conclusions: Circadian rhythms appeared much more rapidly in this infant than previously reported; their rapid appearance was probably facilitated by maximal exposure to sunlight, and regular social cues. These lighting conditions replicate universal infant experience prior to the invention of artificial light.
(NB: social interactions have since been disproven as zeitgebers, they do not entrain the circadian rhythm).
This single-case study has since been partially reproduced in a cohort study of 130 full-term infants, demonstrating that cortisol circadian rhythm, cortisol being the hormone of wakefulness, is established at age 1 month and remains throughout life.
Given that body temperature circadian rhythm appears within one week, the time delay it takes for this primordial rhythm to get established may be dependent on when the newborn starts to keep their eyelids open more often than closed, one hypothesis being that the pupillary light reflex and hence the circadian rhythm system coupling with bright light is already established at or before birth, and hence that the only factor to entrain a newborn's circadian rhythm is to expose their eyes to bright light without the red pass filter of the closed eyelids, although previous studies found that medium exposure (1h) to bright light even with eyelids closed can inhibit melatonin in adults, hence future studies may elucidate this point.
The endogenous (ie, baby's) production of sleep-wake regulation hormones such as melatonin and cortisol appears later around the 8th month of life (see also here), although there are conflicting evidence such as the case study above which found an endogenous variation in melatonin as early as 45 days along with the appearance of the wake circadian rhythm. The pineal gland size and melatonin secretion level stabilize around 1 year of age. Circadian sleep patterns during the first two years in life, and later well into adulthood as well, are strongly influenced by genetic factors. The circadian rhythm is a universal biological process observed in all living organisms, from mammals to plants and even bacteria, mitochondria and unicellular organisms, strongly suggesting this is a crucial trait for survival since it was highly conserved throughout evolution.

Sleep graph from a sleep diary of a baby from 3 months old to 17 months, collected by u/jitney86. This shows how the baby's sleep transitions from a somewhat irregular sleep pattern but with already clear night block, into a highly regular biphasic or triphasic sleep pattern. Note this child was breastfed, which is known to slow down the stabilization of the sleep-wake pattern.

Another sleep graph for an even younger infant from 3 days old to 3 months old, showing a completely irregular sleep pattern at first, then slowly transitioning to a more regularly patterned sleep, with a big block of sleep at night and smaller naps blocks during the day. By u/AtmosChemist.
Although in the literature the "circadian rhythm" is commonly employed in the singular form, there are in fact multiple independent but synchronizable molecular clocks throughout the body and down to every cells (see also here), with each organ having its own clock and time of peak performance, including different brain regions (see also this talk by the same author), and this molecular circadian machinery was observed in all mammals. The circadian system is structured as a loop-on-loop architecture with feedbacks, which both ensures persistence of rhythmicity and local adaptations depending on metabolic needs.
The body has 2 major biological processes to regulate sleep, according to the now well demonstrated empirically Borbély's model:
- the homeostatic sleep pressure process S that relies on adenosine. The homeostatic process S is more like a countdown timer, which tracks how long the individual stayed awake and continues to build up without any limit as long as they stay awake. When this timer reaches beyond a threshold, which means the individual stayed awake for a long period of time, it ensures that individual will feel sleepy enough to get necessary sleep to avoid the risk of dying by prolonged sleep deprivation. In other words, the homeostatic process S is like a failsafe mechanism in case the individual didn't sleep during their circadian night. The more sleep pressure, the more slow wave deep sleep during the sleep session after, without affecting the core body temperature. The process S was discovered in 1984 by Borbély's team.
- the circadian rhythm process C, most correlated with melatonin although it is more tightly coupled with core body temperature which seems to be the core method circadian rhythm phase changes are propagated throughout all cells in the body. The circadian rhythm (process C) is periodic, it doesn't account if the individual stayed awake for way too long, it's like a 24h clock defining different periods: one of activity (circadian day), one of sleep and rest (circadian night) and transition periods in-between, and it always repeats itself periodically no matter if the individual stayed awake or was asleep. This is the reason why if the individual stay awake beyond the circadian night, they will paradoxically feel less fatigued than during the circadian night, as although the sleep pressure of homeostatic process S will still build up, the pressure of the circadian rhythm will lift off. The circadian rhythm is what maintains all living organisms asleep for long periods of time. For example, if the individual wakes up only after sleeping a short period of time, it means they were sleeping in circadian misalignment, outside of their circadian night, so that their body didn't have the support of the circadian rhythm to maintain them asleep, and so they essentially napped.
Although these are two different systems, they do interact with each other (see also here and here and here and here and here). So using a treatment that affects one will also often affect the other (eg, both light therapy and melatonin have been shown to affect both processes). See this video by Thoughty2 for a nice introduction. It is currently accepted that the effect of each system is minimal on the other, with a lab-controlled study demonstrating that the sleep homeostat S does not alter core body temperature and hence nor the circadian rhythm. Other studies found no effect of the homeostatic process on the circadian rhythm at all when observing the effect of napping versus prolonged sleep deprivation in humans. However, this assertion of independence between these two systems must be tempered by the recent finding from a study screening for molecular circadian shifting molecules which found that adenosine analogs such as cordycepin were the most potent by far. Future studies will hopefully elucidate the conditions of this inter-systems interaction.
Neurologically, although research is still ongoing in this area, it appears that the association cortices, especially in the frontoparietal attention network, are particularly sensitive to sleep pressure, whereas the subcortical thalamic and basal ganglia brain regions are more affected by the circadian rhythm.
Given that the sleep process S has little effect over the circadian rhythm process C apart from amplifying sleep induction (aka sleepiness) when both have aligned troughs, we will mostly focus on the circadian rhythm process C in the rest of this document.
The circadian rhythm is primarily governed by subpopulations of neuronal oscillators in the suprachiasmatic nucleus (SCN), with different subpopulations encoding different times of the day (eg, morning, afternoon, evening, night).
Although the SCN is considered the "master clock" as it synchronizes all other, peripheral clocks throughout all organs and cells in the body, it is not necessary for the circadian rhythm to be expressed. Indeed, astrocytes (cells that feed neurons) arguably play an important role (see also here), and circadian shifting by bright light is not impacted by the destruction of the SCN, with another study showing that the ipRGC cells in the eyes alone were sufficient for circadian rhythm and body temperature shifting without needing the SCN, showing that the non-visual effect of light on the circadian rhythm is independent from the SCN.
One of the major established pathways for the SCN's control of the circadian rhythm is by the cyclical activation of secretion of melatonin by the pineal gland via the retinohypothalamic-pineal (RHP) axis's , a "vestigial eye" that is highly conserved throughout evolution. In some reptiles and amphibians, the pineal gland is even directly visible, forming its own "pineal eye" or "parietal eye" colloquially named a "third eye" smaller than retinal eyes, but it is absent in mammals, endothermic archosaurs such as birds and ectothermic archosaurs such as crocodilians and turtles. However, the vast majority of melatonin is produced outside the brain, with the gastrointestinal tract secreting 2 orders of magnitude (at least 400x!) more melatonin in response to food than the SCN. Furthermore, even after pinealectomy, melatonin levels still increase in a dose-dependent manner (ie, proportionally) to oral intakes of melatonin in animals, just like for animals with their pineal gland, and it even restores an entrained circadian rhythm, showing that extrapineal producers of melatonin, likely the digestive tract, are producing and managing most of the circulating melatonin, not the pineal gland.
Hence, it appears from recent empirical evidence that both the pineal gland and the SCN, the two major brain structures related to circadian rhythm modulation, are not required for circadian rhythm modulation and entrainment.
However, there is one biological phenomenon that is tightly coupled, almost indissociable from the circadian rhythm: the core body temperature. Indeed, core body temperature modulation is the primary way that circadian rhythm changes are signalled across all cellular clocks throughout the body, with the circadian clock and the cell cycles being coupled, which demonstrates that core body temperature modulation is a strong tool the body uses to control cells activity, not just their circadian clock. Humans are homeothermic animals, hence core body temperature plays a crucial role in survival, and it is hence maximally internally regulated and shielded as much as possible from ambient factors. Likewise, the effect of melatonin on the circadian rhythm is mediated by its effect on core body temperature (as suspected since at least 2007), with melatonin being an inhibitor fo temperature (see also here). It hence appears that what matters to control the circadian rhythm is the modulation of core body temperature, the various structures such as the SCN and the pineal gland acting as main controllers, but they are not the only ones that can affect the circadian rhythms of various clocks.
---
TODO: add figure and rewrite section below to be more intelligible:
There are rules I derived for how much we can sleep in and out of circadian alignment and per 24h. I am still refining them but essentially, you usually can't sleep more than your ideal amount of sleep. For example, on average adult humans need to sleep 7-9h per night, let's say 8h to simplify. This means, if you fit in this average, that you need ideally to sleep 8h per night to feel fully restored and be healthy.
Now, if you sleep twice per day, eg during your circadian night and during the siesta, you will only be able to sleep a cumulative sum of 8h, for example 5h during your circadian night and 2-3h during the siesta. This is called a biphasic sleep pattern. This can even be a triphasic pattern for young children. This is in line with previous studies finding that children who take daytime naps actually have the same total sleep duration as children who don't.
So if you want to sleep a full night at once, you need to avoid the siesta in the 24h preceding the circadian night. But this only works if you can avoid using an alarm clock, and if you know when is your circadian night. Otherwise, it's much healthier to sleep the siesta (biphasic sleep), it's not for nothing that the body devised this failsafe mechanism.
Another rule is that it's only possible to sleep a full night of sleep during the circadian night. The siesta is limited to 1-2 ultradian cycles shorter than the circadian night. For example, if your ideal sleep duration is 8h, 1-2 ultradian cycle less represent 1.5 to 3h less, in other words 5h is the maximum duration of the siesta (ie, the longest nap that you can do). You will never be able to sleep more outside of the circadian night, unless you have a huge sleep pressure buildup (ie, you didn't sleep for 30h+) or acquired an illness that modifies your core body temperature.
Example of how the interplay between the circadian rhythm C and homeostatic process S can produce different sleep-wake patterns:
6 possible scenarios:
- Monophasic in phase and woke up more than 10h before. Will sleep at maximum the optimal duration (eg, 8h on average for adults) and wake up at the natural wake up time or before when optimal duration is reached. Can only sleep 1 or 2 ultradian cycles earlier than optimal fall asleep time. Can sleep less if slept too late and natural wake up time happens before optimal duration (eg, 6h of sleep = 1 ultradian cycle less).
- Monophasic out of phase woke up 10h before (will sleep max 5h).
- Monophasic in phase woke up less than 10h before. Low homeostatic sleep pressure will make it difficult to fall asleep, but if it happens then the circadian rhythm will maintain the body asleep and it should be possible to sleep a full night if in alignment with the circadian night.
- Biphasic idem 3 item. Then will sleep optimal duration - amount of time slept in the last 10h (eg, if napped for 2h, then can only sleep 6h during the circadian night).
In phase 8h max. Out of phase, 5h30 max incl nap in biphasic, it's the sum over 24h (or freerunning period eg 25h for non-24). We assume no prior sleep deprivation, which could result in increased homeostatic sleep pressure and chaotic circadian rhythm which could make sleep significantly longer or paradoxically shorter.
If we assume the current circadian night is from 2am to 10am:
- if woke up at 3pm, can sleep in phase at 2am, 8h. Wake up around 10am.
- can sleep out of phase 5.5h.
- can sleep partially out of phase between 6am and 8am, resulting in at least one ultradian cycle in phase and hence between 2 and 4h of optimal in phase sleep + 4 or 2h of out of phase sleep respectively totaling about 6 to 7h of sleep. Wake up around 1pm (if slept at 6am) to 2pm (if slept at 8am, we sleep less if sleeping later because more out of phase).
- can sleep biphasic, 2h to 4h before 10pm, then 4h anytime between 2am and 10am, totaling close to 8h (sometimes more up to 9.5h).
- can sleep completely out of phase, 2h before 2am and 3.5h after 10am, totalling 5h30 of sleep.
If woke up at 6am:
- can only sleep 6h from 4am to 10am.
What are zeitgebers?
Zeitgeber literally means "time giver" in german. A zeitgeber is any periodic signal of about 24h (ie, a cycling signal, such as light-dark), which the body can pick up and use to get entrained to (ie, synchronize like a clock to a 24h schedule). Since the circadian rhythm regulates crucial biological processes for health and survival, the periodic signal needs to be reliable (ie, not random) to be a zeitgeber and entrain the circadian rhythm.
Indeed, humans, and all biological systems, have no way to determine exactly the absolute time. Hence, we have biological, chemical and hormonal systems that approximate what can be called a biological clock. But since it's an approximation, it is imperfect: on average, humans follow a 24.2h schedule naturally according to the NIH. The remainder, 0.2h, serves as a margin of error, which is eliminated thanks to external time cues: the zeitgebers. When this margin of error is correctly eliminated, we say that the individual is entrained on a 24h schedule.
Why is the biological clock approximative? Cannot it just run on exactly 24h like mechanical clocks? This is because of synchronization theory. There is no way to design two independent clocks (eg, such as the human biological clock and day-night cycle) that will forever stay synchronized together. Even mechanical clocks end up out of sync after a long enough period of time without being reset manually by the user or automatically by an online system. Indeed, the only way for two clocks (or periodic events) to become and stay synchronized is to link them, to make them dependent with the other in some way, as synchronization theory teaches us.
For example, if you place two metronomes on the same physical support, they will at some point become synchronized with one another. Zeitgebers are the link, the physical support that links our biological clock with the external world, with the sun caused day-night cycle. Without zeitgebers, it would be impossible to be synchronized with the day-night cycle, our clock would just be running on its own time, which is exactly what individuals with non-24 do. Furthermore, since there is no way to design two independent physical clocks with the exact same period, it is hard to see how this could be achievable by biological systems with innumerable SCN neurons with each their own phase and period and hence molecular clock, not to mention peripheral clocks in other organs throughout the body beyond the brain down to each biological cell, and so logically these independent biological clocks must synchronize together, and also with the environmental cycles using external cues, the zeitgebers. And indeed, studies demonstrated that the circadian rhythm emerges as the interaction of populations of clock neurons in the SCN, and in the body (peripheral clocks), with this interaction being tweaked by zeitgebers such as photic inputs (ie, bright light exposure).
Hence, zeitgebers are the essential tool we have to modify the circadian rhythm of humans, and hence potentially to treat circadian rhythm disorders. When something alters the circadian rhythm, it is a zeitgeber (ie, a time cue that alters/synchronizes the biological clock(s)) by definition.
Humans are biologically predetermined to respond more or less intensely to some zeitgebers, which includes the influence on their circadian rhythms. According to the current scientific literature, here is a rough outline of the order of power of zeitgebers on the circadian rhythm:
Light (strongest) > melatonin (strong) ~ food (likely strong) >> exercise (weak) ~ social interactions (weak) ~ sleep behavior (weak)
Light is the strongest zeitgeber by far. Indeed, we have specific photoreceptors, the ipRGC cells, in the eyes which are tailored to react to light to both dilate or contract the pupils and to shift the circadian rhythm. Light is undoubtedly the most potent zeitgeber both for the central clock (SCN in the brain) but also for all peripheral clocks of all the organs throughout the body, and hence bright light is the most potent therapeutic tool for circadian rhythm disorders. Light directly alters core body temperature. Light is also likely the first zeitgeber that the human brain's circadian system is programmed to react to, since there is evidence that ipRGC cells are already mature already at 6-7 months (30-34 weeks) postmenstrual age as shown by the appearance of the pupillary light reflex, which is controlled by the melanopsin photopigment in the eyes' ipRGC cells, the same pigment that controls circadian rhythm shifting (see also here). Note however that it's not just bright light that resets the circadian rhythm phase, but the alternance between high phases and low phases, here the alternance between bright light and darkness, as studies using the constant routine protocol demonstrated that individuals under constant bright light exposure lose entrainment and freerun just like individuals under constant darkness. The alternance between light color (blue versus yellow) also serves as a failsafe mechanism to entrain when light intensity doesn't vary.
Melatonin comes second, as its purpose is to both consolidate sleep and the circadian rhythm (ie, ensure you don't wake up in the second half of your night so you can sleep a full night), and phase advance.
Food is still under research but since the digestive tract produces most melatonin in the body by far, 2 orders of magnitude more than the brain, and also consumes it, food is thus arguably an also very strong zeitgeber. Food is thus an additional source of variation in circadian rhythm.
Other potential zeitgebers such as physical exercise or social interactions are very weak zeitgebers and named zeitnehmer ("time taker" in german) by some authors. Zeitnehmers are weaker than zeitgebers as although they can send rhythmical signals that can help with entrainment, they are also majorly affected by other oscillators through feedback loops. Hence, including them in an entrainment therapy leads to issues as they are often tricky to time appropriately (eg, exercise is more in phase with the circadian rhythm when done in the evening than the morning) and don't provide much circadian shifting or entrainment effects.
Physical exercise produces slight circadian rhythm shifts thanks to muscle contraction which modulates the BMAL1 clock gene in the muscle. The more muscle contraction, the more phase advance is obtained. Although exercise can slightly shift the circadian rhythm (see also here), it does not affect melatonin levels. Furthermore, a systematic review of 23 studies has shown that evening exercise does not affect sleep if done at least 1h before sleep, it instead helps people fall asleep and spend more time in deep sleep, the only exception being high-intensity exercise done under 1h of bedtime, which then caused people to take more time to fall asleep. Hence if you are night-walking (awake during the night and sleeping during the day), it's perfectly fine and actually healthy to do sports during the night, as long as your exercise session is at least 1h before your biological night. The precise effect of physical exercise on the circadian rhythm depends on the minimal core body temperature (CBTmin - can be approximated with wrist skin temperature too):
> When exercise was performed in the period between 4 h before and 1 h after the temperature minimum, there was a phase delay of 1.03 +/- 0.78 h (mean +/- s; n = 6); when performed between 3 and 8 h after the temperature minimum, there was a phase advance of 1.07 +/- 1.23 h (n = 9). [...] Performed at other times, exercise had no significant effect on the phase of the temperature rhythm.
Accordingly, a 2019 systematic review of physical exercise as a therapy for insomnia found only weak objective improvements in sleep parameters, mostly in reduced sleep latency (time to fall asleep), which rather suggests an effect on the sleep homeostat rather than the circadian rhythm, as otherwise it would be the wake up time (dawn marker) that would be modified, since SCN neurons in diurnal animals encode circadian phase in relation to dawn, not dusk like nocturnal animals. Other studies on cohorts found no evidence that physical exercise impaired nor improved sleep, even when done close to bedtime or at night. However, the effect of physical exercise on the circadian appears to be additive with other zeitgebers such as bright light therapy, so they can be combined. Furthermore, an analysis of the PRC curve of physical exercise relatively to the DLMO suggests that exercising in the circadian morning phase advances the phase, whereas exercising in the circadian evening or night delays the phase, which explains why individuals with DSPD who forcefully wake up early to exercise may in fact be delaying their circadian rhythm by exercising during their circadian night. Also, the change in blood pressure due to exercise is greater in the circadian evening than in the circadian morning, which suggests that it may be safer for individuals with DSPD to exercise in the objective evening if this aligns with their circadian morning, instead of the objective morning which may align with their circadian evening or night.
More likely, all potential circadian phase shifting effects of physical exercise are either local (ie, changes clocks in muscle tissues but not the central clock in the SCN), or their effect can only be observed in constant conditions experiments, as otherwise light exposure overrides any small effect obtained from physical exercise. Indeed, a study on mice with a LDLD schedule, which is a way to entrain them to 2 small days in one, found that when physical exercise was prevented during one of the small days by wheel locking, only the intensity of physical increased during the next activity period, but not the phase/timing, which appears to be only defined by the circadian rhythm and not activity/physical exercise.
Social interactions were previously considered to the primary zeitgeber for humans since a 1971 study by Aschoff. However, these results were debated, and it was finally found that this study and others were confounded with uncontrolled light exposure amounts and patterns and furthermore later studies controlling for these factors could not reproduce the effect, so that the current consensus is that social interactions are not zeitgebers, the previously observed effects being due to uncontrolled light therapy:
> Early human entrainment studies led to the belief that the primary entraining agent for humans was not light, but rather social interaction (Wever, 1979; Aschoff & Wever, 1981). However, due to concerns about the design of these experiments (primarily the use of self‐selected lighting schedules) and the subsequent demonstration that light cycles indeed can entrain human circadian rhythms (Czeisler et al. 1981; Wever et al. 1983; Honma et al. 1987a), this belief is now considered unwarranted (Czeisler, 1995).
Nowadays, the well established high prevalence of the non-24 disorder in blind individuals, "in spite of living with strong social cues (e.g., employment, families, alarm clocks and guide dogs) [...] strongly supports the primary role of light in human entrainment" and is another empirical evidence in humans that social cues are not zeitgebers.
It should be noted that social factors can nevertheless affect sleep-wake patterns as demonstrated by the well established social jet lag phenomenon, which is important to distinguish from the inexistent effect on the circadian rhythm, since modifying the sleep-wake pattern does not affect the circadian rhythm.
What about sleep itself (ie, the sleep-wake schedule, which is to schedule a rigorous sleep and wake time)? Past studies found that the sleep-wake schedule may feed back to the circadian rhythm but weakly, and hence more likely qualifiable as a zeitnehmer. However, this and other past studies were biased by uncontrolled factors such as posture, as the participants are usually told to lay down to sleep at the same time as lights are switched off, with posture decreasing core body temperature for at least 2h and lights off also increasing melatonin levels and hence decreasing core body temperature. Indeed, later more stringently controlled studies found no effect: a an excellently designed lab-controlled study showed no effect of sleep (naps) and sleep pressure (complete sleep deprivation) on the core body temperature and hence the circadian rhythm of humans. On the other hand, they confirmed that the core body temperature and distal skin temperature showed great coupling with circadian factors such as the lighting pattern. Likewise, sleep deprivation did not demonstrate any incidence on core body temperature whether in a comfortable ambient temperature nor after cold air exposure (see also here and here). Note however that sleep deprivation does mask proximal skin temperature, but not core body temperature.. In other words, the sleep schedule does not affect at all the circadian rhythm, hence why chronotherapies and cognitive behavioral therapies (CBT) have shown low to no efficacy so far. In fact, it was already observed since 1987 that locomotor activity nor sleep had any effect on the circadian rhythm.
Relatively recently, it was discovered that the lunar cycle influences humans' sleep and melatonin rhythms, with a reduction of 30% of deep sleep during full moon, according to a well designed in-lab controlled human study.
Since we have multiple clocks throughout the body and down to every cells (see also here), we ideally need to entrain a maximum of them for successful robust entrainment. The VLiDACMel protocol attempts to do that with a combination of light/dark therapy, melatonin and food (composition and timing) control.
To achieve greater circadian phase shifting, it is possible to combine multiple zeitgebers, which makes their effects additive, which means that a combination therapy produces more phase shift than any of the components alone. For example, light therapy's effect is additive with melatonin (see also here and here and here and here) and with physical exercise.
When used for therapeutic purposes, especially to treat DSPD and non-24, zeitgebers are also called chronobiotics.
Can changing timezone help?
No it wouldn't work. But if you live in a latitude where there's not long enough sunlight such as closer to the arctica, then moving south can help.
What matters in different world regions is light exposure, not the timezone.
If light therapy doesn't work for you, it won't work better in another timezone of similar latitude or with a similar light exposure. And if light therapy does work for you, you don't need to move to a new latitude, you can just buy a lightbox or light therapy glasses.
As to why it wouldn't work, there is no absolute time, time is always relative (as demonstrated by Einstein's relativity theory). To know when it's day time or night time (and hence sleep time), our bodies use external time cues, formally called "zeitgebers". These include various things such as light, temperature, etc. These are more intense in the day, and lower in the evening and night, and is what hints our body to know the difference between day and night. So our body's rhythm always works relatively to these external cues.
To make an analogy, it's like navigation, when you're lost, you use points of interests to know where you are such as recognizable buildings in the distance and then you can deduce where you should head to to reach your destination, or you can use a compass to indicate the north. Without external tools, you have no way to know where to go, you will randomly walk and just get more lost.
Zeitgebers are the external compass that our body naturally uses to synchronize the circadian rhythm with the day night cycle. When you move to another timezone, the timing of these zeitgebers change, and so your body adapts. But it will adapt the same wherever you go. So after some time you will sleep and wake up at the same times as in your old timezone, but just shifted to the new timezone. The issue with DSPD, non-24 and other circadian rhythm disorders is likely not that they do not adapt to zeitgebers, it's that they adapt incorrectly, it's like having a miscalibrated compass that indicates an offset north pole.
The relative effect of zeitgebers
Every zeitgeber can be good (phase advance) or bad (phase delay) depending on the timing. Phase advance means that the circadian rhythm period is shortened, whereas phase delay lengthen it. This time-dependent effect, which is on top of the dose-dependent effect (eg, intensity of light or dosage of melatonin), is summarized in the Phase Response Curve (PRC) of the target treatment.
Zeitgebers are double-edged swords: since PRC is an intrinsic and universal property of all zeitgebers, present in all animals and even unicellular organisms, this means that when a factor can phase delay, it can also phase advance if exposure happens at another time. For example, if you suspect getting exposed to screens light in the evening phase delays your circadian rhythm, then the same screens light can be used at wake-up to phase advance.
All PRC curves have a tipping point, where the zeitgeber's effect on the circadian rhythm will completely reverse, from maximal phase delay to maximal phase advance or the opposite. Hence, the exact appropriate timing for phase advance or phase delaying depends on the zeitgeber, it's not necessarily at wake up for phase advance or at evening for phase delay (eg, for melatonin the PRC is inversed with the light PRC). For light therapy, this tipping point is the minimal core body temperature CBTmin (with more phase advance after), whereas for melatonin it's the DLMO (with more phase advance before DLMO or more delay after) (see also here). The CBTmin matters for light therapy but not for melatonin, for which only DLMO matters. For physical exercise, the CBTmin is also the tipping point. Thus, adequate timing of zeitgebers is critical to get phase advance effects, otherwise a mistimed zeitgeber can not only be ineffective but even further worsen the delay and hence the circadian rhythm disorder condition.
Here are the simplified PRC curves for light and melatonin effect on humans (image from Wikipedia):
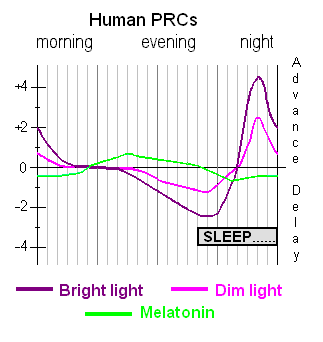
Citing Lewy (1985) explanation on bright light zeitgeber:
> In both diurnal and nocturnal species, certain features of PRCs appear to be universal. When the pulse of light occurs during the animal’s subjective day (based on the activity-rest cycle), hardly any effect is noted. When the pulse of light occurs during the beginning of the animal’s subjective night, the animal will delay the phase position of its subsequent activity-rest cycles. When the pulse of light occurs during the end of the animal’s subjective night, the animal will advance the phase position of its subsequent activity-rest cycles.
The relative effect of zeitgebers on the circadian rhythm can be confusing. We can make the following analogy: it's a bit like a boat, naturally you will float with the river (your natural circadian rhythm), but you can use paddles (light therapy) to row against the river (waking up earlier and earlier when used in the biological morning) or faster along the river (being exposed to bright light in the biological evening because you don't do dark therapy). But you can't just stick the paddles in the water to precisely stay where you are in the river, it doesn't work like that, you have to row in one direction or the other, even if just to stay in place (maintaining a stable wake up time), and where you will end up (your wake up time) won't be super exact and will change with the flow, but you can ensure what direction you go (against or with the river's flow = natural phase of the circadian rhythm). How much earlier you wake up is defined by how strong you row (light intensity), how aerodynamic your boat and paddles are (blue light color and ergonomic form factor to optimize delivery such as light therapy glasses instead of lamps), and how long you row (how long you do light therapy).
The relative effect of zeitgebers is crucial and fundamental for the synchronization of multiple independent clocks, whether the host's circadian clock with an environmental zeitgeber, or down to the synchronization of SCN's clock neurons together. Indeed, if there was no relative effect, in other words no PRC curve, there would be no synchronization.
There are in theory two types of zeitgebers: the type-0 resetters, which theoretically can reset at the time you target regardless of your current circadian rhythm, versus type-1 resetters, which effect is dependent on the intake/exposure time relatively to your current circadian rhythm. In practice, all currently known zeitgebers (and hence treatments) are type-1 resetters. Hence, it's crucial to time all treatments relatively to your current sleep and wake up times, not the target/wished ones. This is true not only for melatonin (see also here), but also light therapy and food. It's not specific to treatments for circadian rhythm disorders, in fact there are now chronotherapeutics as coined by Smolensky et al, an emerging field of scientific and medical study which is finding that virtually all drugs have a time-dependent effect relative to the circadian rhythm, such as antibiotics, with adequate timing of administration during the circadian day both increasing efficacy and reducing adverse effects. For more informations, see the following papers about chronopharmacokinetics and chronotherapeutics: here, here, here, here, here, here, here, here, here, here, here, here and Smolensky, Labrecque, Chronotherapeutics Pharmaceut News 1997:4: 10-7. This makes sense, as it was discovered that nearly half of protein-coding genes are rhythmically expressed in at least one part (tissue) of the human body. This time-dependent effect of zeitgebers is also what makes research about them difficult, as it is easy for scientists, working at usual office hours, to miss time-dependent effects.
So far, we have mentioned only the temporal aspect of zeitgebers (ie, shifting the circadian rhythm). But zeitgebers also modulate the amplitude/magnitude of the circadian rhythm. In fact, both the amplitude and synchronization are simultaneously modulated in a non-linear and non-trivial way as shown experimentally, which can explain the asymmetrical response to bright light, with dimmer lights sufficient to shift the circadian rhythm at (circadian) night but brighter light necessary in the (circadian) day for the same effect.
Is it necessary to use multiple zeitgebers, even if we only have a slightly bigger circadian rhythm than the average, let's say 24.5h? Yes, because not only having multiple zeitgebers allows to have a failsafe in case you miss one of the zeitgebers (eg, forget to take the melatonin pill one day, then at least the light therapy will still help), and also because the body has multiple circadian clocks: the most famous one is the "master clock" in the brain, more formally known as the photoneuroendocrine system, which consists of the suprachiasmatic nucleus (SCN) in the hypothalamus, which is photoreceptive (respond to the photic signal from the ipRGC cells in the eyes) and communicates bidirectionally with the pineal gland which regulates melatonin (side-note: only in mammals did the pineal gland lose its photoreceptive capacity) , but there are also lots of peripheral clocks in other organs (liver, intestines, muscles) down to every cells (mitochondria produce and metabolize melatonin, see the Melatonin section). If a zeitgeber entrains one but not the others (such light not entraining the digestive clock), then the body may still continue to freerun because of the mismatch between the various body clocks and hence prevent whole body entrainment.
Since the industrial era, most zeitgebers are weakened in our daily lives, foremost light due to officies deprived from direct exposure to sunlight and artificial lighting in the evening, which led and is still leading to a widening and delaying of the chronotype distribution except for the very early morning larks, ultimately causing social jet lag for nearly everyone.
A few studies indicate that likely all zeitgebers work by ultimately modulate body temperature: body temperature modulation is the universal signal to reset the biological clocks throughout the body (see also here for a more easy to read article). This is further demonstrated by the fact that light therapy does not need the suprachiasmatic nucleus (SCN) to affect the circadian rhythm, only the ipRGC cells. Since light therapy modulates melatonin levels, and that one of the core melatonin activities is to modulate the body temperature, and given the widespread availability of melatonin receptors throughout the body, it appears likely that light therapy shifts the circadian rhythm by modulating melatonin levels which itself modulates the circadian rhythm clocks over both the central and peripheral systems.
(TODO: add the proposition of an evolutionarily derived algorithm for circadian rhythm entrainment to zeitgebers: https://archive.is/AvoPl )
Seasonal variations in zeitgebers
Or why Bedtime and wake up time are independent (dual-oscillator model)
A common assumption is that human sleep schedule is flexible and can be manipulated by varying - or maintaining stable - the bedtime: an earlier bedtime will lead to an earlier wake up time, and a later bedtime to a later wake up time. This is however contradicted by the evidence.
Although this is commonly studied in seasonal animals such as migrating birds, a lesser known fact is that the circadian rhythm of humans also has seasonal variations, although artificial lighting can eliminate these variations. What is interesting with these seasonal variations is that they allow to observe how the human's circadian rhythm naturally fluctuates in typical sleepers with varying zeitgebers exposure: later, shorter and weaker zeitgebers such as sunlight during winter, versus stronger, longer and earlier sunlight during summer.
This figure (from this review on seasonal variations of the circadian rhythm and melatonin in humans) shows the changes in melatonin secretion start time (onset-time, left graph) and stop time (offset-time, right graph) relatively to the duration of melatonin secretion and season.
What this figure shows is that the start time of melatonin secretion (associated with the fall asleep time), on the left, doesn't change much with the season, despite the melatonin secretion duration (associated with the sleep duration) increasing in general during winter. But on the right, it's shown that the melatonin secretion stop time (associated with the wake up time) changes linearly with the melatonin secretion duration across seasons! So it's the melatonin secretion that stops later during winter, likely due to progressively later sunrise time and hence later start of exposure to bright light, resulting in a longer melatonin secretion. In other words, during the winter, humans sleep longer on average, and we have a longer melatonin secretion that goes well into the morning as the melatonin secretion stop time and wake up time get delayed later according to sunrise time, but not the fall asleep time which remains constant. This is the same phenomenon that underlies the relative coordination phenomenon (see the dedicated section below).
This is a crucial observation, as this is a very compelling evidence that the wake up time is decoupled/independent from sleep onset (fall asleep) time. Indeed, since sunlight is both rising later and setting earlier during winter compared to summer, we could assume that to sleep longer, humans would both sleep earlier and wake up later, with their circadian rhythm being recalibrated to fit the sunlight exposure. But this is not the case, since only the wake up time varies with season and sunlight exposure (onset and duration) but not the falling asleep time. This shows that sunlight (and hence light therapy) primary modulates the wake up time, but not the fall asleep time.
There is a model of the circadian rhythm that is founded on this sleep offset-onset decoupling: the Dual-Oscillator Model of Regulation of Human Melatonin Secretion : "the nocturnal period of melatonin secretion are governed by the mutual phase relationship of two circadian oscillators: one (E) that is entrained to sunset and controls the evening onset of activity and melatonin secretion, and another (M) that is entrained to sunrise and controls the morning offset of activity and melatonin secretion. In this way, when the interval between sunset and sunrise becomes longer, the duration of the nocturnal period of activity and melatonin secretion becomes longer. Results of our research suggest that this model can be extended to humans."
This model is quite old, and is based on findings on animals: "Studies examining the profile of melatonin secretion in rodents following phase shifts to light stimuli have indicated that the onset and offset of melatonin secretion do not always phase shift in a parallel manner. Accordingly, the hypothesis has been suggested that there may be two coupled oscillators, an evening or E oscillator associated with melatonin onset, and a morning or M oscillator associated with melatonin offset (Pittendrigh & Daan, 1976; Illnerová & Vanecek, 1982; Elliott & Tamarkin, 1994; Illnerová & Sumová, 1997). [...] In contrast to the pattern of light-induced phase delays noted in rats above, Elliott & Tamarkin (1994) have reported a tendency for the melatonin offset in hamsters to shift before that of the melatonin onset following phase-delaying light pulses. However, their results following phase-advancing light stimuli concurred with those of Illnerova & Sumová (1997), with the shift in melatonin offset occurring immediately, whereas the shift in melatonin onset advances only after several days of transient adjustment."
Computational modeling revealed that the EM model better predicts the 2 key behavioral phenomena observed with skeleton photoperiods: activity psi-jumps and photoperiod-induced changes in activity phase duration. Although the biological basis is debated, one potential culprit may lie in the SCN neural subpopulations: indeed, it can be schematically separated into two kinds of subpopulations, with the ventral/core SCN subpopulation being more reactive to the phase (timing) of bright light exposure and which expresses period1 gene with a bimodal waveform with two peaks at dawn and at dusk which assumedly tracks day-to-day entrainment, whereas the dorsal/shell SCN subpopulation only expresses period1 gene with a unimodal profile with one peak locked to dusk. The EM model can be seen as the first theory of the circadian rhythm being comprised of multi-oscillators, although we now know that it is an oversimplification, it still allows to predict observed key behavioral phenomena. As a 2011 review nicely summarizes:
> While both photic entrainment and seasonal adaptation arise from a redistribution of SCN oscillatory activity patterns, different neuronal coupling mechanisms are employed, which are reviewed in the present paper.
Although the EM model is based on animals findings, the above results on humans seasonal variations show this is applicable to humans too.
Furthermore, there is evidence from human newborns that the wake circadian rhythm appears earlier than the sleep circadian rhythm, which further supports the hypothesis of distinct circadian pacemakers (oscillators) for the wake up times and the sleep times.
Another evidence in humans are the community studies to delay the college schools start time, to adapt to adolescents biologically induced transient circadian phase delay: when the schools start later, most teenagers will still sleep at about the same time, but will wake up later (TODO: add refs). This is in line with what can be expected if the evening (sleep) and morning (wake up) oscillators are distinct and mostly decoupled.
This is especially interesting as this indicates, by transitivity, that both the wake up time, bright light exposure, minimal core body temperature and melatonin offset (stop of endogenous melatonin secretion) are coupled, whereas the onset of melatonin secretion (DLMOn) seems to be decoupled and may be affected or play a role in photic history, and potentially be more controllable via other means such as with exogenous melatonin pills. Hence, it's no wonder the wake up time is a more reliable estimator of the circadian rhythm than the bedtime or the fall asleep time! Indeed, it was found that the wake-up time is a reliable predictor of the DLMO and the circadian rhythm similarly to the sleep midpoint, whereas the bedtime is not.
Interestingly, this means that the melatonin levels are independent (decoupled) from circadian phase shifting, and indeed that's the case as was later demonstrated (see also here and here and here). In fact, this was first discovered in 1985 by Lewy et al, as they found in humans that there is indeed a delayed effect of bright light on melatonin onset, concluding that bright light both entrained and suppressed melatonin, which prompted them to conceive the clock-gate model integrating this dual effect of bright light on melatonin. The dim light melatonin onset (DLMO) sampling method of the circadian rhythm phase was also orginally devised for this study (see also here). Combined with the fact that the melatonin onset is always delayed of several days after the circadian phase shift, this shows that the start of melatonin secretion (aka melatonin onset or DLMO or DLMOn) is a very unreliable proxy of the circadian rhythm, studies should prefer to measure the core body temperature or at least the wake up time instead.
However, this model assumed that melatonin is the underlying circadian rhythm signalling hormone, whereas recent evidence demonstrated that it's only a relay to modulate core body temperature, the latter being the core signalling pathway for circadian clocks throughout the body. Hence, this model is still valid if we simply replace the assumptions about melatonin secretion by the core body temperature pathway.
This hypothesis that the sleep offset (wake up time) is governed by the circadian rhythm with the sleep offset being delayed, dependent adjustment variable was already advanced by Krauchi et al in 1998:
> Does the SCN regulate heat production, heat loss, or both together? There are only few data available at present to answer this question. Czeisler (1978) has shown that the daily rhythm of heat loss from the extremities (as indicated by wrist skin temperature) is mainly coupled to sleep onset, and can be dissociated from the CBT rhythm when subjects internally desynchronize under free-running conditions. Thus, the heat loss rhythm cannot be the major circadian input of the CBT rhythm, but is rather the dominant contributor to the sleep-evoked component. One preliminary conclusion is that heat production seems to be driven by the SCN, and heat loss is rather the dependent variable which adjusts for heat balance.
This also suggest that any therapy aiming at controlling the bedtime, such as sleep hygiene and chronotherapy, is inappropriate to shift the circadian rhythm, as effective therapies should target the wake up time, such as light therapy.
In summary, the age old assumption that humans can control when they fall asleep is a core idea we got wrong about sleep. Instead, we cannot control when we sleep, but we can indirectly control when we wake up via zeitgebers (such as bright light therapy), which in turns will drag the fall asleep time along with some delay.
(TODO: Distinct Components of Photoperiodic Light Are Differentially Encoded by the Mammalian Circadian Clock, 2020 https://doi.org/10.1177%2F0748730420929217 )
Circadian waveform manipulation: shorter light and bifurcated exposure makes all parameters of the circadian rhythm more flexible
WORK-IN-PROGRESS: this section is subject to vast changes in the future.
Another very important effect of seasonal variations in zeitgebers, especially in sunlight exposure, is the shorter duration of daylight: the days get shorter during winter and longer during summer. The key takeaway is that photoperiod (day length, duration of bright light exposure) affects phase response. But both phase shifting and period plasticity (ie, lengthening/shortening the day length) are themselves just a few subtypes of circadian waveform manipulation, as shortening happens naturally with seasons, but circadian waveform can also be artificially manipulated to cause interesting circadian patterns, such as light-dark-light-dark (LDLD), with interesting properties, such as allowing for very fast (<3 days) phase resetting (ie, 12h phase shift) or stable biphasic circadian alignment.
The SCN is split into two kinds of subpopulations of neurons, ventral/core SCN and dorsal/shell SCN, with ventral SCN adapting faster to bright light input, and dorsal SCN being slower to adapt as it relies on feedbacks from the ventral SCN. The synchronization between subpopulations of SCN neurons is what is thought to underlie the ability to track seasonality, with previous studies finding that individual neurons do not encode short or long days information, but the neural activity at the population level (ie, of a lot of neurons) does encode this information, however newer studies found otherwise, that the dorsomedial SCN does indeed encode period length and is sensitive to retinal inputs. This was found to modulate circadian clock gene expressions, especially period1 which is modulated in ventral SCN in long days to encode both dawn and dusk, whereas the dorsal SCN only encodes dusk in both short and long days (for nocturnal rodents, or dawn in diurnal animals). The slower synchronization (several hours) of the dorsal SCN to zeitgebers compared to ventromedial SCN (under a hour) is likely what allows the SCN to continue to maintain entrainment temporarily in the absence of zeitgebers. Furthermore, a very well designed ex-vivo 2021 study found that the core/ventrolateral SCN network is more susceptible to phase shifts, whereas the shell/dorsomedial SCN network more to period changes: it appears that the ventrolateral SCN responsible for daily entrainment is made to be more sensitive to the timing/phase of bright light exposure and less to period lengthening and is affected under less than a hour, whereas the dorsomedial SCN which encodes seasonal entrainment (long vs short days) is more sensitive to the duration/period of bright light exposure and less to phase shifts and takes several hours to adjust, causing what is termed an after-effects to stimulation such as bright light exposure, also called photic history. These findings suggest that contrary to what was previously thought, circadian plasticity, of both phase shifting and period lengthening/shortening, is also observed at the SCN neuronal scale, not just as an emergent property of networks coupling. In addition, this study demonstrated that clock genes express a sinusoidal waveform in free-running conditions, but a highly asymmetrical waveform with a shorter phase advance portion and a longer phase delay portion when entrained ; and it was also observed that daily waveform changes were observed under entrainment to long days, short days and non-24 periods. Finally, and contrary to what was supposed before, a previous study observed that "ipRGC cells have retinal projections that are widespread across the entire SCN and neuronal activation following light exposure was ubiquitous", which shows that both the ventrolateral and dorsomedial SCN networks equally receive photic inputs, but they just have different intrinsic responses as shown by this 2021 ex-vivo study. The authors further suggest the following: "As the VIP and AVP neurons are respectively located in the ventral and dorsal SCN, this suggests that regionally differential phase and period responses in the SCN might be derived from intrinsic differences between the VIP and AVP neuronal clocks. The period response, inversely correlated with the phase response, could serve to help the SCN recover back to its baseline network phase state from decreased synchrony following phase shifts."
The day length, also called photoperiod and is equivalent to the duration of bright light exposure, is very significant, as it was found to modulate other aspects of the circadian rhythm, such as the phase shift response to bright light exposure, with shorter days (of rodents, hence longer active phase) increasing phase shifts to the same photic input comparing to longer days (ie, longer sleep phases). Shorter days were further found to be associated with a greater synchronization in the SCN subpopulations, especially between the ventral and dorsal SCN, whereas longer days produce a more desynchronized and spread out profile encoding multiple times of the day.
More precisely, short days concentrate the distribution profile of the phase response curves of each clock neuron in the SCN to a shorter timespan in the day (when there is the short bright light exposure), so that they are more easily synchronized and hence a phase shift in the photic input induces a bigger phase shift in the neuronal population since they 1) receive the photic input at the same point of their PRC since they are synchronized, so they get the same phase shift, 2) they echo together with the feedback loops especially using GABA inhibitory and excitatory connections. Whereas in longer days (longer bright light exposure), the clock neurons phase response curves profile distribution is more spread out throughout the day, so that the synchronization is reduced and the phase shift differs greatly between each neuron, leading to a reduced compounded phase shift at the population level.
Shorter total bright light exposure induces a more flexible circadian rhythm. Which is the opposite of what we want to treat non24, as the therapeutic goal is to stabilize the circadian phase. This explains why during winter it's even more difficult to freeze freerunning with non24 compared to summer. But the silver lining is that this may be used advantageously to shift faster to a desirable phase, as a phase reversal (ie, 12h shift) takes much less time when mice have been exposed to a short days compared to a long days. Biphasic days (LDLD) allow for an even faster adaptation to a phase reversal than shorter days. However, these findings were only observed in nocturnal rodents for the moment, more studies are needed to observe whether this also applies to humans and other diurnal animals.
Short days are one example of natural circadian waveform manipulation. Another, but artificial, circadian waveform manipulation is light-dark-light-dark (LDLD).
LDLD days are composed of two blocks of one photophase followed by one scotophase. In other words, two small days in one. For example, LDLD(7:5:7:5) denominates a day comprised of 2 subdays with 7h of bright light exposure and 5h of darkness. Non-24 periods entrainments are called T-cycles, examples: T15 means an entrainment to a 15h day, T30 to a 30h day.
Although short days allows for a significantly faster entrainment to a new bright light schedule than long days, under about 4 days instead of 7 days, LDLD provides additional benefits: not only is it much faster to entrain from long days to LDLD than to short days (1 day vs 1 week), LDLD also allows to entrain to a full phase reversal under just 3 days, hence the total time between the initiation of LDLD and the end of phase reversal is 4 days. Furthermore, LDLD is a highly stable entrainment pattern, so that it can not only serve as a fast resetting method, but also as an alternative sleep-wake schedule for a circadian aligned biphasic sleep, which has potential applications for shift work.
However, LDLD has some limitations: first, it is highly artificial, and although it is resilient to temporary perturbations in the photophases and scotophases, it requires to shield/manipulate one's exposure to sunlight and artificial light therapy to create two distinct photophases and scotophases. Secondly, this biphasic pattern of the circadian rhythm naturally reverts to a monophasic pattern, with the two scotophases joining together, when exposed to constant conditions (either always dark or always bright light), so that for LDLD to be maintained, the distinction between the two subdays is necessary. Thirdly, not all animals could entrain to LDLD, with 3/36 rodents just freerunning with an underlying long day entrained circadian rhythm, and similarly in another study 1/18 hamsters failed to entrain.
Time to adapt to a shifted phase compared to the original phase, per bright light exposure pattern. Long day and short day here refer to objective days (ie, length of photoperiods) not the subjective day, which is the inverse for nocturnal animals (ie, short subjective day for long objective day, and long subjective day for short objective day). Excerpt from Figure 2 of this study.
Now it is important to note that short objective days (short photoperiods) for nocturnal rodents imply longer active periods (longer subjective days), whereas for diurnal animals, for which the retinal-SCN pathway is inverted, short days imply a shorter active period (see also here). Indeed, melatonin and bright light are wired inversely to the noradrenergic vasoconstrictor system in nocturnal animals compared to diurnal animals, so that melatonin signals wakefulness periods and bright light exposure induces sleep, but the circadian rhythm and its linear coupling with core body temperature remain the same as in diurnal animals, with high phases associated with wakefulness periods and low phases with sleep periods. Since the basis of greater phase shifts and greater period lengthening/shortening stem from a greater synchronization of the SCN neuronal ensemble, and that it's the bright light input that projects and affects the SCN neuronal ensemble, then to translate these results from nocturnal onto diurnal animals such as humans, a short photoperiod implying a shorter subjective day (ie, shorter active period) can be expected to produce similar results of increased circadian plasticity. In other words, whereas short days imply shorter sleep periods and longer active periods for rodents, we infer that they translate as shorter active periods and longer sleep periods for humans, similarly to what both rodents and humans naturally experience with seasonal variations in bright light exposure.
This is in contrast to the hypotheses other authors offer, and if ours is correct, then this put into question the practicability and usefulness of short days and LDLD interventions for shift-working humans: whereas short days and LDLD imply longer active periods for rodents while allowing them to obtain wider phase shifts to realign with zeitgebers faster, humans would have to (forcefully) experience shorter less productive active periods and longer sleep periods, with sleep induction and maintenance being known as more difficult to achieve than wakefulness induction and maintenance (ie, it's easier to stay awake longer than to sleep earlier and longer).
Is LDLD producing a really biphasic circadian rhythm, or is it only a masking effect of behavioral activity? According to this early paper, by Gorman et al, original discoverers of LDLD, the observed phenomena cannot be explained by masking. More recent evidence using objective markers of the circadian rhythm, such as body temperature in mice, also observed a bimodal waveform, hence demonstrating that it's not just activity but the circadian rhythm itself that is bifurcated by LDLD.
Phase shifting and period shortening/lengthening are just two kinds of circadian waveform manipulations, but there are several other parameters that were found to be modifiable as an after-effect through light-dark manipulations, with an after-effect being the description of an effect that both appears after and hence as a result of a circadian waveform manipulation, and stays after discontinuation of the manipulation:
TYPES OF CIRCADIAN AFTER-EFFECTS: PERIOD, WAVEFORM, ENTRAINMENT, PHASE-SHIFTING: "The effects of previous states of entrainment on properties of the biological clock are termed aftereffects. Best known are period after-effects—lengthened and shortened free-running period in constant darkness following entrainment to long versus short light cycles (Pittendrigh and Daan 1976) and waveform after-effects—lengthened and shorted active period (alpha) in free-run after short versus long photoperiod. Less well known are after-effects on entrainment—different patterns of entrainment in identical conditions in T22 depending on lighting history (Chiesa et al. 2006) and after-effects on phase resetting—larger light-induced phase shifts in hamsters entrained to short compared with long photoperiods (Pittendrigh et al. 1984; Evans et al. 2004; Glickman et al. 2012; Glickman et al. 2014). The ability to entrain to 30-h LDLD cycles following bifurcation, but not other conditions, represents another type of circadian entrainment after-effect." Ref: https://pubmed.ncbi.nlm.nih.gov/28770653/
- In addition: shorter photoperiod (longer subjective day) for the same total light cycle (same day duration) leads to lengthened freerunning period: "Under constant conditions, animals previously entrained to LD5:19 had significantly longer freerunning periods than those from LD9:15 (Fig. 4C)." https://pubmed.ncbi.nlm.nih.gov/16731659/
- "after-effects on entrainment" refers to photic history. Which includes dim light at night effect of increasing phase shifting and entrainment bounds!
A few practical aspects to implement circadian bifurcation (LDLD):
- Ideally, the two photoperiod (periods of exposure to bright light) are 12h apart (eg, start at 9am for 5h, then at 9pm for 5h), which is a symmetrical LDLD. But an asymmetrical LDLD is fine too, with a psi-difference variance of up to 4h (ie: the second photoperiod can be started just 10h or up to 14h after the first instead or 12h) being found to entrain two oscillators just fine, which shows that once the circadian rhythm is entrained to LDLD, it splits in two functionally independent oscillators, each synchronized to its own phase, which can be phase shifted independently from the other one, so the phase can be adjusted for each photoperiod independently according to the usual rules of phase resetting by light therapy as outlined below. However, note that when using an asymmetrical LDLD, there are a few new rules that appear, such as the phase-leading photoperiod being a stronger marker of entrainment than the phase-lagging one.
- Both photoperiods and scotoperiods are crucial to maintain, otherwise the two oscillators will try to merge together into one, unimodal circadian rhythm (see also here).
- What matters is the transition, the contrast between darkness and bright light exposure (dawn) in humans and other diurnal animals, whereas for nocturnal rodents the SCN neurons track the contrast between bright light and darkness (dusk) (see also here p8).
- Start the first period of LDLD at the circadian wake-up time, ie, the start of the circadian day, as done in studies on mice (here and here). This is because the wake up time is the marker of the circadian rhythm, it's what the dorsal SCN neurons detect to encode period length. If sleeping in circadian alignment, the circadian wake-up time coincides with the behavioral wake-up time, but otherwise it can be masked by the sleep homeostat (ie, build up sleep deprivation).
- To design a LDLD schedule, start from a typical short (objective) day schedule such as a winter day, because the effects of LDLD are more similar to short days (ie, short photoperiod) than to long days (long photoperiod), so we can expect it to work better when the rest periods are as long as short days, as done on mice, instead of having shorter rest periods as what was extrapolated by some authors. In practice, to transpose LDLD schedules from mice to humans, invert the photoperiods and scotoperiods: LDLD7575 --> LDLD5757
- Plan at least 4 days to do a 12h phase shift using LDLD: 1 day to transition from short (winter) day to LDLD (ie, bifurcate the circadian rhythm into 2 functionally independent oscillators), and then 3 days to stay in this LDLD pattern as was found to be the minimum required duration to maintain bifurcation before attempting a phase shift, and then simply remove one of the photoperiod and extend the duration of the photoperiod you prefer, and you will get a 12h phase shift under 4 days via LDLD.
- Figure S4 of this study suggests that napping is not enough, the circadian rhythm needs to bifurcate for the enhanced circadian plasticity to be allowed, ie, by exposing oneself to a tightly controlled and timed light-dark-light-dark pattern.
- Light intensity during photophases (ie, light therapy periods) does not matter, it does not influence the ability to bifurcate, and most studies on LDLD used 300-350 lux not even at eyes level but room level. However, lots of studies found that 0.1-lux green dim light during scotophases significantly improved the ability to bifurcate and the stability of entrainment, compared to both complete darkness and red dim light.
- LDLD may be achievable by simply staying in constant bright light conditions but with a 56% chance of success according to a hamsters study. Furthermore, the study found that animals who once bifurcated could more readily bifurcate again later after merging, which suggests that once bifurcation is achieved once, it becomes easier to achieve the same again even after returning to a unimodal circadian rhythm. However, it's worth noting that bifurcation under constant bright light, which is instead called "splitting", may result in an unstable entrainment at first, with an asymmetrical LDLD pattern with one of the photoperiod freerunning independently from the other one, but eventually reaching a stable symmetrical LDLD entrainment pattern (ie, photoperiods 12h apart).
Although it is currently (as of 2022) unknown whether artificial temporal reorganization manipulations of the circadian waveform such as LDLD would work on humans, scientists suggest it is possible and already see potential applications to improve the health, wellbeing, safety and productivity of shift workers, with some tentative schematics of how such protocols would be done in practice.
For individuals with a circadian rhythm disorder such as non-24, having a bifurcated circadian rhythm can have significant advantages, such as allowing 2 windows of activity, one during the objective daytime and one during the objective night (whereas nightwalkers such as inversely phased non-24 or extreme DSPD will not have activity opportunities during daytime). For those suffering from chronic fatigue due to chronic circadian misalignment, LDLD allows to multiply by 2 the number of activity periods and rest periods, so this probabilistically increases tremendously the likelihood of experiencing productive activity periods, whereas with a typical schedule the next opportunity is only in 24h, here it can be 12h or even earlier depending on the LDLD scheme used.
For individuals with DSPD and shift work disorder, the benefits are obvious, as a LDLD pattern is essentially a robustly circadian aligned biphasic sleep, where the napping sleep period is replaced by a "true" second night sleep, with melatonin production and other circadian night processes that are not activated during naps of non-LDLD biphasic sleep patterns.
The 2021 optogenetics study already mentioned above also found something very interesting: all the effects are reproducible with just short light-dark transitions, as with skeleton photoperiods, but replaced by neuronal stimulation that mimics it. Hence, it appears that to trigger phase shifts and period shortening/lengthening, all that is needed is to apply a contrast to the neurons, this is all they detect. Furthermore, by default, when there is an ambiguity, the SCN networks seem to revert to a shorter day. Finally, it was found that non-24h light-dark schedules (T-cycles) cause SCN desynchrony, similarly to long days, and hence reduced responsiveness to phase shifts. This may explain why individuals with non-24 are usually more treatment-resistant to light therapy, and require longer and more intense light therapy, than other circadian rhythm disorders.
Another particularly interesting finding is that it appears that the period plasticity has an after-effects, with long days causing mice to keep a longer freerunning period after being moved to constant conditions compared to short days ("under constant conditions, animals previously entrained to LD5:19 had significantly longer freerunning periods than those from LD9:15.").
It's worth noting that now that there is unequivocal evidence of the circadian rhythm being composed of multiple oscillators, and that it can be dynamically reprogrammed (ie, high neuronal plasticity), there is nothing that indicate that bifurcation should be limited to a split in two: it may very well be possible to bifurcate the circadian rhythm into 3 or 4 functionally independent oscillators, since this simply implies a SCN neuronal connectivity reorganization, with subcommunities of neurons getting assigned to track specific photoperiods (see also the Balanced Networks model for how neuronal networks can specialize in an unsupervised way based only on stimuli). For those interested in history of science, it seems that one of the earliest if not the earliest evidence of the circadian rhythm being composed of multiple oscillators in humans come from a 1983 study which used a sort of early LDLD-like design, although an earlier work by Wever in 1975 appears to document the circadian rhythm in humans as a multi-oscillatory system (but inaccessible online, could not read content).
These findings on circadian waveform manipulation, especially the greater synchronization for shorter bright light exposure compared to longer light exposure, may imply that there may be a sweet spot for entrainment, with a long enough bright light exposure to synchronize but not too long to remain synchronized to the zeitgeber robustly, but at the same time it's worth considering that longer bright light exposure causing desynchronization shields against unwanted phase shifts since the effect will be distributed across multiple populations of SCN neurons with different PRC phase profiles. Anecdotally, when the present document's author experimented with LDLD, this indeed allowed to produce 2 short days of high attention and productivity under 24h, with entrainment achieved under 2 days, but lost after only 2 days, which caused the circadian rhythm to freerun much faster than even before (about 3h/day of phase delay over the next 3 days under constant conditions - artificial bright light and sunlight were excluded - following loss of entrainment to LDLD and bright light therapy discontinuation). Hence, LDLD can have serious adverse effects in case of entrainment failure. Nevertheless, using 3-4h/daily of standard bright light entrainment (using the VLiDACMel protocol) allowed to reduce the freerunning speed back to its original state under a few days, even after discontinuing bright light therapy. Nevertheless, a longer lasting after effect (ie, longthened circadian period) can not be excluded after LDLD entrainment failure in case the circadian period was not instantly reduced by a VLiDACMel manipulation.
Another field worth investigating is the study of military and commercial submarines personnels, as they are regularly forced to rotate on artificial non-24 T-cycles (eg, 18 hours days) or even split cycles, with or without exposure to zeitgebers depending on submergence.
Synergistic effect of multiple zeitgebers
WORK-IN-PROGRESS: this section is subject to vast changes in the future.
A study has demonstrated a synergistic effect of using multiple zeitgebers, by observing the induction of period genes by light together "with modulations of nuclear receptor activities by drugs and metabolism" and hence "medical treatment strategies which aim for stable circadian rhythms should consider interactions of multiple zeitgebers". More precisely, they found that "the entrainment of a circadian rhythm to two coexisting zeitgebers depends strongly on the phase difference between the two zeitgebers".
Light and dark therapy
Circadian rhythm disorder management involves maximal control of the pattern of exposure to light, which for diurnal animals such as humans includes being exposed to bright light during the circadian morning and day, and avoiding bright light exposure during the circadian evening and night. Furthermore, all zeitgebers work only when they have a periodic component, which is an alternance between high and low phases, for light it's composed of bright blue light phases versus dimly lit/dark reddish phases, as without such alternance, participants lose entrainment (ie, constant routine protocol). Hence, bright light therapy should always be complemented with dark therapy, and both therapies should really be considered a single therapy, a light exposure control therapy or, more prosaically, light and dark therapy.
The next subsections will cover the various parameters and findings about bright light therapy and dark therapy on the circadian rhythm.
Brief history of bright light therapy
Heliotherapy, which is bright light therapy using sunlight (or sunlight therapy nowadays), can be traced back to 15 centuries BC. Renewed interest by pre-modern medicine emerged with the discovery of the importance of UV light for the human body to produce the essential vitamin D during the mid nineteenth century, which led to the UV therapy craze and its excesses. Modern light therapy seems to have emerged around in the late 1970s to 1980s, with research on insomnia and on depression uncovering the previously unknown effects of bright light therapy on the human circadian rhythm and on mood. Indeed, until the early 1980s, it was assumed by chronobiologists that social cues were the main zeitgebers for the human circadian rhythm, with bright light having little importance, despite being the main zeitgeber for animals, until this assumption was disproven first by a review in 1981 by Czeisler et al and then by empirical data in 1986 and in 1989 demonstrating the vast underestimation of the magnitude of bright light circadian resetting effect. The existence of the ipRGC cells in the eyes, the cells that allow circadian rhythm shifting from bright light ocular exposure, was discovered in frogs and mice in 2000 and later in humans, in 2003 by Panda et al.
Norman E. Rosenthal is often credited as the first scientist to have coined the term Seasonal Affective Disorder (SAD) for seasonally-dependent depression, and the use of bright light therapy to treat it, in 1984, from a case study on a depressive patient admitted in 1980. However, Rosenthal is actually not the first to have used bright light therapy to treat depression. Indeed, there is another study published 1979 by Wehr which used bright light therapy to treat depression in a group of human patients. Wehr went on to become a principal investigator and led the study that Rosenthal worked on and which defined the SAD disorder.
The effect of bright light on the circadian rhythm is known since at least before 1966, thanks to the groundbreaking works of Kleitman in 1949 and later by the invention of the free-running protocol of Aschoff and Wever in 1962 inspired by the De Candolle experiments in 1832 successfully making the Mimosa Pudica (nicknamed the "Sensitive plant") free-run under constant dark conditions, which experiment was itself inspired from de Mairan precursory discovery of the existence of the circadian rhythm using the same plant. In 1971, K. Hoffman appear to have been the first to discover the photic history effect as well as the splitting effect on syrian hamster, which he named "hysteresis" at the time. Hence, the knowledge that bright light influenced not only the circadian rhythm of animals and plants but also humans was well established much before 1979 when the effect of bright light on mood was discovered with Wehr's work. A review published in 1983 concludes that there was sufficient evidence of an effect of bright light on humans circadian rhythm similar to animals that this warranted further investigations into the use of bright light as a therapy for circadian rhythm disorders. Another review in 1983 specifically focuses on DSPD as a primary target of bright light therapy intervention. A group study was conducted in 1985 on healthy volunteers. Hence, light therapy was investigated for circadian rhythm shifting and potentially sleep disorders treatment directly subsequently to its use for depression.
In conclusion, the effect of bright light on the circadian rhythm was first discovered before its effect on mood. The first case studies investigating the use of bright light as a therapy were done first for mood disorders (depression, SAD), and then for circadian rhythm shifting on healthy volunteers, before starting trials for sleep disorders the next decade. However, it's important to note that the rationale for using bright light therapy for depression always involved the hypothesis of a circadian dysregulations underlying depression (the critical photosensitive period hypothesis, see also here, later renamed to circadian phase shift hypothesis, or also the phase advance hypothesis) and that properly timed bright light could be an effective treatment to affect both the circadian and mood systems.
Hence, the circadian shifting effects of bright light exposure were amusingly enough discovered more than 40 years before the biological pathway was found, since the ipRGC cells existence was discovered only in the 2010s.
It's worth noting psychiatry (eg, Lewy, Krauchi) and psychology significantly contributed to the early research on circadian rhythm science, core body temperature, and bright light therapy to treat depression and sleep disorders, with both disorders being at the time considered to be of nonorganic cause and hence belonging to these specialties. Kleitman, another psychologist who used physiological tools and designs, explained this peculiarity (studying a purely biological phenomenon like sleep by psychological researchers) by the lack of biological technologies which prevented physiologists to study sleep processes, who implicitly delegated this task to psychologists. These pioneers nevertheless did not restrict themselves to their field but also versed in a variety of other fields such as biology, physics and mathematics, just like Piéron before them.
Artificial light is also intimately related to the modern society's economical and labour organization. The essay "The Biopolitics of Melanopic Illuminance, Magnus Eriksson and Geraldine Juárez, Scapegoat (10), 2017" describes how labour laws are a direct consequence of the invention of artificial lighting, how the 24/7 consumerist society is a byproduct of earlier military experimentations to create sleepless soldiers inspired from migratory birds and hence how not surprising it is that the "sleep is for losers" mindset is systematically implemented in the military up to this day, and how artificial lighting is still used as a torture tool in Guantanamo. Note that "biopolitics" is a term coined by Michel Foucault, a philosopher who studied the history of modern medicine and its sociopolitical use as a tool of power to control populations.
Nowadays, artificial bright light therapy is not only investigated for the treatment of circadian rhythm disorders and insomnia, but also for major depression, Alzheimer, delirium and ADHD.
The medical consensus for the use of artificial bright light therapy in the treatment of circadian rhythm disorders varies as of 2022: considered as an option but without sufficient evidence to be systematically recommended by the American Academy of Sleep Medicine, except for ASPD for which it is the treatment of choice, and it is however a recommended treatment for the french sleep medicine institution SFRMS since 2017, with french experts considering bright light therapy as being "amply validated" for the treatment of circadian rhythm disorders.
Bright light therapy parameters, Luminette and photic history
Light therapy, or phototherapy, is a therapy that consists in being exposed to bright light on a precise timing relative to the user's circadian rhythm. The therapy is usually repeated everyday in practice, although studies demonstrated effects with a single exposure.
Light is without a doubt the most powerful tool we have to manipulate the circadian rhythm. Indeed, in case of conflicting inputs between clocks, light always has precedence over other clocks according to Aschoff, and it entrains all central and peripheral clocks throughout the body. Light is the main modulator of circadian rhythms, sleep and mood. Bright light even affects DNA transcription in the process of genes expression through CREB, which is phosphorylated in response to photic stimuli. Hence, light is the number 1 tool anyone with a (sighted) circadian rhythm disorder needs to try. All other currently available treatments (including melatonin) provide much less circadian shifts than light can (but they can be combined for greater effect).
The medical use of light therapy started in the 1900s with UV therapies which awarded its author a Nobel prize. However, modern bright light therapy only came up later, after the 1950s. The effect of bright light on the human circadian rhythm and its interaction with other vital signs such as core body temperature and heart rate was pioneered by the extensive works of Kleitman before 1966. The discovery of the biological pathway for the circadian shifting effects of bright light came even later, first named the "pre-optic area of the anterior hypothalamus" as it was assumed to be a yet to be discovered neurological structure, until the discovery of the ipRGC cells in the eyes in the 2010s.
However, as the french sleep medicine institution SFRMS stated: "The biological clock can only be synchronized to 24h if the received photic inputs during the day are sufficient in length and intensity and if exposure happens at adequate timings", hence bright light therapy needs to be used in a specific manner to ensure efficacy. It is hence crucial for circadian rhythm sleep medicine to identify the parameters effecting bright light therapy efficacy.
Bright light, including artificial light therapy, affects the circadian rhythm by stimulating the intrinsically photoreceptive retinal ganglion cells (ipRGC) receptor cells (that can be connected to S-Cone cells) mostly present in the parafovea of the macula and nasal regions of the retina in humans (see also here), not the inferior nor superior nor temporal regions of the retina, but according to an animal study, each ipRGC cells in fact axonally innervates bilaterally the suprachiasmatic nucleus (SCN), with ipRGC cells located in the dorsal-temporal region of the retina primarily targeting the dorsal part of the SCN, and those located in the ventral-nasal region targeting the ventro-medial parts of the SCN, although studies on humans so far found no evidence of a dorsal-ventral gradient in ipRGC cells placement in humans, contrary to other animals. The ipRGC (also called mRGC cells or melanopsin OPN4 cells) represent about 1% of all RGC cells on average in a middle-aged human. The ipRGC cells' effect on the circadian rhythm is due to the melanopsin photopigment these cells possess and which make them intrinsically photosensitive contrary to other retinal ganglion cells (RGCs), as discovered in 2003 by Satchidananda Panda et al, in addition to the MW-opsin pigment. The more these cells are stimulated, the more phase advance and melatonin inhibition will happen (as well as a few other hormonal changes such as increased cortisol secretion). The photic input is then relayed by the ipRGC cells to various structures including the retinohypothalamic tract which releases glutamate to stimulate the NMDA receptors on the SCN, which in turns results in the activation of PKA, PKC and CK2 kinases from the calcium influx, which in turns phosphorylate CREB which then serves as a transcription factor for Per1 and Per2, finally entraining various peripheral molecular clocks throughout the body. Although the ipRGC cells relay the light signals directly to the SCN with equal contributions from both eyes, contrary to a previous widespread assumption, the SCN is not necessary for the phase advance effect of light therapy, likely through other unidentified retinorecipient regions, since the destruction of the SCN does not prevent phase advance by light therapy and a subsequent study shown that the ipRGC cells are sufficient to cause circadian rhythm and body temperature shifts without the need for the SCN to be preserved, which shows that the non-visual effect of light on the circadian rhythm is independent from the SCN. However, the SCN is necessary to synchronize all peripheral clocks accurately: when surgically isolated, the SCN is the only organ that can maintain its rhythm, with all other cells gradually going out of sync Furthermore, although ipRGC cells stimulation exquisitively inhibits melatonin in a dose-dependent manner (brighter light inhibiting melatonin more), the phase shift induced by light therapy is decoupled from melatonin: it's possible to produce a big phase advance without any significant melatonin inhibition, and inversely (see also here and here and here and here and here). In other words, melatonin suppression is not necessary for entrainment and bright light does not always suppress melatonin, contrary to what was assumed before. Hence, the goal of an effective light therapy is to optimize the stimulation of a maximum of ipRGC cells and to result in a behavioral phase advance, or a phase advanced core body temperature profile if a more objective proxy is preferred.
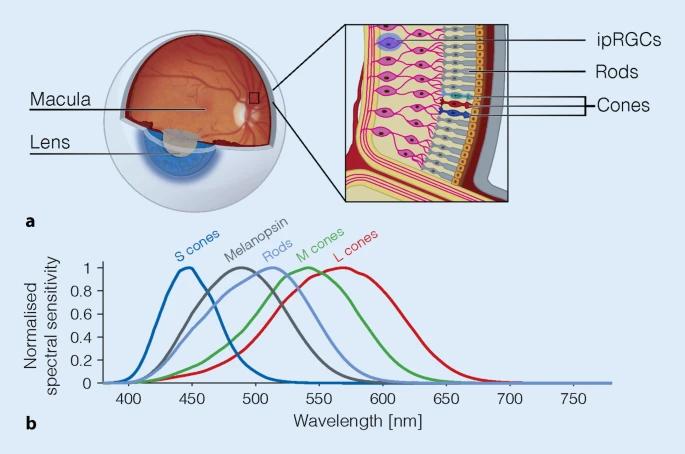
Overview of the retina photoreceptors. The ipRGC cells are mostly located in the parafoveal area of the macula and in the nasal regions of both eyes retinas, and can be connected to S cones which are cone cells optimized to detect blue colored light (although melanopsin cells are distinct from S cones). The peak sensitivity of ipRGC cells (Melanopsin curve) is around ~480nm , more precisely between 479nm and 482nm, and is observed in both humans and animals, and is very different from the peak sensitivities of classical scotopic and photopic visual systems. From the figure 3 of this review under CC-BY 4.0.
Several studies confirmed that rods and cones are unnecessary for circadian rhythm shifting, only the ipRGC cells are necessary, as demonstrated by experiments on rods- and cones-free animals and humans, although rods can contribute a bit to circadian rhythm shifting, and S cones can modulate the response of ipRGC cells depending on the light's color. Indeed, cones can suppress melatonin upon bright light short exposures of green light, but the effect decays exponentially with the duration of exposure, whereas the phase resetting effect is sustained with over long exposures of blue light via the mediation of ipRGC cells, which shows that both cones and ipRGC cells contribute to circadian resetting, but with different and non redundant purposes and mechanisms.
Furthermore, in addition to entraining the central clock (SCN) through the ipRGC cells, bright light exposure also entrains the peripheral (ie, body's organs) clocks. Indeed, although the adrenal gland, cornea, lung, liver, pituitary and spleen still exhibited robust circadian rhythms, it was out of synchronization with the environmental day-night cycle, which shows that peripheral clocks persist without needing the SCN, but the SCN plays a major role of synchronizing these clocks together. In other words, bright light entrains all clocks throughout the body.
Blue light stimulates the eyes' ipRGCs receptors more and produces the most phase advance compared to other colors, with 50 lux blue light producing as much effect as 500 lux white light under laboratory settings (hence a 10x increase in effect!), but amber light was also shown to affect the circadian rhythm (see also here) since the circadian system can also use variations in the light's color as a weak zeitgeber, in addition (or replacement) to light intensity (eg, to continue to be entrained under cloudy sunlight, by detecting if it's blue - daytime - or amber/dark - night time). However, amber light has much less sustainable effect on the circadian rhythm than blue light. Also, blue light constantly suppresses melatonin during the whole exposure, whereas green light does only so temporarily for about 90 min. Blue light inhibits melatonin faster than natural endogenous synthesis cessation, which means that blue light can be used at wake-up to more quickly eliminate sleep inertia due to melatonin left-overs, whereas amber light does not. Blue light alone is sufficient to constantly suppress melatonin as long as the subject is exposed. Blue light not only phase advances the wake-up time but also the sleep timing (ie, falling asleep earlier) as observed by several studies. In other words, light therapy also allows to sleep earlier (ie, sleep onset), likely because of the photic history effect increasing next-morning melatonin concentrations (see below), and hence complementing exogenous melatonin pills, although the effect on sleep onset is not always present as light therapy is more effective to entrain the sleep offset (ie, wake up time), but this may be due to the experimental design as it's necessary to be repeatedly exposed over almost about a week to get this melatonin increase effect because of melatonin secretion phase advance lagging behind by a few days after circadian rhythm phase advance. Sunlight is rich in blue light. Blue light also increases serotonin levels and hence vigilance, particularly at wake-up when sleep inertia is at its highest, and hence bright light is a well-known tool to clear brain fog due to melatonin left overs in the morning as well as having an antidepressant effect likely due to the increase in serotonin levels. Compared to green light, blue light improved the activity of brain areas associated with emotion processing, which demonstrates that blue light is likely more effective to treat depression (eg, SAD) than other colors. A well designed study controlling for equal illuminance and color temperature found that blue-light enriched polychromatic white light resulted in a 50% melatonin suppression for a 175 lux light source, whereas no melatonin suppression occurred with a blue-light deprived white light. Blue light therapy is also better indicated for wearables, light therapy glasses and other low energy devices, since it was estimated that monochromatic blue light alone is about 185 times more efficient for circadian rhythm shifting than polychromatic white light in terms of the amount of photons emitted, and hence energy, required to produce the same amount of melatonin suppression. This is because of circadian light subadditivity, which makes colors opposite on the spectrum such as blue and yellow or green and red to reduce the effect of each other when exposed to both simultaneously. Note however that this work on circadian light subadditivity used melatonin sampling as a proxy for circadian rhythm shifting. Light sources that are more effective at shifting the circadian rhythm are hence those that emit short wavelength light close to 480nm, such as blue LEDs, white LEDs and cold fluorescent lights (>5000K), and oppositely, light sources that have less effects on the circadian rhythm include monochromatic orange or red LEDs, low intensity halogen lamps, and incandescent bulbs.
Light intensity also matters, but with a limited range: the sensitivity bandwidth of the eyes is not the same for visual signals and non-visual signals (ie, circadian rhythm shift): 9 to 10 orders of magnitude for visual, whereas it's limited to 2 orders of magnitude for non-visual, hence the saturation point of light intensity is quite low, with a study showing that 2000lux light therapy already produces maximum phase shift with no additional phase shift with 8000 lux, and another study modelling the phototransduction by the human circadian rhythm by Rea et al suggesting that 1000 lux is already close the saturation point.
This saturation point is different for everyone since we all have different sensitivity to light (up to 50x fold difference!), so that some people saw a circadian rhythm shift and melatonin suppression achieved with light with an intensity as low as 5-10 lux with eyes closed (and even lower lux with eyes open). Also, with only 100 lux light therapy, this produces half of the circadian shifting effects of a 10K lux light therapy, "including melatonin suppression, circadian phase resetting and the alerting responses" (ie, vigilance boost). All these findings show that humans are sensitive to light of virtually any intensity and color, and the maximum phase shift is reached with maximum 2,000 lux, and potentially even below for some people especially those with circadian rhythm disorders if they are hypersensitive to light as some studies found, and hence that there is no need to race for the highest intensity and risk eyes burns, as there is no clinical benefit to expect beyond this relatively low saturation point, as recommended by the SFRMS. On the other hand, this also means that any effort to reduce artificial light exposure (ALAN) in the evening is of primary importance (see the section on dark therapy below).
Of critical importance, humans in industrialized countries are exposed to very little bright light. A study (see also the related PhD Thesis for more details), where the participants wore a light sensor pendant during 1 week, has shown that humans in modern society are exposed for the major part of their 24h cycle to low light (<500 lux for 21 h:27 min ± 23 min) even during daytime! Furthermore, they were exposed to long durations of very dim light during daytime (<10lux for 2h46min) and bright light during nighttime (>1000lux for 26min). Even through sunlight is available during daytime, the participants were only briefly exposed to bright light (>1000lux for 1h18min). This is in line with what previous studies observed: "these values are in the range of light exposure values for young adults in industrialized countries, most of whom typically receive only 20-120 mins of daily light exposure >1000 lux (Espiritu et al., 1994; Hebert et al., 1998; Mishima et al., 2001; Savides et al., 1986)." Hence, humans in modern society are mostly exposed to dim light during both daytime and nighttime, with only brief exposure to bright light both during daytime and to a lesser extent during nighttime, and even long bouts of very dim light exposure during daytime. This likely explains the major reason why light therapy can be so effective, even with low light intensity settings, as well as why dark therapy can be helpful, since humans in industrialized countries can be regularly exposed to >1000lux bright light during nighttime.
This low exposure to bright light can be experienced by anyone with a modern smartphone, as there are "lux meter apps" which use the phone's ambien light sensor on the screen to display how much light intensity the screen is exposed to. This is a very entertaining way to develop a first hand intuition of the real bright light exposure we get in our daily lives, which changes depending on the environment, head orientation, season, weather and time of the day. Anecdotally, the author of the present document measured exposure just in front of a very wide glass window with direct sun exposure over the course of two seasons in Belgium. Results: during autumn, >5K lux was common and >50K lux happened on cloudless bright days, but during winter with raining weather, daytime lux could be < 100lux for the whole day! Which shows that it's not just an issue with industrialization (although it worsen the issue), as virtually direct daylight exposure can still be lower than what is required for entrainment. Hence, daylight is inherently extremely variable on a logarithmic scale (ie, it can jump between several orders of magnitude). With less than 100 lux, this low amount of daylight would likely cause freerunning for any non24 individual. In fact, a theoretical study could indeed accurately estimate the circadian rhythm phase of humans using a mathematical model with logarithmic calculations, and found that more intense light ("larger amount of daylight") was associated with an earlier DLMO.
For stable entrainment, the goal is to oppose the natural daily phase delay of an individual's non-24 circadian rhythm with an equal or greater amount of phase advance, such as by using bright light therapy. Hence, we want to maximize the phase advance to set all chances on our side. To maximize, the goal is to stimulate the ipRGC receptors in the eyes the most, and hence "all studied characteristics of light pattern (timing, intensity, rate of change, duration, and spectrum) influence the circadian system".
Hence, bright light therapy efficacy depends on a set of factors, that current research is directed at optimizing : intensity, duration, spectral composition, time of exposure, non-linear effects (photic history, LDLD, green dim-light at night or red light exposure), orientation. In practice, in the context of optimizing entrainment therapies, these parameters can be classified in 3 broad categories with subparameters (not unlike a previous categorization by Lewy in 1987):
- Maximizing ipRGC cells stimulation:
b- the light intensity, with a linear proportionality between light intensity (in lux) and the ipRGC cells stimulation (ie, how much they will phase advance) as also shown in humans. However, the ipRGC cells are saturated quite fast with a relatively low light intensity, so past this saturation point, there is no benefit from more intense light as demonstrated by a study showing no difference in phase shift between 2K and 8K light therapy.
c- light color modulates the light stimulation on ipRGC cells as well as S-Cones, with blue light stimulating the most and red light the least and green light stimulating cones instead of ipRGC cells and hence losing efficacy over long durations of bright light exposure. Blue light alone appears to be much more effective than blue-enriched white/polychromatic light due to circadian light subadditivity.
- Light exposure timing relatively to the individual's circadian rhythm (ie, the Phase-Response Curve - PRC). This manifests as two practical effects:
b- a longer duration of exposure leads to a proportionally bigger phase advance: a study shown that using a relatively low light intensity of 500 lux but over 6.5h produced a 3h phase advance, whereas 1h of the same light therapy only produced a 1.15h phase advance. A previous 2011 study demonstrated a similar result, with 4h light therapy being more effective than shorter light therapy. Hence, as specifically demonstrated by a 2011 study, a longer duration of light therapy is more effective than increasing light intensity. Furthermore, there is no dead zone in the PRC curve (see also here), which means that there is no virtually no limit to the phase advance obtainable with light therapy, and that light therapy started later (even hours) than the wake up will still be effective (ie, during the circadian morning and circadian day), as long as it's before the circadian evening and circadian night. The VLiDACMel protocol is another evidence found by serendipity: initially light therapy was only of 1-2h in the previous protocol, until the author got fed up of the small effect obtained and decided to try an extreme duration of light therapy to determine beyond doubt if light therapy had any effect at all or if it was the placebo effect, and it ended up being a huge effect past 3h of exposure proportionally to the duration of light therapy.
- Optimize photic history: prior light exposure changes melatonin levels and response to future light therapy, "such that a history of less light exposure leads to a greater response to light" and inversely a history of greater light exposure will protect against unwanted phase delays due to light exposure during the biological evening (see also this review). Hence, repeated light therapy over multiple days will provide more effect than a single session, because a resistance to unwanted phase delays due to uncontrolled light exposure (eg, artificial evening light) will build up over repeated light therapy sessions. Consistent with this study finding elevated next-morning melatonin concentration after at least 5-7 days of bright light exposure, but not with less than 5 days, the author found that repeated exposure during about 10 days is necessary for the light therapy effect to converge to its maximum. This is likely, at least in part, due to the fact that melatonin onset (DLMO) has a delay of several days to catch up with circadian phase shifts, whereas the melatonin offset (stop of melatonin secretion) is instantaneous. Another potential explanation is that the SCN neurons do display after-effects of bright light exposure, with the ventrolateral neurons that encode phase are getting affected under less than a hour whereas the dorsomedial neurons that encode periods take several hours. In other words, entrainment of the wake up time is instantaneous, but the bedtime will continue to freerun for a few days until it finally gets entrained according to the new wake up time.
Point 1 should be taken care of by the light therapy device (especially if it's a blue light therapy glasses such as Luminette). Points 2 and 3 are reliant on user's handling of the device, and how compliant with the therapy they are (ie, to use light therapy daily for the required amount of time).
Photic history (or light history) is a crucial, but complex, phenomenon that remains poorly understood, as the temporal aspect of the circadian rhythm has been historically understudied. Photic history is the phenomenon characterized by how prior light exposure affects how the circadian rhythm will react to future light exposure, as well as other indirect changes such as increased next-morning melatonin levels, by previous days exposure to bright light. It may be through this melatonin regulating pathway that exposure to bright light can produce or eliminate biphasic sleep, which can be naturally induced by a too short exposure (10h) to bright light during the awake period, and eliminated by a longer bright light exposure (16h). In other words, the circadian system possess a memory of prior light exposures. Photic history can be both beneficial or detrimental depending on the timing: light therapy in the morning is less effective if an individual is exposed to light in the previous evening or night, whereas if the participant is exposed to blue light only during the biological morning and use dark therapy in the evening, this increases melatonin levels more than other colors while simultaneously phase advancing more than other colors. Furthermore, prior exposure to bright light during the biological day reduces sensitivity to light in the biological evening, whereas, inversely, prior exposure to less light (eg, only dim light) during the day increases the sensitivity to night-time light, which will more easily cause unwanted phase delays. Exactly both of the previous points are covered as the key takeaway of photic history effect by the SFRMS here. Indeed, the ipRGC cells that are responsible for the circadian rhythm shifting after light exposure were demonstrated to have "larger responses to light stimuli after dim light exposure, and reduced responsiveness to light stimuli after bright background light exposure". Indeed, another study demonstrated that bright light inhibits melatonin more after being exposed to dim light at 0.5 lux compared to 200 lux, which confirms that being exposed to bright light during the circadian day reduces the effect of evening bright light exposure. Aberrant light exposure can cause major cognitive, learning and mood impairment directly through the ipRGC cells, and the opposite is true, with light exposure having an antidepressant effect, and indeed a 2019 systematic review and meta-analysis found that light therapy is as effective as antidepressants for the treatment of both seasonal and non-seasonal (major) depression, with the combination of both being even more effective (see also this other systematic review). This confirmed the findings of the 1979 case study by Wehr et al that phase advances produce an antidepressant effect, and may support the phase advance hypothesis of depression, which proposes that the phase advance of certain circadian rhythm such as REM sleep propensity relative to the sleep-wake cycle is implicated in the pathogenesis of depression. A more fragmented light-exposure rhythm is associated with a more fragmented sleep. A more stable inter-days exposure to light is associated with a more stable sleep pattern in typical sleepers, although it's unclear how this would apply to people with circadian rhythm disorders. There is some preliminary evidence that the effect of bright (blue or white) light is increased by a previous exposure to red light, which suggests that not only light intensity but also color of past exposures modulate the effects of future exposures to bright light.
Photic history may stem from the GABAergic signalling that ipRGC cells can do in addition to the better known excitatory signalling, as GABAergic signalling involves chemical processes that can modify structure and hence memorize at the synaptic level. It was also established that light therapy controls both the DLMOff (stop of melatonin secretion - around wake up) instantly, but also the DLMOn (start of melatonin secretion) with several days of delay, the latter may partially contribute to photic history and explain the delay before the full effects of light therapy are observed. This melatoninergic pathway for photic history seems to not be activated by intermittent light exposure since intermittent light therapy inhibits melatonin much less than continuous bright light therapy despite producing almost as much phase advance. The effect of photic history may span days or weeks. However, photic history does not appear to impact PIPR and hence pupillary light response, but supine position (laid down) does increase PIPR, hence being laid down may increase photosensitivity, and this is of importance for MRI studies.
To summarize, photic history shows that light therapy in the biological morning not only phase advances, but also 1- makes the participant more robust to insomnia by increasing endogenous melatonin levels and hence indirectly consolidating sleep, 2- reduces the sensitivity to phase delaying lights in the biological evening and hence may reduce the need for dark therapy.
Photic history explains why the effect of light therapy snowballs until it reaches its max effect at about the 10th day, because light therapy not only phase advances instantly the circadian rhythm, but also reduces evening light phase delays, so over time the phase advance becomes bigger and bigger. The author is convinced photic history plays a major role in the special effects I have observed with very long light therapy, and that this is a critical parameter to control for optimal therapeutic yields. Indeed, photic history explains the following practical observations:
- why it takes a few days to work: 2 days for the first effects, 10 days to reach maximum phase advance without changing anything during the 10 days, because light therapy is self reinforcing ;
- why the first effects observed are a stabilization of the wake up time, and only later of the bed time.
- why feeling sleepier at the correct time after light therapy because melatonin levels are increased the biological nights after ;
- why a longer exposure increases non linearly the phase advance, because not only the phase advance is linearly increased, but prior light therapy protects against evening light exposure so that sleepiness and melatonin levels will stay at high levels even when exposed to light. This point also explains why non-24 can be reinforced through a vicious cycle of dim lighting in the awake period (which can be inversed with the day-night cycle), which will only reinforce the hypersensitivity to light and hence the circadian misalignment problems. But the opposite is also true, as it can be used to create a virtuous cycle: through photic history, light therapy can make the user less hypersensitive to light (ie, more robust to unwanted phase delays due to bio evening lights).
Although photic history seems to have been mostly forgotten nowadays, it was already known since at least the studies by Rosenthal et al on bright light therapy for SAD in the 1980s (they are the original authors), stating that "patients generally respond to bright light therapy within four days of starting treatment and relapse within four days of discontinuing treatment" and note elsewhere that SAD patients usually respond within "2 to 4 days of initiating treatment", emphasizing the initial delay when starting treatment.
The suprachiasmatic nucleus (SCN) also modulates feeding behaviors, and can promote the consumption of dense food (ie, weight gain and obesity). Hence, light therapy may modulate feeding behaviors (ie, hunger) through the SCN.
Is more light intensity always better? Not necessarily, because there is a physiological limit beyond which light intensity doesn't matter because we already reached ipRGC cells max stimulation. This maximum stimulation limit was quantified and is limited to 2 orders of magnitude (eg, 100-10000 lux or 10-1000 lux, the exact boundaries of light sensitivity are not known and can vary from one person to the next). Since blue light is about 185 times more efficient to stimulate ipRGC cells, it's much easier with blue light to reach the max stimulation of ipRGC cells and hence maximize the phase advance than with white light or other colors. That's why most blue light therapy glasses only use a low lux setting such as 500 lux or max 1500 lux, whereas white light therapy lamps use 10K lux (the reduction in lux also serves as battery saving strategy since 10K lux is too much to run on a battery). Although more light intensity is not always necessary, sufficient light intensity is necessary to stimulate the ipRGC cells sufficiently to get enough phase advance to be entrained. It remains to be seen how little is sufficient for non24 entrainment, but 100 lux light therapy was found to be sufficient to produce half of the circadian shifting effects of 10K lux light therapy. Furthermore consistent entrainment was achieved during the self-experiment with 500 lux. The second subject could stay entrained for months at the time of this writing with only computer screens (but during spring-summer, so might be confounded with sunlight complementary effect). For comparison, computer screens at maximum brightness usually emit about 250 lux.
In addition to light intensity, the duration of light exposure also proportionally increases the amount of phase advance (see also here and here), and there is virtually no maximum limit since there is no dead zone in the light's PRC curve (see also here). The dead zones of a PRC curve refer to the time from the circadian afternoon to the start of the circadian evening, where the phase shifting effect of bright light is diminished, but it is not null. Scientists and clinicians to used to think that light therapy would advance only during a limited timeframe around wake-up, but we now know that, since there is no dead zones, light therapy works for much longer than that can be started much later than wake-up and still works. Also, even if the circadian phase shifting effect is diminished during this late period, the circadian period lengthening/shortening is not, because this effect on the circadian period depends on the total duration of bright light exposure and is encoded by a distinct SCN neuronal population and different retinal ipRGC cells populations than circadian phase. And we can go further by mentioning circadian bifurcation, which shows that we can split the circadian rhythm into two circadian periods using a precise bright light/dark exposure schedule, that involves exposure during what was thought to be the dead zones, and regardless of light intensity, showing once more that the dead zones can in fact be as sensitive as the other zones under some conditions such as circadian bifurcation. In summary, bright light therapy always has an effect regardless of timing, but some timings of administration relative to the individual's minimum core body temperature point (an anchor of the circadian phase) are more effective. This is why increasing the duration of light therapy is much more effective than increasing the light intensity (see also this commentary), as we have much more leeway to increase the phase advance, whereas bright light intensity saturation is reached with pretty low lux levels, around 1000 to 2000 lux. This lack of dead zone in light's PRC curve (see also here) also explains the result found in this study about 10h vs 16h of light therapy producing a biphasic or monophasic sleep respectively, this result shouldn't be possible if there was a dead zone in the light's PRC curve. This disproves the 1960's critical photosensitivity period hypothesis, which posited that bright light could affect the human circadian rhythm only under a specific time window. The temporal aspect of the circadian rhythm, including the duration of bright light exposure, is hence a crucial factor, despite being historically overlooked by researchers as they focused on the spectral sensitivity of the circadian system. The previously held assumption of a dead zone in the light PRC curve may also have played a role.
Phase advance is proportional to the duration of bright light therapy's exposure, with an almost linear increase up to 6.5h past wake-up, hence showing there is seemingly no limit to the amount of phase advance that can be obtained with longer durations of light exposure. Reinterpretation of the results from the Figure 2 of this study.
In fact, although not well known, Czeisler's team conducted a study in 2012 on 14 healthy men with a very long bright light therapy regimen of 5-8h of bright light exposure everyday for 5 days, which allowed them to achieve 8h of phase advance on average. The light therapy setup involved 10K lux lamps on the ceiling, walls and floor, with the subjects being restricted to this room for 5 days. During the evenings, light was dimmed to 5-15 lux. Initially, the astronauts subjects slept from 00h-08h, and at the end of experiment they slept from 16h-00h (target was 14-22h). To ensure light therapy would happen during the phase advance portion of the PRC curve, they used a mathematical model to predict the CBTmin point and planned light therapy to start 1h after the scheduled wake up time, in order to ensure some margin despite the progressive phase advance and hence moving CBTmin. This study further found that moderate lighting of 90-150 lux suppressed melatonin as much as 10K lux, further emphasizing the importance of dark therapy in the circadian evening. Of course, the results were obtained in a strictly controlled lab setting, with perfectly scheduled dark therapy and light therapy, with no interference from sunlight. In a realistic, at-home setting, a reduced magnitude should be expected (eg, maybe 3-4h of phase advance instead of 8h in the lab). This groundbreaking study demonstrates the viability of very long bright light therapy to produce significant phase advances in a short time span on the human circadian rhythm, in line with the current document's author's experiments results. Keep however in mind that this study only spanned one to two weeks, hence it didn't assess the long-term stability of the acquired phase shift, but since they could assess core body temperature (with a rectal probe) objectively confirms the circadian shifting effect and magnitude. One thing to note is that they found much better core body temperature shifting with high lux (10K) than with moderate lighting (90-150) lux, since for the high lux group they found that the core body temperature matched with the scheduled sleep-wake pattern, whereas the moderate light group still had a CBTmin delayed 5.5h compared to the scheduled wake up time:
Another study provided some figures of how much phase delay (not phase advance, which is more difficult to obtain) can be expected for different light therapy durations:
> one hour of bright light exposure (minus the delay under control conditions) resulted in a mean phase delay of 10 min, 2 h of light exposure resulted in a phase delay of 53 min, while 3 h of light during the night delayed the melatonin onset by about 1.5 h (averaged across different intensities).
A recent study published in 2022 on patients having developed a non-24 like disorder following severe brain injury found that the use of very long bright light therapy to simulate a whole artificial day allowed to reduce the freerunning period by 3h, which is a staggering amount!
Interestingly, seasonal affective disorder also requires 30 to 90 min of exposure at 10K lux for most people, although some may need more or less, contrary to the commonly prescribed too short duration of 20 min of exposure which stems from one of the earliest papers on using 10K lux bright light therapy which found that 20 min at 10K lux was equivalent to 1 or 2h at 2500 lux, the intensity used in the original paper on bright light therapy for SAD treatment, but this paper never demonstrated that 20 min was sufficient to treat most SAD patients, and hence this number remains to this day a clinical lore. In fact, it also did not study the circadian rhythm shifting effect of short very bright light therapy neither, but this 1990 study is unfortunately the source that initiated and spread the misconception that bright light therapy need to be highly intense but short, which was later contradicted by Dewan et al's study demonstrating that increasing the duration of light therapy was much more effective than the intensity, especially since the circadian system saturation point can be reached with a light intensity as low as about 1000 lux and that there is no dead zone in the light PRC curve. Another study further strengthen the inadequacy of the current focus on bright light intensity, as it was shown that low intensity light therapy (100 mLux) is sufficient to reduce objective daytime sleepiness as monitored with EEG, with fewer differences with higher illuminance, suggesting once again that duration and timing are likely higher yielding modifiable parameters. A systematic review on the use of light therapy to augment the effect of antidepressants for major depression and bipolar depression found that an illuminance greater than 5000 lux for periods longer than 30min were required to observe a significant effect.
Informally but interestingly, a member of the N24 Discord server, owner of a Beurer TL30, contacted Beurer in 2020 to ask what they would advise to treat circadian rhythm disorders, to which they replied to use the lamp for 4 hours at 1250 lux distance, suggesting they also are aware that long light therapy leads to more phase advance that can help with circadian rhythm disorders, maybe due to this 2011 study.
During the current document's author's self-experiment, it was found that lengthening the duration of light therapy was more effective to get additional phase advance in comparison to increasing light intensity, the latter showing no significant benefits but produced minor but uncomfortable side-effects such as dizziness and headaches due to sudden exposure to bright light. This is in line with the results of a previous study.
The effect of light on the circadian rhythm is not solely due to its inhibition of melatonin, because it was shown that intermittent light can phase advance the circadian rhythm without reducing melatonin secretion, and that a longer light exposure causes more phase advance irrespective of any effect on melatonin. Hence, melatonin is decoupled from the circadian rhythm as shown by several studies .
Blue light therapy's effects (both on the circadian rhythm and the potential phototoxicity) may be dependent on the user's age: the eyes lens (cristallin) obscures with age to a yellowish tint which is acting as a blue light filter and filters more with age, with 60 years olds having an average 2 times blue light filtering as 20 years old, and newborns having no blue light filter. Furthermore, the number of ipRGC cells is reduced with ageing beyond 80 years. The sleep of older individuals is also more fragmented and the circadian rhythm is phase advanced, has a lower amplitude and a shorter period. Hence, older individuals may need longer exposure to brighter light than when they were younger. A 2017 study found that younger adolescents see more inhibition from bright light than older adolescents. Another study in elders found no evidence that age affected the direction nor the magnitude of the circadian rhythm shifting effects of light therapy. However, another study found that melatonin inhibition by bright light varies with age, and interestingly the maximal suppression is achieved at different light colors depending on age, with younger individuals being more sensitive to shorter wavelength (ie, blue light), in line with the observation that the eyes lens obscure with age to a yellowish tint. A 2019 systematic review found that age impairs melatonin endogenous secretion and inhibition by light, but has no effect on the phase advance induced by light therapy. Hence, the light therapy duration or intensity can be adjusted accordingly to the user's age to adjust for the reduced efficiency on melatonin inhibition (ie, brain fog), with younger individuals being more sensitive to blue light, but the circadian phase advance produced by bright light therapy does not change with age. This also explain why children and adolescents are more affected by night-time exposure to non filtered blue light emitting screens such as phones and computers.
Although the phase advance effects of either bright light and melatonin are limited, combined their effects are additive (see also here and here and here and here), so that you can for example get 1h phase advance from melatonin and separately 1h from light therapy, and combining both would give you 2h in theory (in practice it will be less because there is some natural biological variability from day-to-day, but at least by combining multiple therapies you get more leeway to stay entrained despite uncontrollable disturbances).
So it is possible to try to use only light therapy alone, it was shown in lots of studies and in systematic reviews to work without needing melatonin, but of course you will get less effect. Expect about 1-2h of phase advance with each treatment alone (see here and here for light), and combined the effect is additive (melatonin + light therapy can help you to achieve 2-4h in total).
Can the light therapy glasses work even with eyes closed? Yes:
> The eyelid acts as a red-pass filter (Zeitzer et al. 2014) and transmits only approximately 3%–14% of light (Robinson et al. 1991) in a wavelength-dependent manner. Thus, the retinal exposure of light depends on the status of the eyes (open, closed).
> [...] Similarly, Figueiro and Rea (2012) showed how light delivered through eyelids during one hour suppressed melatonin and phase shift DLMO. Both studies suggested that phototherapy may also be given with closed eyes, and even while asleep (Zeitzer et al. 2014).
Source: Systematic Review of Light Exposure Impact on Human Circadian Rhythm, 2019, Chronobiology International
But eyelids filter most of blue light, even in neonates:
> Datalogging studies demonstrated that [...] the eyelid [...] acts as a predominantly red-pass filter, permitting 21% transmission at 700 nm with less than or equal to 5% transmission at 580 nm.
So yes it works, and even with light as low as 5-10 lux while the eyes are closed, but has of course much less effect on the circadian rhythm than with the eyes open, due to both the reduction in light intensity but foremost the red pass filter that mutes most of the blue spectrum. This is exploited by a new kind experimental light therapy device called a "light mask", a light-emitting device in the shape of an eye mask to be worn during sleep and emitting light during the last 4 hours of sleep before wake-up, hence with the eyes closed, which shown some efficacy in phase advancing individuals with DSPD. It's likely that much longer exposures than usual (as they did with exposing for 4h to the light mask) are necessary to get any benefit from light therapy with the eyes closed, since ipRGC cells are much more sensitive to intense blue light, so a higher and longer intensity of red light therapy is likely necessary to compensate for the non-optimal stimulation wavelength.
But eyes closing can nevertheless be useful. If the light is blinding you when you switch on the Luminette, close your eyes at first for a few seconds/minutes and then open them, your eyes will have accommodated by then and the light won't blind you anymore. This should also avoid the dizziness and headaches that can happen when being suddenly exposed to bright light due to sudden increases in cortisol. It may also avoid the changes in the macula induced by sudden bright light exposure in a dim lit environment, by gently allowing for pupil contraction, the pupil area being correlated with melatonin suppression (TODO: and phototoxicity?). Then, when the eyes are contracted, it's better to open the eyes as soon as possible to get the full circadian shifting effect.
The bottom-line about the safety of ocular blue light phototherapy for circadian rhythm disorders and SAD: ocular blue light phototherapy is safe for the eyes, except if you have a photosensitivity disease such as a retinal disease or epilepsy or another disease that requires you to protect from natural sunlight by wearing sunglasses or similar protective eyewear.
What if light therapy does not work for you? Well, in the future definitely researchers and clinicians should try to devise test of circadian photosensitivity, to check if an individual is likely responsive to light therapy before they acquire such a device. One way, that the individuals can already do by themselves, is to maintain a sleep diary, and observe if a pattern of relative coordination can be observed (ie, faster freerunning when out of phase with the day-night cycle, and slower when in phase). Since relative coordination is due to sunlight exposure, this is strongly suggestive of responsiveness to light therapy. Other potential avenues are occular tests, by checking the pupils area or the pupil's contraction reflex to sudden bright light exposure, since it's the same ipRGC cells and their melanopsin photopigment that control both pupil's contraction reflex and circadian rhythm shifting in response to light exposure (see also here). Indeed, genetically muting the melanopsin pigment severely impairs both the pupillary light reflex and circadian alignment. Furthermore, pupil area is proportional to melatonin suppression. In practice, pupillometry can be done with measuring either or both the amplitude and speed of contraction post light exposure as they are strongly correlated, with the light intensity, starting size of the pupils before the test and the age of the participant being irrelevant. A study on DSPD found that the pupil light reflex could diagnose circadian DSPD, as they had a faster pupil contraction speed than those without a circadian misalignment, and with brighter light being more effective to diagnose. This strongly suggest that individuals with DSPD are hyper photosensitive, and it's safe to assume the same for non-24. Interestingly, individuals with autism were also found to display a pupillary light reflex with more amplitude and correlated with their sensitivity to stimuli (sensory processing disorder), which is even more intriguing considering circadian rhythm disorders are very common for people with autism to the point some scientists suggest that circadian rhythm disorders underlie autism. Smartphone-based camera and LED flash apps have been demonstrated to provide an objective and equivalent assessment as infrared-based pupillometry, with reduced cost and increased versatility (see here and here). In fact, there is already a test to quantify the sensitivity of ipRGC cells in the eyes by ophthalmologists or optometrists: the maximum post-illumination pupil response (PIPR) after blue light exposure of variable light intensity or via a chemically induced test.
Interestingly, although apriori it is assumed that blind individuals with non-24 would not be responsive to light therapy, a study of 21 participants with blind non-24 found that 2/3rd were responsive to bright light, since their circadian rhythm demonstrated a relative entrainment. This may be explained by the fact that the visual and non-visual (circadian rhythm shifting) pathways are distinct, and hence that blind individuals may very well have their visual pathway damaged but their non-visual pathway intact. But then why are these individuals free-running (non-24) if they can still be entrained by light? Because since they lack their visual pathway, they are prone to stay in darker environment (ie, to position themselves less in places and in an orientation that is more illuminated). Hence, light therapy may represent a worthwhile intervention for blind individuals with non-24 too, and future clinical trials should investigate that.
A clinical trial found that bright, blue light therapy was found to increase effective connectivity within the DMN (awareness network) in patients with a mild traumatic brain injury (mTBI), who often suffer from post concussive symptoms including mood impairments which can improve with bright light therapy. Indeed, "mood dysregulation, including lower self-reported happiness and associated positive emotions, appears to be closely intertwined with the brain’s default-model network (DMN)." It remains to be seen whether patients with disorders of consciousness could also benefit from bright blue light therapy.
There is an emerging body of evidence, pioneered by Van Someren's team, that bright light therapy can reduce the cognitive impairments and may reduce the disease progression of Alzheimer’s disease and related dementias (ADRD) by consolidating sleep and the circadian rhythm. This neuroprotective effect is arguably not directly due to light therapy but by the reduction of sleep deprivation and circadian misalignment, as well as the increased melatonin secretions via photic history, which is a strong body-wide antioxydant.
To conclude, we will cite Figueiro et al, on the importance and challenges of bright light exposure control:
> The challenge for lighting researchers and professionals is that they have been so closely tied to thinking about a particular building – i.e. a single place where one needs to see tasks and perceive ambience instantaneously. Circadian hygiene is not instantaneous, but cumulative. Today, because people have luminous displays and active lives that change their 24-hour pattern of light and dark, they do not have a single lighting entity that is responsible for total 24-hour light exposure patterns, and therefore cannot address 24-hour light exposure issues. As shown by Rea et al.,68 however, it is the temporal relationship between the total circadian light–dark and activity–rest patterns that needs to be measured and controlled to reduce circadian disruption.
Indoor room lighting is not sufficient. For example, a study on undegrad students found that blue light enriched light therapy relieved post-lunch dip impairments in mood, subjective sleepiness and performance, contrary to normal indoor lighting.
Is exposure to sunlight through a glass window sufficient for light therapy? A study shown that glass windows do not filter all UVA, up to 75% pass through, although the glass color matters as green or a sunlight filter blocked all UVA, laminated glasses reduced UVA, and UVB is totally blocked by all glasses. Since UVA is close to the blue wavelength which is maximally stimulating the ipRGC cells, there is a possibility that some of this blue spectrum light is filtered too, reducing the effectiveness of sunlight therapy via glass windows. However, light intensity is still far more than enough usually to stimulate the ipRGC cells. The light intensity can easily be checked by using a smartphone lux meter app (see the next section), to ensure that there is enough light intensity (lux) to saturate the ipRGC cells.
Bright light can also affect the metabolism directly by regulating insulin, as bright light exposure during the circadian night can cause decreased insulin secretion and overall failure of pancreatic islet cells as shown in a rats study
Lastly, there is room for future discoveries on light therapy and circadian rhythm shifting, as studies on mice identified at least 6 different subtypes of ipRGC cells. Whether the subtypes have different functions and stimulation thresholds and conditions remain to be explored.
TODO: add the new insights on how the adenosinergic pathway mediates the circadian rhythm regulating effect of bright light: https://doi.org/10.3389/fphys.2022.1085217
> The mechanisms by which light acts on the circadian clock are broadly understood as a linear pathway from the eyes through to transcriptional changes in the SCN, recently reviewed in (Ashton et al., 2022). SCN cells receive light input via synaptic connections with the pRGCs. This then initiates a cell signalling cascade within SCN neurones which acts to reinforce, and if necessary adjust the phase of the clock, either by causing an advance or delay of the clock timing. Glutamate and PACAP, released from pRGC terminals act upon the molecular clockwork within SCN neurones via intermediary kinase cascades to converge on the cAMP response element binding protein (CREB), which is activated by phosphorylation at Ser133 and Ser142 (Ginty et al., 1993), resulting in the rapid and transient elevation of transcription of light-responsive genes such as Fos, Dusp1 and Egr1, whose expression is closely correlated with behavioural phase shifts (Jagannath et al., 2013a). The canonical clock genes Per1 and Per2 also exhibit a phase-dependent increase in mRNA expression, peaking around 1-2 h after light exposure, with Per2 peaking later than Per1. The modulation of Per1/2 by light is currently thought to be the pathway by which the core clockwork is regulated. However, the effects of light on the circadian clock are highly dynamic and dependent upon the time and intensity of light exposure, and other factors including sleep/wake history. How these different stimuli are integrated to achieve entrainment remains largely unknown.
> [...] A few neurotransmitter and neuropeptide signalling pathways including melatonin (Redman, 1997), vasopressin (Yamaguchi et al., 2013) and adenosine (Antle et al., 2001; Jagannath et al., 2021) have been shown to modulate circadian entrainment (modifiers of entrainment reviewed in (Golombek and Rosenstein, 2010)).
> [...] Adenosine signalling through A1 and A2A receptors encodes sleep need (Lazarus et al., 2019). However, several groups have shown that adenosine, and adenosine receptor antagonists including caffeine, directly regulate circadian timing in several rodents and in humans, independently of their effects on sleep/wake physiology (Oike et al., 2011; van Diepen et al., 2014; Burke et al., 2015; Ruby et al., 2018).
> [...] Furthermore, we showed that the significance of adenosine signalling is to encode sleep/wake history to the clock and modulate its response to light in mice.
Other effects of non-UV bright light therapy
In mice, ocular stimulation with blue light (463 ± 50 nm, 20 kJ/m2) for at least 10 days also promotes hair growth, but not green light (522 ± 50 nm) nor when the optic nerve was destroyed:
> Fan and colleagues elegantly showed that daily blue light (463 ± 50 nm, 20 kJ/m2) for 10 days stimulated hair follicle anagen, leading to a significant hair growth compared to unexposed animals. Green light stimulation (522 ± 50 nm), at the same dose, also stimulated hair growth but less effectively. Blue light-induced hair growth was lost when the optic nerve was crushed and reduced in animals with rod and cone degeneration. Interestingly, in Opn4−/− mice, blue light stimulation did not affect hair growth compared to wild type animals. Mechanistically, blue light stimulation to the eyes increased systemic sympathetic activity and norepinephrine levels in the skin, and activated hedgehog pathways in wild type animals. All these effects were lost in Opn4−/− animals [194].
It is well established that vitamin D is secreted by the human body in response to skin exposure to UV light. But in addition to UV photosensitivity, it was recently discovered that human skin also expresses neuropsin pigments OPN5, which makes skin also photosensitive to non-UV bright light, and that the human circadian system may also integrate inputs from extraocular opsin photopigments similarly to other mammals, birds, fishes and amphibians. Skin photosensitivity to non-UV bright light was shown to be dependent on OPN5, not OPN4 (melanopsin, as in the eyes ipRGC cells).
> Circadian clocks within mammalian skin control the response to UV light [42, 43], as well as cell cycle progression in hair follicles [36] and keratinocytes [44, 45], and response to physical injury [46, 47]. The current results would suggest that light modulation of these clocks through OPN5 function may significantly influence these physiologies. Further, these results suggest that mammals, like fish [48], amphibians [49], and birds [17, 50], utilize extraocular opsin photopigments for direct, light-dependent regulation of circadian clock function in some peripheral tissues. This challenges the widely held dogma that peripheral circadian rhythms within mammals are synchronized exclusively by the master SCN circadian pacemaker via ocular photoreception [51, 52] and suggests that mammals also use local light sensing in peripheral tissues for this purpose."
This can help us answer the question of whether skin exposure to bright light, without exposing the eyes, can shift the circadian rhythm? Apart from the production of vitamin D via exposure of the skin to sunlight's UVs, which are mostly filtered by glass windows, but which has nothing to do with circadian rhythm shifting except for the indirect effect of a vitamin D deficiency on sleep processes, recent studies and reviews demonstrated that the human skin cells express neuropsin pigments OPN5 (ie, extraocular opsin photopigments) which can directly affect the circadian clock of peripheral tissues, such as hair growth, without inputs from the suprachiasmatic nucleus. The authors note that skin exposure to short-wavelength light also triggers some hormonal secretions such as β-endorphin and urocanic acid production. However, as the authors note, the maximal stimulation was for these skin photopigments were reached for UVA wavelength, and did not observe "significant phase shifting activity with light of 475 nm or 525 nm, a wavelength range encompassing maximal sensitivity for melanopsin (Opn4) and rhodopsin (Opn2)". This means that dark therapy does not require avoiding skin exposure to bright light or UVA light apriori, as such light only affect the exposed skin tissues local clock, not the main circadian rhythm clock (SCN) and does not inhibit melatonin. Furthermore, exposing skin to the artificial light therapy's light such as from Luminette will not shift the skin local clock, since the OPN5 pigment is not sensitive to this wavelength but to shorter ones. Nevertheless, the authors of the mice study found that the mice could entrain to a 24h schedule with only their skin being exposed to UVA light, which suggests that, in the absence of all other timecues, the skin may feedback into the central clock in some way. It's also conceivable that the skin tissues local clocks may get in circadian misalignment with the master clock and other peripheral clocks under specific conditions (eg, differently timed UVA and blue light exposures).
Why light therapy glasses?
Summary: Any light source that stimulates the retinal ipRGC cells will modulate the circadian rhythm, but they have different pros and cons: sunlight is the strongest by far but is incontrollable and not sufficient during winter because of late sunrise and bad weather, indoors light is often not intense enough and exposure changes when we move between rooms, (cheap) light therapy lamps are often underpowered and too cumbersome to use for long durations. Wearable light therapy devices, such as light therapy glasses and especially those with long battery like Luminette, are a well rounded alternative that offer very long exposure under all circumstances even when in bed or while moving, ensuring the same reproducible bright light dosage all the while and regardless of the weather and season. Outdoors sunlight should still be used with reason (ie, avoid sun burns and skin cancers) whenever possible, with artificial wearable light therapy devices used indoors in all situations.
Now that we saw how light therapy works, and what are the main parameters for efficacy, it is worth asking what is the best light source or best light therapy device for light therapy.
Anything that can stimulate the eyes' nasal hemiretina ipRGC cells will activate the retinohypothalamic-pineal pathway and the SCN, and hence modulate the circadian rhythm, so that all light sources are viable and effective. However, different light sources will have different usage cases and pros and cons.
There are several potential light sources: the ubiquitously available and most powerful outdoors sunlight ; indirect indoors sunlight ; light therapy lamps ; wearable light therapy devices such as light therapy devices. We will evaluate the merits and limitations of each light source below.
What about outdoors sunlight? while it is true there is no stronger bright light source, it is hardly controllable and very inconvenient. Just like as in photography and videography where it is known that sunlight is an irreplaceable and uncopiable light source, artificial light sources are widely used despite being MUCH less powerful, because they are infinitely more controllable. When you make a movie, you don't necessarily want a yellow tinted sunlight lit scene, you may want to create tension with dark green hues for example, which are not possible with sunlight. Sunlight is dependent on the season, weather, and your circadian phase. Indeed, if you are severely out of phase with sunlight as is likely the case if you have DSPD or non-24, then sunlight exposure may phase delay you or sunset may occur before you fulfilled the duration of bright light exposure you need. During winter, sunrise happens much later than you may need, past the start of your wished morning, so then artificial light therapy is necessary. It is also much more convenient and effective to use artificial light therapy devices, because you don't need to jump out of bed and get outside for HOURS, you can just put on your wearable light therapy device on your head as soon as you wake up and keep wearing it the whole circadian morning and day as you need, for the exact duration you need.
Case in point: it can often be heard by people suffering from unmanaged, freerunning non-24 that they are insensitive to bright light, because they can get exposed to summer sunlight for days or weeks without being affected. I can state with certainty that this is a misconception, because I experienced the same. When my own non-24 disorder was not managed, I myself believed that I was insensitive to bright light, as i could stay in a camping in summer for weeks and still freerun and even sleep right under the noon sun for hours and risk dying of sunburn (which did not happen thanks to my wife who moved me into a shaded area when she found me unexpectedly asleep outdoors). In fact i always was photosensitive, but mistimed sunlight exposure did not seem to affect my circadian rhythm because it had counterproductive phase advance and phase delays effects at the same time because of being exposed both in my phase advance and my phase delay parts of my light PRC curve. In other words, the exposure to bright light was not properly calibrated to my needs, just like taking the wrong dosage if a drug can make it completely ineffective. This is very different from controlled exposure, which is possible with outdoors sunlight, but is much easier with wearable artificial light therapy devices. Hence, sunlight therapy can be attempted as it is free and available almost everywhere, but this is much more cumbersome and impossible to fully control for optimally individually tailored light therapy, hence inefficacy of sunlight exposure is not predictive of inefficacy of artificial light therapy at all.
There is a common argument that circadian rhythm disorders are caused by our sedentary lifestyle of living indoors, that these are caused by our lack of exposure to outdoors sunlight. This is an oversimplification, and of course as often with oversimplifications, it is false. Although lack of outdoors sunlight exposure due to our modern urban lifestyles do indeed certainly worsen circadian rhythm disorders, circadian rhythm disorders require an extreme amount of bright light exposure that is not possible under normal circumstances (which is supported by recent evidence such as findings that PIPR metrics are much higher in people with DSPD and even more in non-24 - ie, less bright light pass through the retina). For example, the present document's author require 7-9h of daily bright light exposure to be entrained to a 24h cycle (because otherwise he has non-24). The common argument suggests that similar effects could be obtained by direct sunlight exposure. But this is not humanly possible. This would mean staying 7-9hrs outdoors, under ALL seasons and weathers, and including not only when it's cold, but when there is direct sun exposure, hence with ample opportunities for life threatening sun burns and skin cancers, since sunlight also contains ultra-violet light (UVs) that artificial light therapy devices do not (side-note: this is why artificial light therapy devices cannot help to produce vitamin D contrary to sunlight, as UVs are necessary for Vitamin D synthesis). This common argument simply forgets that during all ages, humans always were frail to environmental conditions and always sought to live in structures to protect themselves, first by living in caves when they could not build, and later by building their own houses. Hence, suggesting that people with circadian rhythm disorders should live outdoors all the time is not only unrealistic, it is fantasist, as humans never lived this way and are not built to live this way.
What about indoors sunlight exposure? Well, if you use a lux meter, you will observe that sunlight loses intensity very fast, and often houses don't have the best orientation or structure to allow for unfettered exposure to sunlight. This is because light loses intensity as a quadratic function of the distance to the light source, so the more indirect the light source, the more dim it will be when it will reach the end point. Our eyes do not see this quadratic loss, because our vision is logarithmic, indoors sunlight is usually very dim. A simple lux meter app on any modern smartphone will be sufficient, as nowadays ambient light sensors on the front are made to mimic the human eyes sensitivity to light intensity very well, as this is very important for smartphones to adequately auto-adjust the screen brightness given ambient light conditions. Comparing the lux meter app readings with our subjective impressions often give a very surprising idea of the difference between what we think we perceive, and the real metrics. For example, we may see a shaded area in-doors as being just half as bright as outdoors, when in fact it is 10-20x less intense (from 6000 lux to 300 lux for example). The smartphone screen should face towards the same direction as your eyes would be. Try also to keep the smartphone at the same place, but just change slightly the orientation around, it is often surprising how much the lux reading changes with just a slight change in orientation, but this indeed represents how light bounces on walls and how much intensity can be lost in the process (because then not only there is the quadratic loss of lux intensity as a function of distance from the light source, but also walls can absorb some of the light). As a general rule of thumb, 500 lux should be considered the minimum exposure at eyes level to get effective bright light therapy (this is also the minimum offered by the Luminette glasses), but your mileage may vary. Note that behind glasses, most UVs are filtered (so almost no risk of sun burns nor skin cancer in theory).
Now let's assume you are in an in-doors environment with high enough lux exposure to sunlight, let's say a work office with a wide bay window. In theory, if you get exposed to bright light during your circadian morning and day with whatever light source, that's going to be effective in theory given there is enough light intensity (lux). So here this environment looks great, you don't need any artificial light therapy device? Maybe you can reduce the usage of artificial light therapy devices, but I would argue that you still need them. Think about in practice: if you have a bay window, sure it works when you are in front, but it does not when you are commuting in the subway, when you go to the toilets, when you go to get a coffee, when you go to lunch, when you go to the meeting room to meet with partners or the rest of the team, etc. All these moments add up pretty fast to hours of missed light therapy you could have done with a wearable device, and that's so many hours that won't be helping your phase shifting or entrainment: the effect is vastly different between 2h versus 4h of bright light exposure. This is why wearable light therapy devices are strongly recommended although not absolutely necessary for the VLiDACMel protocol, because the core innovation is to maximize duration of exposure to bright light.
Beyond the huge advantage of being wearable (and hence mobile - they move anywhere with you), wearable light therapy devices also are much more effective than cheap non-wearable light therapy lamps because those are underpowered: almost any light therapy lamp under $200 that claims to emit 10K lux such as Beurer's in fact require you to stay very close to the lamp to get this intensity, literally the nose in the lamp. They also require you to stay immobile the whole time you use them, and if you need a very long duration of exposure such as several hours, this is unrealistic. Given wearable light therapy lamps are about the same price and are much more comfortable and polyvalent to use, they actually allow you to do long light therapy.
When we look at the theoretical advantages of wearable light therapy devices, this design offers the opportunity to optimize most of the parameters of bright light therapy that are currently known to be important, including enrichment in blue light and a comfortable form factor (glasses) which guarantees an adequate, invariant and reproducible distance and angle of the LEDs relatively to the eyes as to optimally stimulate the ipRGC cells with the same light intensity and spectral composition with no risk of color diffusion over distances. It can be argued that although light therapy glasses fundamentally provide the same therapy as light therapy lamps/boxes, light therapy glasses ensure reproducible dosage of the light regimen, such as light intensity and spectral composition, much like how constant and precise dosage ensure the greatest efficiency of drugs. This is why there are anecdotal reports of individuals achieving entrainment with a simple light from a computer screen, a make-up mirror with bright light, and also classical light therapy lamps for Seasonal Affective Disorder such as the Beurer TL30 lamp, but often short lived as it's difficult and cumbersome to optimally and systematically use light therapy lamps under the exact same conditions (eg, distance), whereas results are much more reproducible and sustained longer with light therapy glasses.
The previous paragraphs answer why wearable light therapy devices are recommendable compared to other light sources. But why Luminette? along with the FeelBright cap light therapy, they are the most clinically validated (in independent studies) wearable light therapy device. It also has the longest battery when emitting 500 lux at eyes level: 12h when brand new, 9h after wear after 2+ years of daily intensive usage. Other light therapy glasses or wearable light therapy devices may be as effective, but the first thing to check is the battery duration, and at the moment there is none with a battery that lasts longer than 4h. In any case, there is no light therapy device that is medically certified for this purpose, as there is no ISO standard for light therapy yet.
In conclusion, because of consistency and reproducibility (ie, maintains same light source-eyes distance), comfort of the form factor (ie, can be worn at wake up, in bed, and while moving), this is why wearable light therapy devices such as light therapy glasses are so much more effective than natural sunlight or non-wearable light therapy lamps. Wearable light therapy devices are a marvellous technology as they essentially miniatirize and make portable a sunlight proxy. Of course, whenever possible, direct sunlight exposure should be preferred (within reasons to avoid sun burns and skin cancers - because sunlight contains UVs contrary to artificial light therapy devices), but artificial light therapy should be used in complement whenever necessary and especially for circadian rhythm disorders.
In practice, it is crucial to be able to use light therapy as soon as (natural) wake up for maximal phase advance and to clear up brain fog and sleep inertia. It's much more comfortable to just don some wearable light therapy device while still laying in bed, than to jump out of bed to get exposed to outdoors sunlight. Of course, when outdoors, sunlight (even when cloudy or rainy) will almost always be much more intense than artificial light therapy, so that it is unnecessary to wear light therapy glasses while outdoors, but light therapy glasses should be worn anytime indoors, as we often underestimate how little lux there is indoors, and especially how this changes with just a slightly different orientation.
Note that there is an emerging alternative with hyperphotosensitization drugs, which allow to get increased effects from natural sunlight. While this is a certainly interesting and useful development, it remains that artificial light therapy devices are much safer, can be used for much longer and have no tolerance, so they can be used as often as necessary (ie, 7-9hrs per day on a daily basis as in the present document's author's case). And they are much less expensive to produce and buy.
Note that there is currently a lack of validation data for light therapy glasses in whether they are superior to light therapy lamps, because no such test was done, the only tests were done for controlled exposure in labs with equivalent lux, which shown that light therapy glasses such as Luminette are at least as effective as clinical-grade light therapy lamps, but no comparison study was done in natural living conditions against low quality lamps. Also worth noting is that this lack of validation is also because there is currently no standard for light therapy, as although the main paramaters are known, the optimal values for these parameters are yet to be determined, so since there is no standard, there can be no validation.
Is light therapy a psychological effect?
Of course not, the neurological retinohypothalamic-pineal pathway is well documented and since the 2010s the retinal ipRGC cells existence is well established, so we know that the effects of bright light on our brain and body are biological, physiological. Even before this documentation in humans, the effect of bright light in animals and plants were well documented before, and since then the circadian rhythm has been found to be one of the few universal mechanisms present in all biological systems at all scales (from the cells level to the organisms level).
Because of this physiological mechanism, psychological dispositions do not matter except in compliance. This means that if compliance is ensured (ie, sticking to using light therapy when prescribed), there will certainly be an effect, regardless of beliefs.
For example, when I tried light therapy glasses, I was extremely skeptical, as I had already tried a lot of other therapies encluding melatonin alone, extreme sleep hygiene (down to identical food composition and timing every days and staying at home all the time) and even light therapy with a 10k lux light therapy lamp, with no long term success.
So I tried the glasses firmly believing that they would be ineffective like the rest, and to prove so, I thought I would use them for a very long time at the highest intensity and observe no effect. But this is when I observed the exact opposite: an extreme phase advance of 4-5h! And when I repeated the experiment, the phase advance increased by hours each day! It was the first time ever that not only my circadian phase did not freerun, but went backwards. This was all despite me firmly believing it would not work, and being the most skeptical yet out of all past treatments.
It's worth mentioning that I was the least skeptical when doing sleep hygiene as, at the time, I was a firm believer that everything was achievable through sheer will, but empirical evidence proved this thought process to be a very inadequate way of selecting effective circadian sleep therapies (and very wrong and ineffective in general).
Luminette usage tips
Photos of the Luminette 3, which has a longer battery and 3 different light intensity between 500 and 1500 lux (on earlier versions only the maximum was available):

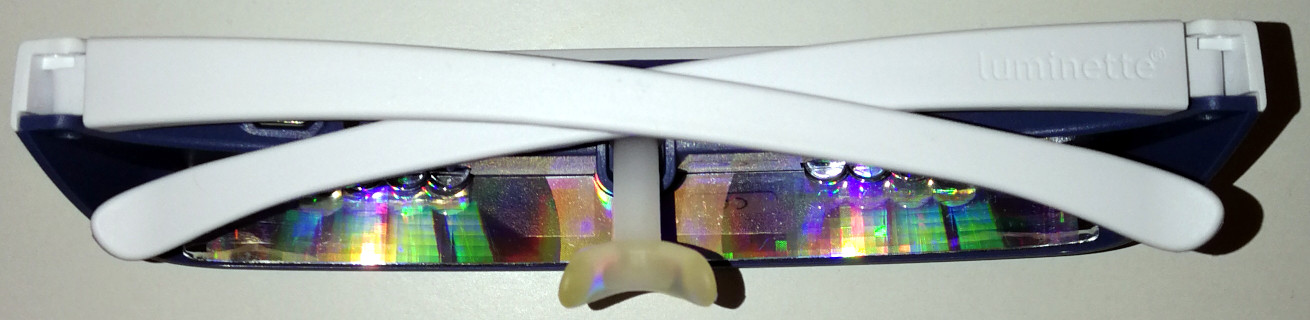
Luminette can be put on top of prescription glasses (personally tested with huge aviator-style glasses), and they use a battery that lasts for 5 days with 1 to 2 uses per day (= 1 to 2h with the 500 lux light intensity setting). Re-timer are also made to fit over prescription glasses (but did not test myself). However, make sure the prescription glasses do not have a blue light filtering coating, otherwise they will reduce the effectiveness of bright light therapy.
Before use, it's necessary to remove the blue filtering film on the hologram, some people don't notice it. The hologram should be silvery without the filter. Follow the quickstart instructions here: https://www.myluminette.com/en-us/instruction-manual
How to properly place the Luminette (picture from here and property of Luminette):
The FAQ further describes: "How can I tell if the Luminette is correctly positioned? Luminette is correctly positioned if the blue light reaches the lower half of your eyes when you look in a mirror. If this is not the case, adjust the Luminette by placing the nose rest into the slot."
Interestingly, the Luminette also says the following, which in the author's experience seems quite accurate: "How long will it be before I notice the effects ? The “boosting” effect of the Luminette® is almost immediate. After a few sessions, you’ll feel your energy returning and your mood improving. If you are using Luminette to rectify a sleep phase disorder the results will become noticeable between 4 to 5 days."
The Luminette already includes rubber (in blue) on the edges so that it can firmly stay fitted on the head:

However, for users who have an active lifestyle, this may not be sufficient. Inexpensive rubbery accessories to fixate glasses are available online, and most are compatible with the Luminette. Here is a selection tested by the current document's author:

The black rubber band should increase friction on the ears and reduce slippage, but this does not work better than the already rubbery blue bands designed into the Luminette v3.

The concept of a white disc is a simple yet effective one, it effectively prevents slippage in upstraight head orientations and it is very comfortable, almost unnoticeable both visually and sensorily (the disc is not felt on the ears). This is highly recommended if the goal is to just prevent slippage in upstraight, work positions. But if the goal is to wear the glasses while doing physical activities that involve sudden high amplitude movements or more extreme orientations such as facing the ground, then other solutions will be preferrable.

This L-shaped accessory prevents slippage in most orientations, even when facing down. However, it is highly uncomfortable, and makes fitting glasses on the nose much more difficult and unnatural.

An alternative to the disc-shaped accessory, this 9-shaped accessory does not reduce slippage more than the disc-shaped one, but it is significantly more uncomfortable due to the little spike at the bottom of the 9.

Finally, there are holder straps, which are the most reliable accessories to ensure glasses won't slip and fall on the ground. However, they may not fit perfectly to the head, and they make fitting glasses a bit more cumbersome and uncomfortable, although much less than the L-shaped accessory. This is the solution that the current document's author preferred and settled for. To further improve the fit and reduce slippage, the disc-shaped accessory was also added. This allows for the glasses to mostly stay in place just by the disc-shaped accessory, and the holder strap remains a last resort safety net in case the glasses may fall during sudden orientation changes.
Note that these accessories are not necessary, they are convenience items that significantly improve usage comfort for intensive users (the author started using them after more than 1 year from starting the daily use of Luminette).
The nose rest piece needs to be replaced about every 6 months. These can be ordered for 10 euros apiece from myluminette.com official website (direct link here for US, here for UK, here for France). Make sure to select the appropriate model of your Luminette, as by default it selects V2 but V3 is also available. It's possible to order several pieces at once, in case you plan on using Luminette over the long term.

On the left a nose rest piece for Luminette v3 after more than 1 year of use, versus a brand new piece on the right.
When the Luminette breaks, no LED lights up even during charging, they just become fully inert. Nothing happened, the day before i used them only 1h, then went to an appointment so i left them on a table. So there is still lots of battery left and there was no mechanical damage. Hence, it's an electronics components failure for sure.
In case the Luminette fails to light up, the producer Lucimed offers two aftersale options:
- For 2 years, the Luminette is under warranty and can be repaired by Lucimed for free, if a purchase receipt can be provided and the use is not considered "abnormal" (ie, very long bright light therapy would likely be considered outside of warranty but I'm not a lawyer).
- Beyond the 2 years of warranty or in case of "abnormal" use, Lucimed offers to repair the Luminette for 35 euros if possible, or if not they will offer a discount to buy a new pair.
There is a third possibility: self repair or a repair shop. The author of the present document successfully conducted a simple self repair, see below.
For an idea of how long the Luminette can last, my Luminette v3 broke after 1 year and 2 months of extensive daily use (1-3h the first 3 months, 3-6h the next 3 months, 5-9h the rest). The symptoms showing it was broken was that no light lit up, neither when clicking on the button nor the recharging LEDs when plugging to an electrical outlet. For those using the Luminette as long as i currently do (5-9h daily), i expect the Luminette breaks under about a year. For those using it less eg 1-3h daily, it should last longer.
To fix it, I tried a self-repair approach. I opened the Luminette using a torx screwdriver (I have a 10 euros multi pieces screwdriver for electronics). This is enough to remove the case and to see the upper part of the electronics board with the batteries, it's possible to unscrew further to remove the electronics board and access the down side but it only includes the LED components so that's mostly unnecessary, especially since no LED was lighting up which meant it wasn't an issue with the LEDs but with something else on the board that made the whole system dysfunction, not just one specific LED bursting.
Then, I simply used an electrical contact cleaner spray with a straw, both on the upper part of the board and on the down side of the board (there is a thin slit that allows to use the spray if you plug the straw in on the spray for more precision). I heard this is a common technique used to "repair" or at least extend the life of electronics boards, as often it's simply a problem of a short circuit appearing somewhere for some reason. I then let the board dry up for 20 min with the case still open, and finally I saw the charging LEDs light up cyclically about every 5-10 seconds (the glasses weren't plugged to the electrical outlet so that's the first time I see these LEDs light up like that on their own). These LEDs lit up without clicking on the device, I guess they are a debug code to signal that the case is open or that there is some issue with the Luminette, there is no information about it in the user manual unfortunately. I closed down the case, screwed back the torx screws on the glasses corners (attaching with the legs), and the glasses were fully functional again. I am not sure how long they will remain operational, but I'll let you guys know if it happens again. The board seem pretty simple, so I'm pretty sure this can be relatively easily fixed by repair shops and engineers. UPDATE: a few days only after, the issue reappeared and the Luminette broke down again.
If someone from Lucimed (Luminette producer), or someone who would like to make an even better light therapy glasses, is reading this document, here are my suggestions to improve:
- allow usb passthrough use while recharching (currently it switches off automatically when plugged in to a charger) - this would allow for very long light therapy session without worrying about whether the light glasses were charged the day before.
- more optimal led placement (eye level on the eyes sides, ie on the glasses legs) to stimulate the ipRGC cells in the nasal part of the retina but not the macula at all (reduce potential blue light phototoxicity since it only affects the macula). There are ipRGC cells in the parafovea, but why try to stimulate them when there are also lots of them in the nasal area and it's much further from the macula that is more sensitive to light damage? Another advantage would be ergonomics, since the LEDs would be placed in the horizontal sides of peripheral vision, instead of vertically, so the main body frame could be much more outside of view (although it will still need to be somewhat bulky to include the electronics board and battery). An issue would be robustness, as glasses legs are less robust than the body.
- more optimal blue light peaks at 479nm, the theoretically optimal wavelength for ipRGC cells stimulation, while reducing risks of blue light phototoxicity that ranges at least from 400 to 480nm (more towards 400nm and much less towards 480nm).
- stick with allowing 3 light intensities, from 500lux to 1500lux, as 500 lux is sufficient for most circadian rhythm disorders, but higher intensities and longer exposure may be necessary for older individuals to pass through the cristallin more obscured with age. More is likely useless for most individuals as it will produce too much side effects, and lower is no better than computer screens (which often emit 100-250lux at max brightness).
- At least maintain the battery capacity of Luminette 3. The long battery is necessary to sustain long light therapy sessions. The long battery is an overlooked feature but is critical for the efficacy of a light therapy device.
- If possible and if it doesn't reduce the comfort too much, increase the battery capacity of Luminette 3, as to allow very long light therapy sessions using the 1000 lux (medium) intensity setting. Currently, only the low light intensity (500 lux) allows for up to 11h of light therapy daily, the medium intensity setting cut that in half down to 5.5h max daily. There are however physical limitations, as cold light that emit more blue light and stimulate the ipRGC cells more also consume more energy.
- Maybe transitionin to monochromatic blue LEDs with peak at 480nm (but filtered below to reduce eyes health hazard) would provide tremendous benefits, with all the rest of the device kept the same. Indeed, because of circadian light subadditivity, which makes colors opposite on the spectrum such as blue and yellow or green and red to reduce the effect of each other when exposed to both simultaneously, monochromatic blue light stimulates the ipRGC cells and cause more phase shifts more than any other colors. It is also much more energy efficient, with 50 lux blue light producing as much effect as 500 lux white light under laboratory settings (hence a 10x increase in effect! Monochromatic blue light alone is about 185 times more efficient for circadian rhythm shifting than polychromatic white light in terms of the amount of photons emitted. Hence, using monochromatic blue LEDs could tremendously increase the battery duration with one charge, or the battery size could be reduced to produce an even leaner glasses body, and also reduce the amount of glare the user is subjected to since less lux are necessary to produce the same amount of phase shifts, ie, the same amount of non-visual stimulation can be achieved while impairing 10x less the visual stimulation (maybe even driving with these glasses may become possible!). However, there can be concerns about the eyes health hazard that monochromatic blue LEDs may cause compared to white LEDs, and also the amount of benign but uncomfortable side effects such as dizziness, especially during very long exposure as is recommended in the VLiDACMel protocol.
Safety of blue light therapy
Is light therapy dangerous for the eyes, more precisely the macula?
To answer this question, we must understand how light can affect the eyes.
An excellent review by Christophe Martinsons outlines the 2 types of known risks: thermal and photochemical. Only the 2nd type, photochemical — which underlies blue light phototoxicity —, is confirmed and is well studied, and was described as follows:
> Type 2: the damage is a photoretinopathy caused by phototoxic reactions in the RPE, following an acute exposure to blue light. Blue light excites lipofuscin by producing reactive oxygen species and free radicals, causing an oxidative stress to the RPE cells.
This and another similar review and guidelines document allow to understand that light therapy safety is a factor of intensity (and hence eye-to-light-source distance) and color and duration of exposure, with some colors requiring less intensity and shorter exposure duration for the same phototoxicity. All light colors can be phototoxic with high enough intensity or long enough exposure duration. And when a light emitting device is certified safe for the eyes, it's only in the bounds of a specific duration according to a regulatory grid.
According to the same review, blue light phototoxicity spans the wide wavelength range from 380nm to 580nm, with a maximum around 437nm (see Figure 1 in the review). However, phototoxicity is not only a function of light wavelength (color), but also of dose of administration, which itself is a function of the light source's intensity, distance to the receiver's eyes and surface exposed. As shown in Figure 5, for light sources emitting above the 460nm range, the dose required for phototoxicity is high (100 to 1000 J/cm²).
The photobiological safety of blue light is hence defined according to the radiance (brightness of a light source) and duration of exposure, as defined by the ICNIRP standard:
This figure shows that the lowest the blue light source's radiance, the longer the user can be safely exposed to it. More specifically from the same review:
- Risk Group 0 aka Exempt Group: no photobiological hazard under foreseeable conditions. Exposure limit is not exceeded within 10,000 s.
- Risk Group 1: Low-risk group: products safe for most use applications, except for very prolonged exposures where direct ocular exposures may be expected. Exposure limit is not exceeded within 100 s.
- Risk Group 2: Moderate-risk group: products generally do not pose a realistic optical hazard if the aversion response limits the exposure duration or when lengthy exposures are unrealistic. Exposure limit is not exceeded within 0.25 s (aversion time).
- Risk Group 3: High-risk group: products pose a potential hazard even for momentary exposures. Exposure limit is exceeded within less than 0.25 s.
More precisely according to the IEC/EN 62471 regulation, the Risk Group 0 aka Exempt Group need to show the absence of the following risks:
- an actinic ultraviolet hazard (Es) within 8-hours exposure (30000 s), nor
- a near-UV hazard (EUVA) within 1000 s, (about 16 min) nor
- a retinal blue-light hazard (LB) within 10000 s (about 2,8 h), nor
- a retinal thermal hazard (LR) within 10 s, nor
- an infrared radiation hazard for the eye (EIR) within 1000 s.
(All those E and L acronyms should have the other letters subscripted.)
Notice how the lowest risk group, the risk group 0, states that the exposure limit to blue light needs to not be exceeded within 10,000s, which equals to 2h47min. This means that even beyond 10,000s of continuous exposure, exposure to light sources of this category may be perfectly safe, but it depends on the radiance. But we know with a certified device following this regulation that under 10,000s, the blue-light hazard should not be an issue. At radiance 100 or below, the risk stays in group 0 up to 100,000s, where this figure ends. This is why this category is also called "exempt group" as it presents no photobiological risk even with very long exposures. Note that radiance is not the same as luminance (lux), although luminance can be calculated from radiance. The point is that, from the point of view of a consumer, to assess the safety of a light source, it's necessary to assess both the luminance and the duration of exposure, as the safe duration of exposure will change depending on the luminance setting for light sources where it can be varied. Indeed, LEDs phototoxicity is dose-dependent on luminance (ie, light intensity). Ideally, light therapy devices such as light therapy glasses need to be classified in the risk group 0, which is the case for Luminette. But note that the whole device is classified, not just one parameter, hence we can assume that the worst parameter was tested. In other words, in the case of Luminette for example, we can assume that the device was deemed safe for use at the highest luminance (1500 lux) for at least 2h47min, which means logically that lower luminance settings (500 lux or 1000 lux) can be used safely for much longer. In a mice study, it was found that even just 24h of continuous exposure of pigmented non dilated rats to blue light emitting LEDs (455-465nm) already produced some retinal damage, however, cyclical exposure (ie, with a light-dark pattern) over 1 month only caused visible damage on albino mice with dilated pupils. On the other hand, white LED light caused less phototoxicity than blue-only LED, although it's unclear whether the study maintained the same melanopic illuminance, hence maybe the difference in phototoxicity lies in a difference in blue light emission.
According to European Union and FDA regulations, and also systematic reviews by scholars (see also here), if the device is filtering UV light and the intensity is not too much, and the user does not have a macular disease, then light therapy should be safe. The goal of this document is not to list all devices, but at least Luminette is validated under the all these regulations. However, the french ANSES considers that blue light phototoxicity starts is between 450-470nm (they include the effects on the circadian rhythm), and Luminette has a peak of blue light at 468nm according to the manufacturer Lucimed. A study on mice has shown that <440nm blue light is highly phototoxic with cell damage observed, but with 480nm minor cell damages are still observed (albeit much less than with <=440nm). Other studies on primates also observed macula phototoxicity with wavelength of 460nm and 465nm respectively, but a subsequent study demonstrated that even a 7000 lux exposure of primates' retina to LED contact lenses only caused temporary toxicity which was completely restored 14 days after the experiment. Phototoxicity is difficult to assess, as phototoxicity is a combination of factors that do not linearly add up so it's not possible currently to give any threshold. Knowing that blue light therapy is optimal ~480nm (more precisely between 479nm and 482nm), and that preliminary data on mice suggests that the 480nm does not produce any meaningful eye damage unless genetically modified, then it should be possible to design a theoretically safer blue light therapy glasses enriched at ~480nm. Nevertheless, keep in mind that the light therapy devices emit lower light intensity (up to 10K lux) than the sun (up to > 100K lux) by an order of magnitude, and sunlight is rich in blue light, so there is no doubt that light therapy has a lower impact than sunlight on eyes health. But phototoxicity should be assessed on a case-by-case basis, as some apriori unsuspected light emitting devices such as some frontal led lamps are in fact phototoxic according to the ANSES.
In addition, bright light exposure may be contra-indicated for some individuals, especially those with increased photosensitivity or an already present eye disease. A lot of drugs can induce photosensitivity or even drug-induced ocular disorders, making such treatments incompatible with bright light therapy, see this review for a list of such drugs. Individuals with dry eyes may be more at risk. Another review states that "people born without crystalline lens (aphakic) or having received intraocular lens implants (pseudophakic) are exposed to a greater amount of retinal blue and UV light compared to phakic subjects exposed to the same light source", and hence aphakic and pseudoaphakic people should avoid light therapy. Albinos individuals, who lack pigmentation, should likely also avoid bright light therapy, especially with blue LEDs, as well as individuals with often dilated pupils (eg, because of other medications or a genetic mutation).
Although blue light therapy with a european or FDA safety certification should be safe for use under the stated exposure duration, a recent study found that (green) light therapy devices (more precisely the Re-Timer) modified significantly the macula under 7 days of usage, with only 30 min of bright light exposure per day at wake up. However, the experiment design needs to be considered: the participants were maintained in a room constantly lit under 10 Lux, which is very low (1 lux = light emitted by 1 candle). Hence, this study did not just test the effect of bright light therapy, but more precisely the effect of sudden bright light therapy in a constantly dim environment. This is known to increase the effect of the bright light on the circadian rhythm through photic history, so increases in eye damages would not be surprising. Furthermore, this design forced the participants to have dilated pupils, which is known to multiply the effects of light. Hence, there needs to be more research to elucidate whether 1- the significant changes that were found are clinically significant (ie, can they lead to diseases or they are just natural body adaptations that are not indicative of any disease), and 2- whether these observed effects are due to pupil dilation, in other words if the user takes some time to adapt their eyes beforehand (eg, by being exposed to a more lit environment or by closing the eyes the first few minutes while under the bright light, to let their pupils contract) these effects would disappear.
Another study further explored what parameters could make blue light a photohazard. It was found that only the blue-only emitting LEDs induced eye damages in mice and maybe also the blue component of white light emitting LEDs, but not white light emitting fluorocompact lights (FLC). There is however evidence that blue only light therapy is much more effective than white light enriched with blue light therapy due to circadian light subadditivity, this suggests that blue only light therapy devices like Ayo may be more effective than blue-enriched white light therapy devices such as the Luminette, although there is at the moment clinical trials published only for Luminette.
All that said, light therapy using certified devices by current health regulations is considered a safe therapy by the AASM, and with their 2015 guidelines mentioning a study finding no adverse effect in season affective disorder patients who used light therapy for 6 years, hence suggesting long-term safety too. Indeed, light therapy with safety certified devices is no more dangerous than being outside on a sunny day.
But the safety is only guaranteed under the bounds of the duration of exposure that was tested and certified. Since treating circadian rhythm disorders require a (much) longer exposure than expected by the fabricant and regulators, extended continuous use of light therapy with very long exposure sessions may present a eye hazard. Hence, for very long light therapy sessions, it is important to stick to the lowest effective intensity, 500 lux or maximum 1000 lux, in order to reduce the risk of eye hazards, since this review (and this guideline document) shows that such an intensity and with the blue light spectrum are very unlikely to cause eye hazard even for very long exposures.
So, where is coming from the common misconception that blue light is toxic to the eyes? This may stem from blue light phototherapy devices used in dermatology. Indeed, these devices are much more powerful than their ocular counterparts originally designed for seasonal affective disorder (SAD), so that the dermatologic phototherapy devices, called photodynamic therapy (PDT), emit a much stronger light that is designed to cause skin damage to force it to regenerate. Hence, obviously protective eyewears are necessary to use dermatologic phototherapy. But ocular blue light phototherapy is designed to be projected into the eyes, and hence they are much weaker in intensity and filter UVs, so much so that they can only emit a fraction of what human eyes are exposed to with indirect natural sunlight.
In summary, if you can withstand sunlight exposure, light therapy glasses are much much safer and so should not affect your eyes anymore and likely much less than (direct and indirect) sunlight. Some people have retinal diseases or weaknesses, but these people are usually aware of their photosensitivity and also avoid sunlight. If you don't avoid sunlight, then there is no safety issue preventing you from trying light therapy.
In practice, check if the light therapy glasses is certified CE 0459 in Europe, which determines light therapy products, and IEC 62471 internationally or EN62471, for eyes safety. For example, Luminette was certified CE0459 in Europe and as a group 0 aka exempt group under IEC62471 "without photobiological risk".
In the past, it was suspected that light therapy may cause mental distress in prone individuals. However, a historical review found that current evidence suggest that light therapy does not increase the frequency of hypermanic nor hypomanic episodes in people with bipolar disorder, so it is doubtful that light therapy would cause any mental distress in other less susceptible individuals.
Sensory processing disorder involves extreme subjective stimulation from sensory inputs, such as bright light. It is more common among children and commonly comorbid with autism and ADHD and other disorders that are often associated with circadian rhythm disorders. First, it's important to note that there is some debate about whether this is really a disorder or simple child behavior. However, there is a growing suspicion that people with circadian rhythm disorders are hyper photosensitive (ie, more sensitive to bright light), which would fit with the idea of a sensory processing dysregulation, or more prosaically an individual specificity in photosensitivity. Indeed, studies have shown that there can be a 50-fold difference in photosensitivity between individuals, and this study was done on healthy volunteers, not on people with circadian rhythm disorders who may display even more extreme differences. Furthermore, it was demonstrated that children with autism are hyper photosensitive. Another clue is that sensory processing disorder seems to be strongly associated with seasonal affective disorder (SAD), the latter being known to be associated or even caused by the lack of bright light, and other studies showing that extreme sensory processing disorder symptoms are associated with a major depression disorder. (Aside: sensory therapy was rejected as ineffective by a systematic review, suggesting that sensory processing disorder is not just a subjective disorder but caused by an objective dysregulation, such as hyper photosensitivity maybe, that is not modifiable with psychological tools) Given than light therapy is effective not only for people with a circadian rhythm disorder but also with children with autism and ADHD, who frequently also have a sensory processing disorder, unless the patient complaints, the use of light therapy does not appear to be contra-indicated for people with a sensory processing disorder. Furthermore, modern light therapy using light therapy glasses emit a much less intense light between 500 and 1500 lux, which is roughly equivalent to a sunlit dusk (an overcast cloudy day is 1K-5K, a cloudless day is 30K up to >100K), which is unlikely to cause any uncomfortable effect if the patient can sustain exposure to cloudy daylight. It's however safer to approach light therapy for these patients by first using only the lowest light intensity, if necessary for a longer duration to compensate. It's also easy for the patient to check beforehand if they can sustain such an intensity of light by using a Lux Meter app on their smartphone, and measuring if they are already exposed to such light intensities.
There are very few studies on photohazard in children, for obviously difficult ethical issues. According to Lucimed in private communications, light therapy glasses such as Luminette can be used from 12 years old onward.
Some individuals with sighted non-24 have been reporting moderate side effects to very long bright light exposure as described in the VLiDACMEL, see here.
Interactions between drugs and light therapy
Some drugs can affect the responsiveness to light therapy.
Vitamin A is necessary to synthesize all opsins in the eyes, including the melanopsin pigment necessary for ipRGC cells and entrainment to bright light to work, hence it is necessary to ensure adequate levels of vitamin A, via supplementation if needed. Vitamin A also increases the photosensitivity to UV light which can be harmful, hence caution is required with direct sunlight exposure when supplementing with vitamin A. Throughout the animal kingdom, there are thousands of different variants of both visual and non-visual opsins (see also here), each with their own specificities.
Several ADHD and antidepressant drugs cause an increase in photosensitivity, which increases the risk of negative side effects when exposed to bright light (see the section on Hyper-photosensitization pharmacological therapies).
Anti-histaminics block entrainment of the circadian rhythm to bright light, and hence cause anyone to freerun (ie, wake up later and later, and likely sleep longer but not because of tiredness as is commonly assumed but because of the circadian rhythm progressively shifting):
> In phase shift studies, histamine given centrally seems to entrain the activity rhythm in the same way as light impulses and inhibition of histamine synthesis seems to block the entrainment by light.
Anti-histaminics are hence contra-indicated for individuals with a circadian rhythm disorder. Histamines have a bidirectional relationship with the circadian rhythm, the histamine levels being regulated by the circadian rhythm as they are high during the active period (day) and low during the sleep period, and the circadian rhythm being modified by histamines production potentiating the excitability of the neurons responsible for the entrainment to bright light (and more as recently discovered). This explains why "the intensity of symptoms and disease severity show a 24 h pattern in many immunological and allergic diseases" (see also here). Hence, comorbid diseases that produce histamines such as allergies and inflammations such as for example dyshydrosis due to a fungal infection may deplete histamines and worsen the circadian rhythm disorder. Other drugs that can block histamine H1 receptors such as Trazadone should also be contra-indicated.
Beyond the effects on circadian rhythm, anti-histaminics also modify sleep and wakefulness architectures, by increasing slow wave (deep and reparative) sleep and REM (dream) sleep stages durations, and also decreasing vigilance when awake. This contributes to the often reported effect of anti-histaminics making the subject feel more tired and sleepy. This is in fact a known side effect of some antihistaminics and is why the FDA disadvises the use of antihistaminics when driving.
However, the author suspects that antihistaminics may not necessarily completely block entrainment to bright light but partially: it may depend on the dosage of the antihistaminics drug and intensity and duration of bright light therapy. With a longer exposure to a brighter light, it may be possible that entrainment to bright light may still be achieved even under use of antihistaminics. Also different classes of antihistaminics may produce different results, although the studies mentioned above suggest that any histamines inhibitor will partially or completely block entrainment to light.
The role of histamines in the entrainment to bright light may explain why low doses of aripiprazole (see also here) was found to be an effective treatment to entrain individuals with DSPD, as aripiprazole activates the H1 histamines receptors, and thus increasing the responsiveness to light therapy. Hence, H1 histamine activating drugs may be a potential class of drug to complement light therapy.
Interestingly, it seems Vitamin D inhibits melatonin, maybe because the body does not expect to get exposed to Vitamin D unless there is sunlight with UVs, so we can hypothesize that a feedback loop was created between Vitamin D and UV exposure, which can make Vitamin D activates some of the pathways that bright light exposure can. What is surprising is that Vitamin D is secreted from skin exposure to UV light, not the eyes and not from non-UV bright light, so the interaction is quite complex. Given this finding, vitamin D supplementation should be avoided in the evening, as intake in the circadian morning should be favored.
Do-it-yourself, a cheaper alternative for light therapy?
First off, if you can afford light therapy glasses but are just wary that they may not work and hence to spend money for nothing, take note that most light therapy glasses manufacturers offer a money back guarantee of 30 days (such as Lucimed's Luminette), and since light therapy should show efficacy after 10 days max, this means that if you have plenty of time to test for free if it doesn't work out. The rest of this subsection describes cheaper, but less effective, alternatives.
Nevertheless, Luminette can be expensive especially in non occidental countries. But keep in mind that therapies and management devices for chronic illnesses usually cost magnitudes of order more, we are lucky that for non24 the two most effective treatments, melatonin and bright light, are non patentable and hence still reasonably affordable.
If you are really low on money and can't afford light therapy glasses, I strongly disadvise buying a light therapy lamp. Sure, there are inexpensive ones available, but there are three major downsides:
- the cheapest light therapy lamps are not very powerful, they say they emit 10K lux but it's only at point-blank with no range, and with lux approximately decreasing quadratically with the distance, it means that if you are just a few centimeters away from the ideal distance, you will get very low lux (low light intensity).
- they can't realistically be used to treat circadian rhythm disorders, since circadian rhythm disorders need long bright light therapy of several hours. With lamps, you need to stay just inches in front of them, and it's hard to do anything in front given how close you need to stay to the lamp and the angle you need for your eyes to get properly exposed.
- a major drawback is that it's only one lamp, whereas both of our eyes have ipRGC cells (the photoreceptive cells that shift the circadian rhythm depending on their exposure to light). The more ipRGC cells that are stimulated, the bigger circadian shift that happens. Unfortunately, with only one lamp, if it's set on the side of your peripheral vision, it will only stimulate one eye's ipRGC cells (since they are located in the parafovea of the macula and the nasal part of the retina, see also here). In other words, a lamp can only produce half of the results of light therapy glasses. Ideally, you would need 2 such lamps and adequately placed, but at this point light therapy glasses are as expensive and much more comfortable to use and they stimulate both eyes cells, while also having a more optimized spectral composition (ie, light color enriched in blue).
Ideal placement of 2 light therapy lamps, on the sides of both eyes so that the light can reach the nasal region of both eyes' retinas. Image from this study.
What about the downward slope angle?
It is commonly assumed that natural sunlight enters the eyes from a top-down fashion, and hence that light therapy should aim to do the same for optimal efficacy, although this ignores the mechanistic of light rays that in fact bounces up on surfaces and hence enter the eyes from all angles (otherwise we wouldn't be able to perceive objects in the environment).
Although there is one review claiming that optimal light therapy should be angled downward at 15°, there is no reference provided to support this statement. Nevertheless, it may be traceable to this 2003 study in humans, which found that illuminating the inferior part of the retina inhibits more melatonin than exposing the superior part. However, this study did not consider the nasal nor temporal parts of the retina, and it is arguable that potentially the nose and eyebrows may have affected the results by producing a hardly controllable shade in one of the conditions, and furthermore, the condition where they illuminated the inferior part may have inadvertently illuminated the nasal part of the retina since the authors did not control specifically for lateral exposure but only vertically.
A later study demonstrated that there is no evidence of a dorsal-ventral gradient in ipRGC cells placement in humans (although there is for mice's ipRGC cells - see also here -, as well as for their S-cones), which casts doubts on the 2003 study's results. Most other studies, both before and after, only investigated the laterality of the retina's non-visual photic response, finding that exposing the nasal part of the retina produced more melatonin inhibition or circadian shifting than the temporal part of the retina (see here, here and here). But finally, a well controlled 2021 study resolved the debate once and for all: by using specially crafted half and full-discs, either emitting blue or amber light, they could target in isolation 4 different hemi-fields of the retina: temporal, nasal, inferior and superior, with the discs ensuring the nose and eyebrows would have no effect on exposure with no uncontrolled shade. With this well-controlled setup, the study found that only the nasal hemi-field of the retina exposure to the same blue bright light produced more melatonin inhibition (35%) compared to other hemi-fields (~20% each). Hence, this study determined that the nasal part of the retina is more sensitive to bright light, not the inferior hemi-field, and hence that the nasal hemi-field should be preferentially targeted by artificial bright light therapy devices. For example, just as did the study from which the above figure was extracted, light therapy lamps should optimally be placed at eyes level, and in the peripheral vision as to allow for the light beams to optimally reach the ipRGC cells in the nasal-macula area in the retina, with the vertical angle being an negligible factor, as long as the light emitters are placed high enough to pass above the receiver's nose and illuminate the nasal hemi-field.
Finally, because biology is rarely this simply and nature rarely create entirely redundant systems, each ipRGC cells in fact axonally innervates bilaterally the suprachiasmatic nucleus (SCN), with ipRGC cells located in the dorsal-temporal region of the retina primarily targeting the dorsal part of the SCN, and those located in the ventral-nasal region targeting the ventro-medial parts of the SCN. This means that while targeting the nasal part of the retina causes the more melatonin inhibition, period lengthening/shortening may be better controlled by exposing the temporal region of the retina, and likely illumination of both the nasal-inferior hemi-fields and the temporo-superior hemi-fields would allow for a more complete manipulation of the circadian phase, by both shifting and period manipulation.
An inexpensive and potentially more effective strategy than light therapy lamps is to use computer screens at their maximal intensity directly at natural wake-up for 1h to 3h, but longer is better. Indeed, it is easy to stare at screens for long periods of time (contrary to light therapy lamps), and the longer duration can compensate partially for the lack of light intensity. Not only the distance to the user is similar to a light therapy device (contrary to TV screens which may be too far to get enough lux), but also the user can stare directly at the screen, which light will stimulate directly a maximum of ipRGC cells in the macula of both eyes. Hence, the ergonomy of screens, which allows for staring directly at the light source and for long duration of time, makes them a quite good candidate for inexpensive bright light therapy but with limited efficacy compared to light therapy glasses. Then, the rest of the day, the user can get exposed to natural sunlight to increase the duration of light exposure without any device. In the evening, dark therapy is crucially necessary as otherwise the benefits will be erased by the evening phase delay produced by screen exposure, so the screens must be dimmed and blue light filtered to avoid the circadian phase delay effect opposing the circadian phase advance obtained in the morning. This "computer screen blue light therapy" can also be combined with melatonin in the circadian evening to increase efficacy. Anecdotally, this specific combination therapy allowed the author's father to stably entrain during the autumn, spring and summer. Note however that screens may not be sufficient in low sunlight seasons such as winter, due to the reduced intensity of natural sunlight, so light therapy glasses are certainly more reliable.
To ensure your computer screen is useable as a light therapy device, use a lux meter app on your smartphone, this will use the smartphone's light sensor to measure light intensity. Direct your phone towards your screen set at maximum brightness, and position it at about the same height and distance from the screen as your eyes would be when you use it. This measure will reflect what your eyes will perceive. A screen emitting at least 100 lux should be sufficient to get half of the circadian rhythm shifting obtainable with a 10K lux light therapy device.
Are computer screens safe as light therapy devices? They are made to be stared at, and furthermore they emit relatively low bright light (~250 lux at max brightness on the screens the author could measure), and since they are widely used worldwide, if this was unsafe there would be epidemiological data on diseases caused by screens. Nevertheless, the french ANSES stated that data was lacking on chronic exposure to cold light emitted from screens, so that it could not conclude about its safety or dangerosity.
Although DIY light therapy devices can certainly shift the circadian rhythm, they are certainly much less efficient and so the amount of phase shift obtained will be drastically smaller compared to the optimized light therapy glasses such as Luminette, as experienced by this reddit poster.
Is the sunlight sufficient or even better than light therapy lamps as some practicians suggest? Generally, no, but sometimes, sunlight an acceptable light therapy, if we keep in mind these limitations of sunlight therapy:
- Sunlight is highly variable: not only on a day-to-day basis depending on if there are clouds or not (refer to this table, showing that cloudless sunlight is indeed more intense (~100K lux) than a light therapy lamp (~10K lux), but if the sun is cloudy or if you stay inside your flat then it can actually emit less light (<1K lux) and with poor blue light content (as shown in figure 2 of this review)), but also on a seasonal basis, with winter sunlight being of course usually lower in intensity than spring or summer sunlight. Hence, although light therapy can be done for free using sunlight during the summer and spring, it is much more advisable to use an artificial light therapy lamp during autumn and winter to ensure a robust and consistent duration and quantity of exposure to bright light every day. The fact that sunlight produces variable lux intensities depending on the weather and presence of clouds makes it a very bad tool for consistent therapies because the resulting effect on the circadian rhythm will vary uncontrollably from day to day and from seasons to seasons, it's like using a drug with a varying dosage everyday, no sane practician would ever suggest to do that. You don't need to trust the table linked above, you can test for yourself by using a lux meter app on your smartphone, this will display the lux you are exposed to (these sensors are linear, hence they should be reliable enough for lux in the range 100-10K).
- Sunlight is inconvenient: a proper exposure to sunlight requires to go outdoors, as indoor sunlight filtered by windows is much less intense and can easily and frequently be lower than artificial lamps. An optimal bright light therapy is done as soon as one's wake up, as more circadian rhythm shifting effect is obtained when exposed to bright light close to the minimal core body temperature (CBTmin) which happens 1-2h before natural wake up. Getting sunlight directly at wake-up is inconvenient, as you need to jump out of bed and go outside as soon as possible, which may not always be possible depending on your other commitments, and also is subject to be exposed to unfavorable weather conditions such as rain and snow.
- Sunlight is overkill: Sunlight is indeed the strongest light therapy, especially when cloudless as it can emit up to 120K lux, no artificial light therapy device can come even close. But having that much lux (light intensity) is unnecessary: the eye's ipRGC cells' sensitivity range spans only 2 orders of magnitude, it's nowhere close to the 9-10 orders of magnitude of the visual pathway. Since it was shown that most people's non-visual (ipRGC) sensitivity to light starts from a 5-10 lux (and sometimes even lower), this means that the saturation point for maximal ipRGC cells stimulation must be around 1000 to 10k lux depending on the individual. And indeed, a study found no additional phase shift using 8K lux compared to 2K lux, whereas increasing the duration of light therapy from 1h to 3h led to significantly increased phase shifts, which demonstrates that the light intensity saturation point is low. On the other hand, a study found that 100 lux causes 50% of the max stimulation of ipRGC cells in their participants, suggesting that the saturation point may be even much lower for some individuals. Hence, a light therapy device of 1000 to 10K lux is plenty sufficient to maximally stimulate the ipRGC cells and shift the circadian rhythm, as the sunlight won't provide any meaningfully bigger circadian rhythm shifting effect compared to an artificial light therapy.
- Regularity and duration of bright light therapy are crucial: Since the saturation point is easily reached with artificial light therapy lamps, it's important to focus on regularity and duration of the light therapy sessions. Indeed, a longer session of 1-2h of artificial light therapy will always shift more the circadian rhythm than a shorter 20-30min of sunlight: "a longer period of moderate intensity light may be more effective than a shorter exposure period of high intensity light". Since regularity of exposure to a sufficient amount and duration of bright light is crucial, during the winter season (relatively to the geographical location of the user) an artificial light therapy device will certainly provide much greater benefits than the highly variable sunlight.
- Just like food needs, all humans have "spectral diet" needs that are similar but different for each people, with some people needing more light intensity (lux) just like some people need more vitamins or proteins in their diet.
- Note: do NOT directly look at the sun because it can damage the eyes otherwise! Even looking at reflections of sunlight in the snow can cause eyes damage!
If you still want to use sunlight as your first bright light therapy, at least make sure to use a Lux Meter app on your smartphone and direct your phone's screen towards where you are facing, to check if you get enough sunlight exposure to be effective. A lux value of 500 is the minimum, but at least 1K to 1500 lux or more should be preferred, since sunlight does not have the optimal spectral composition all the time (ie, it lacks blue light after the morning and on cloudy days).
Here is an objective protocol to assess whether sitting outside on a cloudy day is effective enough for you:
- Download a lux meter app on your smartphone (such as this one for Android).
- Go to sit outside during a usual cloudy day
- Launch the app and turn the phone's screen towards the direction you would face if you would stay sitted here (taking into account of what activity you would be doing usually: for example if you would most likely be scrolling on your smartphone then direct the phone's screen towards the ground, not the sky). It's important to direct the phone where you would be looking so that the light sensor embedded in your phone's screen captures the light in the direction you would be facing.
- Read the measurements. If it's at least 500 lux, then it's likely effective for your circadian rhythm. If it's below, then you can go home. If it's lower than 250 lux, you can get 250 lux by looking at your computer screen at full brightness, so no need to stay outside for that. Between 250lux and 500 lux can usually be achieved with bright indoor light fixtures.
Ambient lighting may also be used, and several companies are starting to offer "circadian lighting design" services. The color temperature (CCT) of a light, in kelvin, needs to be higher to emit more blue light, and this excellent paper provides a mathematical equation to precisely quantify how much melanopic illuminance can be obtained for different color temperatures. This means that the colder the light color is, the more blue light will be emitted, and the more circadian effect they will produce. However, there are three major issues with ambient lightings. First is the distance and orientation to the user's eyes, and this can hardly be controlled robustly with a fixture to the ceiling or to the wall. Secondly, colder light (higher MELR) consume much more energy than warmer colors for the same photopic utility (ie, how much it lights up a room). Thirdly and finally, ambient light fixtures are often not designed to reduce the lower part of the blue light band, which can be a photohazard (ie, harmful to the eyes). Finally, note that a study on albino mice found that a cold-white 6300K LED already produced some retinal damages under specific conditions.
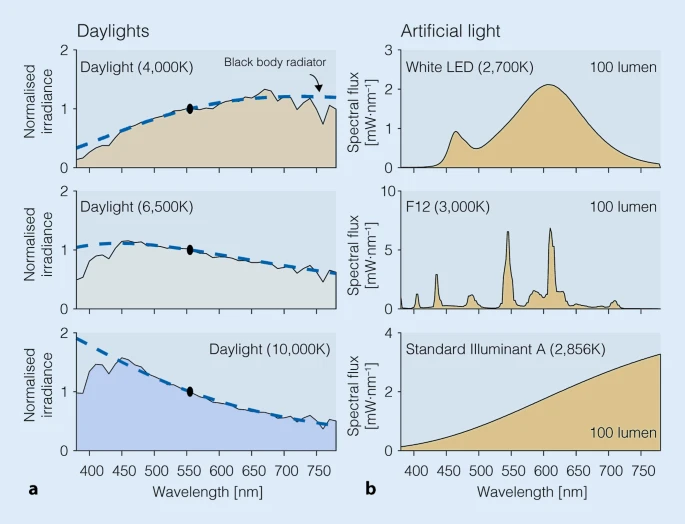
Spectral power distributions of common light sources in our environment, illustrating how little blue light (400-490nm) can be emitted by some light sources including clouded daylight, but especially artificial lights. Figure from this review under CC-BY 4.0.
If you want a cheaper alternative, there are the Re-Timer glasses (cost: ~$120), which I did not try. Re-timer 's green light is sufficient to phase advance, but since it's using green light it doesn't increase vigilance so it doesn't change the feeling of subjective sleepiness in the morning, contrary to blue light which directly reduces sleep inertia and increases vigilance. It was also shown that although green initially suppresses melatonin, the effect is not sustained and melatonin levels recover after 90min even if still exposed to green light, contrary to blue light. Indeed, the study found that the phase shifting response to green light (555nm) is mediated by cones and is only temporary as it decayed exponentially with duration of exposure to bright green light, whereas blue light produced phase shifting over long duration light therapy with no such decay mediated by melanopsin ipRGC cells which are more responsive to long durations of blue light, which means that only blue light therapy can be used for long and very long bright light therapy. Using green light is a strange choice because green light is more effective at pain reduction and about 75% less at shifting the circadian rhythm compared to blue light (TODO: find the source), whereas blue light is optimal for vigilance and circadian rhythm shifting. Currently, only Luminette, Psio and Ayo make light therapy glasses with completely blue light and hence are the most effective light therapy glasses on the consumer market currently. Also both have several independent studies demonstrating significant phase advance (see their respective websites or google scholar), whereas Re-timer only has one as of 2020.
Another light therapy glasses alternative is Psio (cost: non disclosed publicly). It uses blue light similarly to Luminette (although it's pure blue LEDs, no white light, whereas Luminette uses white light enriched with blue light), but with the difference that the light is pulsed (aka intermittent light therapy). Although intermittent light therapy should be as efficient to induce phase advance than bright light therapy, it may produce less melatonin reduction, hence you would not get the vigilance boost that blue light provides, nor potentially the advantages in increased melatonin levels due to photic history.
Can you make your own light therapy device? I would strongly disadvise against. For two reasons: it's difficult to tweak exactly how much lux you will get and you won't get blue light, or if you do, you risk eye damage. Indeed, blue light therapy glasses project light in the range that is partially phototoxic, as it's also the same range (450-490nm, with an optimal peak at ~480nm , more precisely between 479nm and 482nm) that is necessary for optimal stimulation of melanopsin receptors. To reduce the risk of exposure to very phototoxic blue light wavelengths, the method found by light therapy device manufacturers is to add filters to bandpass filter the lower ranges of the blue light wavelengths with UV and near UV lights filters (UV = UV-B and UV-C, near-UV = UV-A, which is up to 400nm). For example, the Luminette enriches white light with blue light with a 468nm wavelength. Also, reducing the light intensity helps, so it's not surprising that blue light enriched lamps and glasses are calibrated to project lower lux (usually 500 to 1500 lux, compared to 10K white light therapy lamps). The phototoxicity is mostly an issue if the light source is directly looked at (ie, when the blue light beams hit the macula, which is what allows central vision), hence another solution is to avoid looking directly at the lamp and placing it in the peripheral vision, as shown in the figure above.
Let's say you can make a blue light boosted DIY lamp with the right amount of lux (that you can somewhat measure with smartphone apps). Then you cannot assess as easily if the color spectrum (ie, blue and green light emission) is correct and safe, because light spectrometers cost thousands of dollars, which defeats the purpose of DIY for cost effectiveness. Finally, you would also need to add a UV and near-UV light filter and also a complex blue light filter to let only the 479nm wavelength pass through (which would likely be a quite expensive filter to buy), and hope you're doing it right so that it's not harmful to your eyes. At this point, if you're worried about safety, it's just much easier and better to buy a certified light therapy device.
If you really do want to try to make a DIY light therapy lamp or glasses, then it's necessary to use a spectrometer to ensure that the light produced by your device is not emitting in the blue light phototoxic range. See for example this study and this review on the current regulations and equation to calculate phototoxicity of solid-state lighting (LEDs).
Finally, to optimally stimulate the ipRGC cells in both eyes, you would need at least 2 lamps. As written here, one DIY lamp of adequate lux would cost $50 (not including the necessary UV and blue light pass-band filters), for 2 it would be $100. At this point, there's not much advantage in terms of cost to buying a light therapy glasses such as Luminette 3 (229€ brand new, 150€ in second hand but very rare since Luminette 3 came out only recently in December 2019 - note it used to cost 380€ when it first came out in 2006) or a Re-Timer Gen1 (120€), and those devices are already designed to provide the right amount of lux and blue/green colored light to optimally stimulate the cells in both eyes, and their safety is certified. If the added cost is still too much, the Beurer TL30 lamp costs ~35€, for 2 then it costs 70€, which is a cheaper option to DIY. And I know of some people who got effective phase advance and entrainment using a Beurer TL30 daily for several hours so it's effective, you can even mail Beurer to tell you how to optimally use their lamp for circadian rhythm disorders.
So in the end, I think DIY is just too easy to mess up, and we already have devices that work and are affordable. If you just want something cheap to try light therapy asap, just either use a computer screen at max brightness, because they are made to be directly looked at so you know it's safe, or buy a Beurer TL30, which is less efficient than light therapy glasses in particular Luminette 3, but it's better than nothing.
For the mathematically inclined engineers who would like to conceive their own optimal DIY light therapy device, this review provides an excellent outline and references to the major models to modelize and optimize circadian rhythm phase shifting using bright light therapy. Especially look into melanopic illuminance.
However, there are some online tutorials for very interesting now kinds of do-it-yourself light therapy devices, such as a DIY square lamp mimicking sunlight through a window (with parallel light rays!), and which could be a very promising piece of furniture to equip rooms without a window and make them more hospital for humans, as humans biology requires daily sunlight exposure and hence cannot stay in a room without a window, but this solution could serve as an artificial replacement. Note however the author of the present document could not test the device and so cannot vouch for it, and furthermore given the technical documentation, the LEDs used in this furniture are too powerful and likely phototoxic since the range of wavelength covers the 380-780nm range with a peak at 452nm!
Camping can be an alternative but it's not a free lunch. Camping can indeed help if the circadian rhythm is not too out of phase with the external day night cycle (see also here and here) by getting exposed to sunlight and hence reaping the phase advancing effects of sunlight therapy. However, if the circadian rhythm is too delayed (eg, sleeping around dawn) then the light exposure can actually fall on the phase delay part of one's light PRC curve, which would only worsen the phase delay. Interestingly, it was found that modern occupations since the industrial era (ie, working in offices) leads to a reduced light exposure during the awake time (see also here), which was previously shown to cause biphasic sleep. Strategically, camping may be used in complement with artificial bright light therapy, by first starting artificial bright light therapy to obtain some phase advance, until it is sufficient to be reasonably aligned with the day-night cycle so that exposure to sunlight would happen during the phase advance section of the light PRC curve (ie, after the middle of the circadian night = minimal core body temperature point). Anecdotally, a reddit member with DSPD reported this strategy and its results: after a few months of artificial bright light therapy with Luminette (source: private communications) and melatonin which already produced an impressive 7h phase advance (wake up from 5pm to 10am) but with no improvement beyond this point, the phase advance could be pushed 2h further (to 8am) after just a weekend camping trip.
Dark therapy and blue blocker glasses
Definition and overview of dark therapy
Dark therapy is the strategic avoidance of light exposure, usually in the biological evening and night. Why is this necessary and how important is it? As explained in the Zeitgeber section above, zeitgebers are double-edged swords, anything that can phase advance your circadian rhythm can also phase delay it. Furthermore, bright light has a special property of lacking a PRC dead zone, so that it always affect the circadian rhythm, whatever the time of administration, which means that control of bright light exposure at all time is a necessity. This is the purpose of dark therapy as an adjunct to bright light therapy.
To quote a 2019 systematic review on light therapy giving a succinct definition of dark therapy: "To avoid unwanted changes in the circadian phase or night-time sleep, light exposure in the evening and at night as well as in the morning needs to be controlled, as even the longest wavelengths (631 nm) or intermittent light exposures do induce circadian resetting responses." In other words, dark therapy is the logical complement of light therapy: whereas light therapy involves increasing exposure to phase advancing light (in terms of timing relative to one's circadian phase, duration, color, etc.), dark therapy involves decreasing exposure to phase delaying light.
Furthermore, beyond the mitigation of phase delay, the alternance of light and dark phases is what signals the periodic component of the zeitgeber, in other words, it's necessary to have dark periods for entrainment to bright light to work, as demonstrated by "constant routine" protocol studies where typical sleepers lose entrainment under either constantly dark or constantly brightly lit environments.
Since bright light is the strongest zeitgeber and hence most helpful treatment, due to its dual effect on circadian waveform shaping (including phase shifting and period lengthening) aas well as melatonin suppression (which suppresses subjective feelings of sleepiness and increases sleep fragmentation), it can also be the most detrimental factor if the subject's eyes are exposed to bright enough light during their biological evening. Hence, "just as light exposure can shift circadian timing, so too can the strategic avoidance or reduction of light". Indeed, for typical sleepers, as soon as the lights are switched off, both melatonin and body temperature start a fast change towards their high and low phases respectively in preparation for sleep, illustrating how dark therapy directly affects the circadian rhythm.
For individuals with a circadian rhythm disorder such as DSPD or non-24, a stricter discipline in following dark therapy is necessary compared to typical sleepers, not because of "unhealthy sleep habits", but because they are more prone to phase shifts: when exposed to shorter subjective days of bright light, the "master clock" SCN neurons have a tighter phase distribution which makes them more prone to unwanted phase shifts of mistimed zeitgebers. In other words: the less entrained (to a long day), the more prone to phase shifts of a bigger magnitude.
Although rare, some individuals with DSPD even reported that dark therapy alone was sufficient for them to achieve their target phase advance. However, the most common use case is an adjunct therapy to light therapy, as a way to gain additional phase advance on top, of or preserve the phase advance obtained via, light therapy.
Hence, dark therapy serves two purposes:
- to allow for melatonin to get secreted without inhibition from bright light,
- to avoid unwanted phase delays by bright light exposure during the circadian evening and night, an effect of bright light that is independent from melatonin suppression.
There are two broad categories of tools to do dark therapy:
- either by using wearables such as blue blocker glasses as recommended by french sleep medicine institution SFRMS, which are glasses that filter out blue-green light, and can also dim down light intensity if VLT filter (same kind of filter used in sunglasses) is included.
- either by changing environmental light such as by using of blue filtering and brightness dimming apps in combination with switching off ambient lights.
The first approach is preferable if the user has no control on ambient light (such as when it's necessary to start dark therapy away from home), the second approach is more convenient but needs more preparation to buy adequate ambient lights and hence can be more expensive.
Due to photic history, dark therapy is also important to increase the effectiveness of light therapy in the morning. There is also some preliminary evidence that the effect of bright (blue or white) light is increased by a previous exposure to red light, which is recommended during dark therapy, hence there is some evidence of not only a reduction of unwanted phase shifts by doing dark therapy, but also of increased responsiveness to future bright light therapy treatments.
Dark therapy is also the only currently available method to preserve the non-receptor dependent antioxydative action of melatonin, since this requires huge doses of melatonin (about 8mg/kg/day in humans) that are currently undeliverable to humans. Indeed, the digestive system produces 100x more melatonin than the brain, hence the dosage of melatonin pills, albeit sufficient to activate the brain receptors and induce sleep, is not nearly sufficient to have antioxydative properties. The only solution is to preserve the endogenous melatonin secretion of the digestive system.
Theory for effective dark therapy
Light exposure and timing relatively to the circadian rhythm accounts for 71% of the variability in circadian rhythm shifting, hence the importance of controlling light exposure to control the circadian rhythm.
Since blue light shifts the circadian rhythm the most and constantly suppresses melatonin during exposure, it is especially important to filter blue and green lights, using blue color filters or blue blocker glasses. Indeed, a well designed study controlling for equal illuminance and color temperature found that blue-light enriched polychromatic white light resulted in a 50% melatonin suppression for a 175 lux light source, whereas no melatonin suppression occurred with a blue-light deprived white light of the same illuminance and color temperature. Furthermore, since green light can also phase advance, albeit with a limited range as duration is capped since the effect is mediated by cones rather than ipRGC cells, and temporarily suppresses melatonin secretion for ~90 min, green light filtering is also preferable. Amber and red filters and glasses are generally effective to filter out blue and green light and avoid melatonin suppression due to ipRGC cells unwanted evening stimulation.
Light intensity is also crucial, and any effective dark therapy includes the dimming of light sources. By how much do lights need to be dimmed to avoid affecting the circadian rhythm? 100 lux was already sufficient to cause 50% of the max stimulation of ipRGC cells in a study, while in another <30 lux was sufficient for 50% melatonin suppression, and another found that 100 mLux (melanopic lux) significantly reduced objective sleepiness, with higher illuminance having little more benefits. In another study, most participant's non-visual (ipRGC) sensitivity to light started as low as 5-10 lux (and even lower in another study), even with the eyes closed (see also here)! More precisely, another study found that human melatonin suppression occurs at ~10 log photons cm(-2) s(-1) at 460 nm. Given the high sensitivity of the human eye to low intensity non-photic inputs, it is likely that most people are exposed to bright light at inadequate timings in their circadian phase, which calls for the design of circadian lighting in home and work environments. This was tested in a naturalistic setting, which allowed to observe that half of the studied homes had bright enough ambient lighting to cause a 50% melatonin suppression at night, but, interestingly, not with other individuals under similar lighting conditions. This shows that not only is the lighting environment an issue that can be improved, but there is also differences between individuals' natural photosensitivity that make them more or less prone to the detrimental circadian shifting effects of ambient lighting. Furthermore, it was demonstrated that a late evening administration of melatonin does not prevent the phase delay induced by concurrent bright light exposure, so that the delay induced by an inadequate bright light environment cannot be solely compensated with melatonin intake, the lighting environment needs to be redesigned according to the human circadian physiology. In summary, this means that any light intensity will likely affect the circadian rhythm, although less with more dimmed lights, and some individuals are less photosensitive than others at equal light intensities, which means that the design of ambient lighting where humans live or do their activities crucially needs to be done with the human circadian physiology in mind, even at low light intensities, and there are indeed several companies providing such services.
Nevertheless, the difference between individuals' photosensitivities is an issue to implement dark therapy in practice, Indeed, how much dimming of light sources is necessary for effective dark therapy is highly variable between individuals depending on their photosensitivities. It was shown that people have different sensitivities to light, with some being hypersensitive to light while others are hyposensitive, as some individuals see their melatonin levels suppressed by half with light exposure of an intensity as low as 6 lux for the most sensitive individual to 350 lux for the least sensitive, hence a ">50-fold difference in sensitivity to evening light across individuals"! And this was done with typical sleepers, there may be an even greater variability for individuals with a circadian rhythm disorder. Circadian light hypersensitivity is common for individuals with DSPD (see also here and here), with an estimated 47% of DSPDs being light hypersensitive, and non24 (see also here), which can compound with the mistimed intrinsic circadian rhythm with the day-night cycle which makes these individuals more prone to the sensitive parts of the PRC (ie, the timing when light has more shifting effect on the circadian rhythm). The photic history is also variable between individuals.
Light hypersensitivity is positively correlated with the pupil area, with larger pupils allowing more light to enter the retina and hence more melatonin suppression. Hence, having wider pupils may be a sign of increased sensitivity to evening light, and hence of needing a stricter dark therapy. A study shown that the pupil's contraction reflex speed in response to bright light could detect DSPD. And indeed, a novel ophtalmological or optometrist test was devised with this principle at its core: the maximum post-illumination pupil response (PIPR) after blue light exposure after variable light intensity or chemically induced test. The purpose of this test is precisely to quantify the individual's photosensitivity to blue light, which is often termed circadian light as it is the light spectrum that affect the most the circadian rhythm. However, due to the novelty of this procedure, it is rare to find a clinicial who can offer it.
The DSM-5 recognizes the possibility of light hypo/hypersensitivity as a predisposing factor of DSPD and non-24: "predisposing factors may include a longer than average circadian period, changes in light sensitivity, and impaired homeostatic sleep drive. Some individuals with delayed sleep phase type may be hypersensitive to evening light, which can serve as a delay signal to the circadian clock, or they may be hyposensitive to morning light such that its phase-advancing effects are reduced".
Although it is often assumed that hypersensitivity and hyposensitivity are symmetrical, in that if someone is hypersensitive to light, they are so for both advancing and delaying. But that is not necessarily the case as the PRC can be nonlinear, as Czeisler et al hypothesized in the 1980s drawing inspiration from the fact that all humans have naturally asymmetrical light PRC, making it easier to phase delay up several hours rather than phase advance. Indeed, it was empirically demonstrated that the response to advancing or delaying cues is asymmetrical: on average, humans have been shown to have a range of entrainment (ROE) — which is the range of day time that one can maintain — to have been estimated between about 23h to 28h or 22h to 28h, which is why it's easier even for typical sleepers to rather sleep several hours later than to wake up even just 1h earlier than usual. It is worth noting that the range of possible entrainment varies depending on the specie. And indeed, we now know that low light intensity light in the evening is sufficient to phase delay, whereas brighter light and longer exposure are necessary in the morning to phase advance, which confirms the asymmetry of the circadian rhythm entrainment range, which makes it easier to phase delay than to phase advance.
This nonlinear response to light may partially be due to photic history ("light after-effects") mediated by period plasticity in the dorsomedial SCN network, which can be manipulated advantageously to modify light sensitivity. It was shown that exposure to only dim light in the biological day made the participants hypersensitive to light in their biological night, and oppositely that being exposed to bright light during the day reduces the phase shifts induced by night-time light. Hence daytime light therapy has a protective effect against evening light, and reducing evening light improves the response to daytime light therapy: "the more daylight, the weaker the impact of articial light in the evening/at night". This is because the SCN neuronal phase distribution gets widened during long days, and hence the various neurons will be at different points of their own PRC curves which will reduce the magnitude of light-induced phase shifts as demonstrated in empirical studies comparing long days (desynchronized SCN networks) vs short days (synchronized SCN networks) and this inverse relationship between phase shifts magnitude and period lengthening magnitude an intrinsic property of SCN neurons, and can be triggered by effecting contrasting (photic or electrical) inputs on these neurons. In practice, this means that people who are more prone to circadian photosensitivity may reduce it by increasing the duration of their daytime light therapy. Inversely, if the goal is to get bigger phase advances, a shorter day should be prefered. This also explainr parts of the reasons why very long light therapy seems to be so effective, by giving some additional protection against phase delays, and why dark therapy further improves the efficacy, especially when starting the therapy's protocol as we can hypothesize that individuals with circadian rhythm disorders are more prone to unwanted phase shifts due to mistimed zeitgebers when they are not yet entrained by the therapy.
Furthermore, another important consequence of the photic history is that light exposure in the previous evening will impair the effectiveness of light therapy in the next morning, and also decreases melatonin levels on the next days, and not just the evening when the light exposure happened. Indeed, light exposure not only phase shifts, but also modulate period length in different regions of the SCN. Hence, the darkness periods (scotoperiods) are as important as the light period (photoperiods), and hence why dark therapy increases the efficacy of light therapy. This is another reason why dark therapy always go hand-in-hand with light therapy, as both therapies mutually strengthen their efficacies.
In summary, all parameters of light therapy and properties of the effects of light on the circadian rhythm are also crucial to consider and control for an effective dark therapy: light intensity, light color and photic history.
It's important to understand that likely every levels of light intensity and color will affect the circadian rhythm, there is no 0 lux condition apart from staying in isolation in a pitch black room. But this is unnecessary, what matters is that the evening delay is much less than the daytime phase advance. For example, as described above, it was shown that being exposed to bright light during the day reduces the phase shifts induced by night-time light, with the opposite being also true. So this is all a matter of balance: to phase advance the circadian rhythm, either reduce the night time lights intensities, or increase the daytime light intensity (and exposure duration), both can result in equal benefits. Or both can be done to get even more phase advance.
As a mind image, picture the following: daytime light drags the circadian rhythm phase to the left (earlier time = phase advance), whereas biological evening and night time light drags the rhythm to the right (later time = phase delay), they are opposing forces and it's possible to tip the equilibrium one way or another by changing one or both forces. In addition, you can picture some kind of inertia in their movements, to illustrate the concept of photic history: when the circadian rhythm is pushed onto one direction because of strengthening one force, the circadian rhythm will continue to drift a bit in this direction even after the force stopped (eg, after doing weeks of light therapy, missing one day will not affect the circadian rhythm much).
Although the above is the general rule, as always, there are exceptions: in the highly experimental field of LDLD studies, a very unnatural circadian waveform shaping intervention that involves splitting the circadian rhythm into two subparts (not just bimodality by napping but two smaller cycles inside one big cycle), exposure to 0.1-lux green dim light during scotophases significantly improves the ability to bifurcate and the stability of entrainment, compared to both complete darkness and red dim light. Hence, there are non-linear effects of the exposure to dim light at night that are still not fully understood nor explored, and may provide additional means to tailor dark therapy to suite various purposes.
Several studies demonstrated the usefulness of dark therapy glasses, aka blue blocker glasses, and are hence recommended in complement to bright light therapy to treat circadian rhythm disorders including DSPD, ASPD and non-24 by the SFRMS.
Until the early 2020s, research on the effect of bright light and especially dark therapy in humans has been hindered by the difficulty to collect accurate data on exposure, as no device can be placed on the eyes, so alternative sites must be considered. Also, it is difficult to design sensors that reproduce the human eye's sensitivity profile both in terms of intensity and spectrum (color). However, in the early 2020s, melanopic lux (mLux) was devised and was found to be the most accurate predictor of the effect of light on the human circadian rhythm. One commercially available device implementing this technology is the LYS button necklace.
Given the biodynamics of melatonin natural secretion and suppression by bright light, dark therapy should be started 2-4h before natural fall asleep time. This is supported by evidence from a study of home lighting, which found that increased melanopic illuminance 3h before bedtime was correlated with increased wakefulness for 90 more minutes past bedtime.
Dark therapy in practice
In practice, an effective dark therapy consists of avoiding: bright light, blue-green lights, and at least a few hours before the natural bedtime. Hence, dimmed red light is acceptable. And no exception: even a short exposure to bright light will suppress melatonin and phase delay the circadian rhythm! Even with eyes closed!
Since pupil adjustment to light/darkness and circadian rhythm shifting are causally linked, because both are mostly modulated by the ipRGC cells, pupil dilation is a sign that the dark therapy is done optimally. In practice, this is known since a long time by astronomers, who use red filtered light to avoid pupils contraction which hinders looking at dim light sources such as stars. In scientific studies, low level light therapy (LLLT) consists of providing light therapy but with red light instead of blue or white light, with the red LLLT light used as placebo control to measure the efficiency of blue light therapy. In other words, if you can't see in the dark, there's likely still a too bright light source in your environment that you need to dim down or replace by something else such as red filtered light. Eyes refractive errors such as myopy or emmetropy do not change how the ipRGC cells work.
Furthermore, red light therapy (LLLT) may even be a treatment to help mitochondria in the retina's photoreceptor cells to repair faster and hence improve declining eyesight in aged (>40 years old) individuals. Indeed, "mitochondrial density is greatest in the retina's photoreceptor cells, which have high energy demands, [...] as a result, the retina ages faster than other organs, with a 70% ATP reduction over life, causing a significant decline in photoreceptor function as they lack the energy to perform their normal role."
However, although red light indeed does not inhibit melatonin contrary to blue light, 40 lux of red light is sufficient to change cortisol and alpha amylase levels, which suggests that there may be other non-visual pathways mediating these physiological changes induced by light beside the well-known one mediating melatonin inhibition through the suprachiasmatic nucleus and the pineal gland (but take this result with a grain of salt as it was published by one study in Hindawi, a predatory journal, this needs confirmation). Another study also finds that even polychromatic white light at 175 lux but with reduced blue wavelength light does not inhibit melatonin. Hence, environmental red or amber lighting is likely acceptable during the circadian night, as red lighting guarantees limited blue wavelength light, although polychromatic white light with reduced blue light may be acceptable too.
For dark therapy to be effective, it's necessary to shield from all bright light sources at all times during the circadian evening and circadian night. Indeed, even short exposures can inhibit melatonin (although less than long exposure) and phase delay (as much as a long exposure), hence a short trip to the bathroom, a common case, can erase all the efforts done before this night. For room lights, RGB LED bulbs such as Yeelight 1S are inexpensive options. For rooms where it's difficult to change the bulb, such as bathrooms, small push button portative lights with a red plastic filter taped on top can be helpful as secondary lights for evenings.
For screens, it's possible to install f.lux or another blue light filter app. These apps are effective, but not sufficient, as it's also necessary to dim the screen brightness to the minimum and also of course dim environmental light sources (lamps). Indeed, both light intensity and color matters, it's not enough to just filter blue light, or to dim down the light intensity, it's necessary to do both.
Here are the effect of blue light filtering apps and screen brightness dimming, as indicated by this excellent review:
> Smartphone use may delay sleep onset. One factor is the light emitted by their screens, but another may also be its entertaining character or related psychological effects, or both. Using the “night shift” mode of modern smartphones, the colour balance of the screen can be shifted to “warmer” and orangeish colours depleted in short-wavelength light. On a recent iPhone 7, this amounts to a reduction of melanopsin activation by 67% at full display brightness. This might seem like a large reduction at first, though by simply dimming the smartphone to its minimum level, the melanopsin activation can be reduced to less than 1% of the activation at maximum display brightness.
And this quote is for smartphones, which have a much lower minimal brightness than computer screens because they need to save power for extended battery duration, and so they try to save on hardware backlight power. In my experience, using a smartphone with the Twilight app for blue filtering and dimming light to the minimum on the phone and a bit more using the Twilight app allows to use the smartphone with little impact on the circadian rhythm or feelings of tiredness, without needing to wear blue blocker glasses. Configured like that, a smartphone is probably safer to use for reading than a book, because the bed lamp you need to light your book can also shift your circadian rhythm.
Furthermore, using a blue light filter software changes the content color, which can be troublesome. Escofet and Bara shown that dimming light sources and screens is more effective than filtering blue light, although arguably the combination of both is more effective.
On computers, unfortunately most computer screens do not dim much the backlight or even at all, as they rather use a variable flickering scheme - called Pulse-Width Modulation, so it's preferable to rather use a smartphone or wear blue blocker sunglasses. Indeed, if the screen uses PMW, then it is always backlighted at the maximum intensity it can, but it is simply intermittent so it visually looks like it's dimmed, but your eyes still receive as many photons and hence a PMW screen acts just like a pulsed light therapy device. Thus, if your screen uses PMW, it cannot be used in your biological evening without risking unwanted circadian phase delays and melatonin inhibition (ie, not feeling sleepy). Prefer to use your smartphone, which usually don't use PMW, since it's less effective at reducing battery consumption than really dimming the backlighting. If you plan on buying a new computer, you can check whether it uses PWM by reading notebookcheck reviews.
If your screen is not using PMW but cannot dim as much as you would like, some apps such as Nelson Pires' Dimmer can be used to add a transparent black window that will mimic a brightness reduction, but it won't actually reduce the backlighting, so the reduction of lux won't be optimal but it will be better than without dimming.
Although screens are often incriminated, and indeed need to be adjusted for evening use, ambient lighting by lamps plays a major, likely greater, role in unwanted circadian rhythm shifting. Indeed, a study found that half of the studied homes had bright enough lamps to suppress melatonin by 50%, although the exact suppression varied a lot for each user (0% to 87%), and greater exposure to evening light was associated with increased wakefulness later bedtime. Of note, energy-efficient lights produced nearly double the light intensity and melatonin suppression than incandescent lighting. Hence, home lighting can certainly affect the circadian rhythm, but not of everyone, some are more sensitive than others, and hence it's unpredictable how much lighting affect a specific individual. This further supports the importance of wearing blue blocker glasses evening indoors, in case the user can't fully control (and dim or switch off) ambient lighting.
A very convenient alternative, instead of installing softwares and modifying all ambient light lamps, which may not be possible in some settings (eg, work office lights, sunlight), is to wear blue blocker glasses or even better blue blocker SUNglasses aka red-tinted laser safety glasses.
Blue blocker glasses are just a wearable device that allows to do dark therapy while being independent from environmental conditions, ie, there is no need to turn off room lights, although dimming their intensity can still be preferable. Blue blocker glasses are amber/orange glasses filtering blue and partially green light, but does not dim the light. You can use them when you are out at a friend's house for example, or if someone is at your house for a dinner or something, you can still do your dark therapy while keeping the lights on for your guests, or simply to read a book without changing your light (else you need to use a red light bulb, which is inexpensive but makes it hard to read). Just like for the typical dark therapy, blue blocker glasses should be used 2-3h before habitual/natural bedtime to allow for endogenous melatonin to build up.
Prefer industrial-grade blue blocker glasses that are orange or red tinted, as they provide complete filtration. Are "comfort" "blue light filtering glasses" or coating for prescription glasses that some brands sell sufficient, that are generally yellow-tinted? Yellow tinted glasses can only filter UV light, not blue light, whereas orange tinted can filter most of blue light and some of green light, and red tinted filters the whole UV, blue and green light spectrum, hence yellow-tinted "comfort" glasses are inadequate to filter blue light. The french national institute of health (ANSES) also confirmed this empirically in a report, finding that the tested "comfort blue light filtering" coatings provide only "very variable" and "weak or inexistent" blue light filtering that "cannot possibly" help to maintain melatonin secretion. Comfort glasses and coatings, generally yellow-tinted, are inadequate for the purpose of dark therapy, and only orange or red-tinted industrial-grade glasses should be used, no coatings.
If possible, it is strongly advised to choose blue blocker glasses that provably filter the whole spectrum of both blue and green light (from 400nm up to about 560nm), as ideally, an adequate blue blocker glasses must also blocking green light (here's a testimony) and not just blue light. Indeed, green light can phase advance and can temporarily suppress melatonin secretion temporarily for 90 min, and melatonin suppression prevents the appearance of feelings of sleepiness and decreases sleep quality by increasing sleep deprivation. Another thing to consider is how much the blue blocker glasses are covering your eyes: if they don't cover adequately, light coming from the sides can still reach your eyes, and actually most of the ipRGC cells (the ones that shift your circadian rhythm) are located in the nasal region, so light coming from the sides of the eyes are very effective at shifting your circadian rhythm. To determine the filtering efficiency, it's simple (but expensive): use a spectrometer behind the glasses under the sunlight. However, spectrometers are quite expensive. Fortunately, there are a few published tests you can refer to, such as the Blubox review, which shows that both UVEX (cheap industrial safety props maker) and Blublox (more expensive but filters a bit more) filter most of the blue and green light bands. Consumer Reports concluded that only the UVEX Skyper glasses blocked nearly all of blue light out of 2 other more expensive glasses. There is also this other spectrometer review with more brands (and it's also a tutorial on how to make your own DIY blue blocker glasses). Industrial-grade laser safety european or FDA certified red-tinted glasses are also a safe kind of blue blocking glasses, and they block the entire UV, blue and green spectrum.
A good and inexpensive (~$20) brand of blue-green blocker glasses is the UVEX line of glasses with the SCT Orange coating. Here is a photo of the blue blocker glasses UVEX S0360X Ultra-Spec SCT Orange:

Blue blocker SUNglasses are a variant of blue blocker glasses, but in addition they have a transparent black shading layer to dim down light. Hence, they both filter blue (and green) light and also dim any light source (which includes devices where you can't install a blue filtering app, such as alarm clocks, TV screens, etc... but also natural light sources such as sunlight).
Here is a DIY blue blocker sunglasses made out of a UVEX Skyper blue blocker glasses, with added 5% black shading/tinting filters for cars windows (one filter outside and one filter outside = 2 in total, 5% means that 95% of light is filtered), simply taped onto the frame:


And here is what it looks like to look through a blue blocker SUNglasses (so it's also dimming down the light - in practice it can dim down sunlight so much that it looks like it's night, which is perfect):
Without:

With the blue blocker SUNglasses (same ISO and photo parameters as the picture above - note how we can see the shape of the neon tube, just as if it was itself less intense, which also shows that obviously you should NOT drive when using the blue blocker SUNglasses):

If it's cumbersome to do your own blue blocker SUNglasses, it is possible to find laser safety glasses with a certified wavelength filtering range and red lenses. There are laser safety glasses for all ranges of light colors (wavelengths), for dark therapy what is needed is to block both blue and green lasers, hence the lenses should be red. The advantage with red tinted glasses is that they filter the whole blue and green wavelengths range, whereas orange tinted glasses such as UVEX only filter blue color and a bit of green, but the remaining green colored light can still affect the circadian rhythm, albeit less than blue light. Additionally, the laser safety glasses should also be described as having a reduced "visible light transmittance" or "Daylight Transmission (VLT)", which means that it will not only filter blue-green light but also dim down the intensity, just like sunglasses.
Here is a picture of a laser safety glasses filtering the 190nm-550nm range (ultraviolet, blue and green colors), optical density OD 4+ and Visible Light Transmittance: 30% (shopping page - note that sometimes these are sold as "laser epilation goggles"):

And here is a comparison without and with these laser safety glasses, using a camera with fixed photo parameters (note how the interior of the neon can be seen only through the glasses, similarly to the DIY blue blocker SUNglasses above):


This shows that the laser safety glasses are a good alternative to the DIY blue blocker glasses if you want a more streamlined and less hacky solution that you can bring outdoors.
Whenever possible, prefer to use blue blocker glasses that have additional panels on the sides, which is called a "wrap-around glasses", and on top and below the glasses, to better filter unwanted peripheral lightrays from the sides, such as can be found on the UVEX Ultra-Spec SCT Orange and the laser safety glasses:

Word of caution: not all blue blocker glasses are alike. Consider all other kinds of blue blocker glasses as unreliable, especially the "comfort" glasses or coating on prescription glasses. Most are made for "comfort", to filter the blue light from screens and "reduce eye fatigue". But not from sunlight, which is very different and hence these will be insufficient for any other purpose than filtering screens light (ie, maybe not even sufficient to filter room lamps). Comfort blue blockers are very different from industrial-grade blue blocker glasses, the latter being made (and advertised) to protect from light hazards that can damage the eyes of industrial workers. So if you see blue blocker glasses being advertised as comfort devices, with photos of people in front of their screens, then likely they are not efficiently filtering all of blue and green light. If the glasses are tinted yellow, they are of very low quality, don't choose that, prefer orange/amber glasses. On the opposite, if the glasses are presented as safety wear for industrial workers, they are likely much more filtering.
If you have prescription glasses, you can choose the Uvex S0360X Ultra spec SCT Orange, which very comfortably fit on top of any prescription glasses (tested with large aviator-style prescription glasses) and still covers from all directions of light. The Uvex S0360X Ultra spec SCT Orange uses exactly the same material and hence has the same blue and green light filtering efficiency as the Uvex Skyper tested here. Indeed, these two models are the only with SCT-Orange dying (SCT stands for Spectrum Control Technology), as indicated by pages 45-47 of this document. All UVEX dyes protect from 99.9% of UV too. UVEX is a brand of Honeywell. The UVEX glasses were used in several studies on light and dark therapy (here on non-24, here on night shift workers). Just like the Skyper, the UVEX Ultra Spec SCT Orange one can be adapted to SUNglasses by adding shading films.
In practice, I used at first 2 pairs of UVEX blue blocker glasses:
- one with shading film that I use to dim down uncontrollable light sources when there is sunlight or at the office when I work late to dim down the bright neon lights,
- and another one without a shading film (so just the blue blocker glasses as-is) that I use in the evening at home to simply filter the blue and green lights.
Later, I started using exclusively the red tinted laser safety glasses, as they are essentially a commercially available blue blocker sunglasses. They work wonders, especially indoors and for the evening/night/morning setting, however they cannot filter sunlight completely.
To check if dark therapy is done right, check if your eyes pupils are dilated just like as if you were in the dark (ie, you should be able to see in the dark right away if you temporarily switch off all light sources, without waiting for any pupil adjustment). This test works because it's the same photoreceptive cells in the eye (the ipRGC cells) that are both modulating the pupil's dilation and circadian rhythm shifting in response to light exposure. Hence, if you can see in the dark while using a device or doing an activity, it's ok for dark therapy and it can be used during the 3h before bedtime. Otherwise, if you can't see in the dark, it means some light emitting device needs to be adapted/removed.
Alternatively, it's possible to use a lux meter app on any modern smartphone with a light sensor, direct the smartphone's screen towards the direction you face, and in an environment adequately set for dark therapy, the reading should be below 10 lux and ideally below 1 lux (= a candle flame's light). A reading of 0 lux is ideal as it doesn't mean that there is really 0 lux detected but rather that the received light intensity is below the detection threshold for the smartphone's light sensor, which means it's likely negligible.
Dark therapy can also be automated by adapting the environment, such as by getting smart RGB LED bulbs, such as Yeelight S1 (~15 to 20 euros apiece). These bulbs can be connected to a wifi router, and programmed to automatically change color to a red dimmed light at specific hours, or at anytime the user wants via a smartphone app. Yeelight is a major brand from Xiaomi, not just a copycat, and actually with Phillips Hue taking inspiration from Yeelight.

Yeelight S1 smart RGB LED bulb, scheduled to automate dark therapy by turning into a dimmed red at specific times set by the user.
Transition/progressive/photochromic lenses not adequate because they require UV, hence they work only against direct sunlight (ie, not through a window, and not with artificial light either). Hence they are not adequate.
Alternative for prescription glasses: laser safety clip on amber glasses, if they filter from 400nm to 550nm then it should be good enough for dark therapy, and they should be certified since it's for laser safety hence you are guaranteed they will filter those wavelength.
If UVEX glasses become unavailable in the future, look for laser safety amber or red glasses filtering from 400nm to 550nm with certification, this should be as efficient if not more than the UVEX glasses, and laser safety glasses will always be available in the future as laser is a generic technology that will always be useful.
Shading films quality can be assessed with their VLT percentage, but for clip-ons it's less standardized, you won't know how much dimming you'll get so it's impossible to say what clip-ons to use exactly. If you want more reliability, use shading films with a defined VLT percentage. If you want a more ergonomic and easy solution, use clip-ons with flip-up so they can easily be put on and flipped up if you want to stop using the shading without removing the clip-on, this will work well on prescription glasses and you can combine with the UVEX S0360X on top. However, clip-ons are incompatible with the UVEX glasses themselves, whether S0360X or Skyper, they are simply too small and misplaced for these big glasses, so the clip-ons not only look weird but they don't dim light from all the peripheral vision. Use clip-ons only on prescription glasses, not on the UVEX glasses. If you want the UVEX glasses to be shaded themselves, use shading films for car windows with 5% VLT and tape them on the UVEX glasses as shown above.
Eyes iris color does not appear to influence the hypersensitivity to evening light exposure. Blue blocker glasses can also be used to protect the eye from the phototoxicity of near-UV blue light (405nm) and the study's authors would recommend them for "high-risk populations, such as people with dry eye, contact lens users, the malnourished and the elderly".
Beware of LED ceiling lamps, especially the cheap ones, as they are often made of only blue-light emitting LEDs combined with a yellow encasing, but so they are rich in blue radiations.
(Side-note: although the idea to make the blue blocker glasses into sunglasses is my own, a previous blog post independently communicated a similar idea, to make "amber tinted shaded glasses" to treat circadian rhythm disorders)
Softwares for dark therapy
Although softwares are suboptimal and insufficient for an adequate dark therapy (see the previous section), they can nevertheless help. Here is a list of recommended softwares to complement dark therapy:
- Dark Mode extension on Chrome, Firefox, Opera and Edge.
- Alternative: Can set Dark Mode on whole Windows interface, and Chrome will adapt and show a dark mode by default then.
- LightBulb for blue light filter on Windows (but not as good as a blue blocker glasses), Twilight on Android.
- FreshEyes extension for Chrome to change colors to make them more visible when using a blue light filter.
- Nelson Pires' Dimmer v2
- Lux meter app on a smartphone with a light sensor, to more objectively check the light intensity in the environment.
Review of wearable light therapy glasses (October 2023)
Criteria
The following is a review of the technical specs of light therapy glasses available or advertised as of September 2021, last updated in October 2023. Since the author does not have the possibility to test every devices, the review focuses on whether the devices technically fits the criteria for an effective light therapy to manipulate the circadian rhythm.
The goal of all light therapy devices to be effective is to maximally stimulate the ipRGC cells in the eyes. Hence the crucial parameters for an effective light therapy device are:
- orientation (ideally on the sides).
- light intensity (>= 500 lux, as computer screens can deliver ~250lux already).
- light color (blue stimulating the most and red the least and ~480nm , more precisely between 479nm and 482nm, being optimal - note that long duration of bright light therapy can only phase shift with blue light therapy, as green light (555nm) lose phase shifting effectiveness exponentially with duration).
Furthermore, efficacy of light therapy is affected by the duration of exposure, hence a long battery and having a weight as light as possible is beneficial for very long bright light therapy exposure and better manipulate the circadian rhythm, as it seems that circadian rhythm disorders need much longer daily light therapy sessions (1-9h) compared to seasonal affective disorder (30-90min).
Hence, this review will focus on the following technical specs:
- Light color wavelength in nanometer.
- LEDs position/orientation.
- Battery duration.
- Form factor: full glasses or lightweight "half-glasses" (just a rail sitting on the nose). Half-glasses styles should better fit with prescription glasses and be lighter, but full glasses can provide more features such as interchangeable lenses to double as dark therapy glasses.
- Safety: UV filtering and nanometer wavelength >= 480nm.
- Published studies to back up this specific device's efficiency (and not just some general papers about light therapy).
- Price.
- Other particularities such as blue light filtering coating (so double as dark therapy glasses).
Blue light emitting glasses
These glasses allow to provide the full spectrum of blue light therapy effects, such as maximal circadian rhythm manipulation (185x more compared to other colors), mood improvement and depression treatment, energy boost (cortisol release).
- Luminette V3: the tried and true light therapy glasses. Since their first model in 2006 after 4 years of research (see also here) that the Lucimed did at the University of Liège in Belgium, they made 2 other iterations. The Luminette v3 came out in December 2019, it emits continuous white light combined with an enrichment of 468nm blue light (see also here). The leds are placed above the eyes in a rail that goes far enough to the sides so that rays are projected to the nasal area of the retina, although the placement could be more optimal if LEDs would be placed on the sides on the eyes to maximally stimulate the nasal part of the retina. The LEDs are numerous, more than with any other currently available light therapy glasses. It has 3 different light intensity settings (500 lux, 1000 lux and 1500 lux). After 8 months of daily usage, the battery lasts 11h to 12h of continuous light therapy with a single charge. The form factor is "half-glasses", so that it's only a rail that sits on top of the nose, so that prescription glasses can comfortable fit underneath, even large ones (tested with aviator-style large prescription glasses). Safety-wise, the light is UV-filtered, but the blue light is a bit on the low-end as below 480nm it can become dangerous, so Luminette with 468nm is on the edge but Lucimed could tweak Luminette v4 to target ~480nm instead, which would be even more optimal to stimulate ipRGC cells while simultaneously reduce safety issues. It nevertheless complies with current USA and European (CE)'s safety regulations. Luminette v2 is very similar to Luminette v3 but has less battery (6h according to a user's test) and is more bulky, but otherwise works as fine as long as you recharge the battery every day or 2 days. There are several studies on the Luminette devices for sleep and circadian rhythm disorders, and there are more underway for example for depression treatment. The Luminette has been successfully used for entrainment by several individuals with non24 (including the author), and also by some individuals with DSPD, with even one formal study on DSPD, a rare occurrence for currently available light therapy glasses. Hence, Luminette is currently the most scientifically backed light therapy glasses available on the commercial market. Luminette has been certified as presenting no risk for the eyes according to international regulations (see also here in french for european regulations). Price: 229€ in Europe, $199 in USA (there were discounts up to 25% during past years black fridays). User manual is available online here. Make sure to remove the protective blue film on the hologram before using the Luminette, otherwise the white light won't be enriched with blue light, and a few non-24 users reported a drastic decrease of performance when the film was present. Note that the fact the device is emitting white light may make it more prone to cause minor side effects of sudden bright light exposure, such as feeling the light to be the strongest compared to other light therapy devices and a reddit user who reported experiencing headaches even with the lowest intensity on Luminette v3 did not experience them with the Ayo blue-only light therapy glasses while getting more phase advance with shorter durations of exposure.
- The company Lucimed has added a new product called Drive, which allows to do blue light therapy while driving, as it does not impair vision. It costs 199 euros in Europe.
- Psio: continuous and intermittent blue light, with simultaneous audio stimulation for relaxation. It emits 470nm blue light, projected from LEDs at the top of the glasses but reflected on the lens and hence with rays entering the eyes from all angles, including towards the nasal area of the retina. No information about battery duration. Form factor: full glasses. Price unknown (need to contact the seller to program an appointment). One study is available on melatonin inhibition, but not on circadian shifting. Safety-wise, there are no UV, and 470nm blue light should be safe, although it could be safer and more optimal by increasing the wavelength to 480nm.
- Ayo: blue light emitting glasses (no white light). These are among the most well-known competitors to Luminette (and actually are more common). The blue light is in the 470-475nm wavelength range, which is closer than Luminette to the optimal ~480nm wavelength to maximally stimulate ipRGC cells, and with "an irradiance of 250 μW/cm2" and 500 lux to 1500 lux depending on the selected intensity. LEDs placement: 2 LEDs directly above each eye (4 LEDs in total), and diffused (partly) with a plastic rail. The orientation of the light rays does not seem ideal to target the nasal area of the retina, the LEDs seem to rather target the macular, which is not ideal. Form factor: lightweight half-glasses, so can likely fit all prescription glasses. Safety-wise, the wavelength is high enough to ensure there is no blue color phototoxicity, and the light is also certifiably UV-free and infrared-free independently by the TÜV Rheinland. It complies with USA and European (CE)'s safety regulations. Battery life is limited to 3h per charge according to documentation, or more precisely 210min/3.5h according to a user's practical test (thank you to ConsciousBluebird473), so that very long light therapy is excluded with this device, hence this device may be sufficient for most cases of DSPD, but not for severe DSPD nor non-24. Although there are no peer-reviewed study on this specific device yet (but there is one non-peer reviewed bachelor report on depression), there is some evidence that blue-only LEDs are more effective for circadian rhythm shifting than white light because of subadditivity, the Ayo device may be more effective than Luminette, although blue-only LEDs were shown to be more phototoxic than white LEDs, but only in albino mice (not in pigmented mice). Anecdotally, a reddit user reported the Ayo blue-only light therapy glasses produced more phase advance with shorter durations of exposure with no side effect such as headaches compared to Luminette v3. Another user reported the Ayo glasses to be more comfortable and lighter to use, with similar effects as Luminettes. However, reviews on Amazon report increased headaches using Ayo and reduced effectiveness compared to Luminette, in addition to weaker electronics that last less (see here, here and here). Price: $299 (299 euros) brand new Ayo, $199 (169 euros) for Ayolite (same as Ayo but without the app nor wireless charging). It's recommended to disable the ambient light sensor dynamic adaptation feature as the efficacy of variable light therapy intensity has not been studied. See also this review.
- Propeaq: continuous blue light of "468nm at an intensity of only 35 lux". They have a full glasses form factor, so that they maybe won't fit with prescription glasses underneath. The light intensity, if not an error (they may have meant lumen, which is the amount of light that the LEDs project and may result in bigger lux at the eyes level since the LEDs are "1.5cm away from the eyes"), is too low to see a significant circadian rhythm shifting effect. No indication about LEDs placement (no orientation info). No indication about UV filtering, but apriori this is not necessary if the LEDs only project blue light of 468nm. The glasses come with 3 sets of lens so it can double as dark therapy glasses with red lenses, and it also offers unique "blue glass" lenses with a sunshading to filter sunlight except for the blue light wavelength that can passthrough 100% unfiltered. However, this may be dangerous to the eyes, as the blue light from sunlight is too intense, and with the filtering of other wavelength except blue light, this may subjectively induce the user to feel less exposed to intense and possibly eye damaging sunlight than with regular sunglasses, so that more tests are needed to assess whether these 3rd set of lenses are safe to use. No information about the battery. The Propeaq glasses cost 199 euros. No study on this device.
- Pocket Sky: continuous blue light with a wavelength between "460-480 nm". No information about light intensity (lux). Form factor: very lightweight half-glasses, with LEDs placed as an overhead rail, which is good enough as it extends to the sides. Due to the very slim design, the device only carry a very thin battery, which can lasts for 3-4 sessions of 20min duration each. Beside the lack of light intensity information which precludes the possibility to fully assess the effectiveness of the device, furthermore the FAQ states that the device automatically starts when extracted from its case, and stops automatically after 20min. This precludes any use for circadian rhythm shifting, as several hours of light therapy are necessary. However, a very nice feature is its "sunrise simulation": the device starts to progressively light up for the first 20 seconds when activating the glasses, which allows to gently let the eyes' pupils accommodate to the light therapy. This is a very interesting feature that would be a welcome addition to all other light therapy devices, as this can greatly reduce the risk of side effects due to sudden bright light exposure, but meanwhile as a user you can workaround this lack in other devices by keeping the eyes closed for the first 20s when switching on the light therapy glasses, which will also allow the pupils to gently accommodate. Another nice feature is that the glasses' case also serves as a recharging station, which is a very nice idea to ensure proper storage of the device when not used and to reduce the risk of breaking it. Price: 165 euros in pre-order. No specific study on this device. See also this review.
- Sula: continuous blue light with wavelength in the "Blue-Turquoise (470-480nm)" range. Form factor: full glasses, which apriori cannot fit with prescription glasses underneath, although they offer to replace the lenses with prescription lenses if asked to. No information about light intensity (lux) nor LEDs placement (and hence orientation). Includes blue light filtering lenses, so that they can double as dark therapy glasses (although it seems they use a coating, which are much less effective than orange/red tinted lenses). No specific study on the device but they provide an accurate bibliography of general studies on light and dark therapy. No information about the battery. No price nor public availability for now.
- Lumos Lux: founded by Stanford researcher Dr. Jamie M. Zeitzer who is a long-time researcher in light's effect on biology and circadian rhythm, and he indeed published a lot of papers on the topic. The video at the bottom with Dr. Zeitzer is a very good accurate introduction to light therapy. However, there is no precise information about the glasses: the wavelength nor light intensity are detailed, although it seems clear the glasses emit blue light. The device intends to allow for a full control of the light exposure, hence the form factor is full glasses, and it includes blue light filtering lenses, so that the glasses can filter all blue light and fully control the exposure. Blue LEDs are placed on the glasses legs and reflected on the lenses, so the orientation is adequate, maybe even optimal. No specific study on this device. This device is promising and is certainly conceived by an academic specialist in light therapy and circadian rhythm science, but there is simply no info on which we can assess whether the device can be effective. Price: unknown, and device not available for sale yet.
Green light emitting glasses
Although green light has shown some efficacy to shift the circadian rhythm, it has shown less effectiveness than blue light for both circadian rhythm shifting and cognitive effects (mood and energy), both in terms of magnitude and duration (the effect of green light on melatonin suppression is limited in duration to 90min as continuous exposure will see reduced effect, whereas this does not happen with blue light, which means that long bright light therapy cannot be done with green light therapy glasses). Green light is currently investigated to reduce pain.
- Re-Timer 2: green-blue 500 nm dominant wavelength light emitting light therapy glasses. LEDs placement: bottom, which is not an optimal orientation to attain the nasal part of the retina. Only 4 LEDs are used, similarly to Ayo. Detailed specifications are available, which is a welcome approach that should be followed by all manufacturers (instead of providing only marketing speech). Light intensity is from 315 lux (143 µW/cm²) to 506 lux (230 µW/cm²). Light pulse is 50 to 166 hertz. Battery duration is "up to 6h" according to the Re-Timer website, but a user reported that this is inaccurate as the battery lasts in fact much longer, about 14h with one charge, which is on par or slightly longer than the Luminette 3, and is hence sufficiently long for circadian rhythm shifting. Form factor: half-glasses. Safety-wise, these are the safest light therapy glasses, as the wavelength is high enough to ensure there is no risk of blue light phototoxicity (since the light is actually green - although one study suggests it may not be that simple), and the light is UV-free. Price: 199 euros. As of 2020, there are two studies on this specific device: one study showing it can shift the circadian rhythm and another one about its effects on eyes health.
- Pegasi 2 (Dream Glasses): green light therapy glasses. LEDs placement: top with a short rail that doesn't go to the sides, which is not ideal but ok, it should be able to stimulate the ipRGC cells in the macula and some in the nasal part of the retina, although suboptimally since the rail is too short. There is no technical information, no information on the battery, and even their photobiological safety EN62471 seems fishy as they mention they have it but they do not provide a link to the full document and it cannot be found elsewhere on the internet. Price: $198.99.
- FeelBrightLight: Likely the oldest portable light therapy brand, it offers a green light emitting visor (a light therapy emitting device attached to a cap) instead of glasses. It was one of the only 2 selected and recommended light therapy devices by famous pioneers in circadian science and medicine such as Josephine Arendt in a 2010 report to the Canadian Air Force.
- Dayvia Sun Activ: green light therapy glasses. Very lightweight, 3 intensity settings. Not much technical infos. Certified safe according to european regulations. Price: 178 euros.
Final word
Although the author did not test other glasses than the Luminette, competition in this field is highly welcome and hence if you would like to try one of the promising blue light therapy glasses, this will allow not only to test alternatives but also support the development of these alternatives. Sessions last only for a maximum of 30 min before the glasses turn off, but they can be turned on again.
Light therapy is certainly a therapy worth trying if no contra-indications (epilepsy, retina diseases, photosensitivity).
Anecdotally, so far from all those who tried on reddit or discord, there's no one who did not get a significant effect on their circadian rhythm, but this does not mean they were all entrained, as for some light therapy was not sufficient (especially when there are comorbid diseases which worsen the circadian rhythm disorder such as restless legs syndrome).
If you found another light therapy glasses not present in this list, make sure they are certified with CE 0459 in Europe, which determines light therapy products, and IEC 62471 or EN62471, for eyes safety.
Melatonin
The many biological functions of melatonin
Melatonin has a ton of different biological functions, hence why it is qualified as a "pleiotropic agent" (meaning "many"). These functions can be classified under two broad families: 1- the receptor-dependent actions, where melatonin plays an indole hormonal role of circadian rhythm and wakefulness-sleep regulation by activating the melatonin receptors, functioning as an endocrine, autocrine, paracrine molecule, 2- the receptor-independent (extracellular) actions, where melatonin does not need any receptor and will directly act on the cells to protect them from oxydative and inflammatory stress. Let's focus first on the circadian rhythm and sleep effects.
Although melatonin is often dubbed the "hormone of sleep", this is a misconception, as melatonin is rather a "hormone of darkness". Indeed, in all species, whether diurnal or nocturnal, melatonin is always secreted when it's dark (usually night time), which in humans triggers sleep related processes, whereas in nocturnal animals such as nocturnal rodents, melatonin does the opposite by increasing wakefulness. In other words, melatonin is a marker for darkness and hence is always secreted when its dark, but diurnal and nocturnal animals are wired oppositely to produce opposite effects on the sleep-wake schedule. Indeed, melatonin and bright light are wired inversely to the noradrenergic vasoconstrictor system in nocturnal animals compared to diurnal animals, so that melatonin signals wakefulness periods and bright light exposure induces sleep, but the circadian rhythm and its linear coupling with core body temperature remain the same as in diurnal animals, with high phases associated with wakefulness periods and low phases with sleep periods. This inversion in the circadian rhythm machinery not only affects daily entrainment but also seasonality, as nocturnal animals have longer activity periods during winter when days are short and shorter activity periods during summer, whereas the opposite is observed in diurnal animals (see also here).
Melatonin can both phase advance the circadian rhythm by binding to the melatonin type 2 (MT2) receptors, and consolidate sleep and the circadian rhythm (ie, avoids fragmentation and waking up too early by ensuring the body stays asleep during the 2nd half of the biological night). It can also induce drowsiness by binding to melatonin type 1 (MT1) receptors, in other words it helps with feeling sleepiness (see also here). There is also a type 3 (MT3) receptor that melatonin (but not other drugs like ramelteon) stimulate.
According to the latest findings, it is currently theorized that one of the main biological purpose of melatonin is to be a circadian rhythm stabilizer, and not a zeitgeber, potentially through a feedback loop regulating the suprachiasmatic nucleus firing rate. This holds true for exogenous melatonin too, although it likely depends on the formulation: instant-release melatonin for circadian rhythm shifting, prolonged-release melatonin for primary insomnia (ie, sleep consolidation). Hence, it may not be biologically meant to shift the circadian rhythm, although it can be used for this purpose as shown by PRC curve studies.
Melatonin modulates, and is modulated by, the circadian rhythm by decreasing the core body temperature via vasodilatation at distal skin sites such as the hands (see also here). Body temperature changes are the primary way the body signals circadian clock changes throughout all cells, notably in response to bright light exposure through the SCN (although it was shown that the SCN is unnecessary as ipRGC cells are sufficient to induce acute temperature changes), and indeed melatonin works the same. Since at least 1992 it's known that endogenous melatonin levels are inversely coupled with peripheral (limbs) temperature (heat transfer to limbs is a way to reduce core body temperature, so increasing limbs temperature actually decreases core body temperature), and later that core body temperature reduction caused by melatonin is dose-dependent, with supraphysiological doses of melatonin being especially hypothermic. A 2001 study on elders found that 3mg of exogenous melatonin produced hypothermic effects whereas 0.3mg did not. Later, another study shown that only doses higher than 1mg of exogenous melatonin could produce hypothermia. Body temperature modulation was already suspected since at least 2007 to be the primary signalling pathway of circadian clock changes and synchronization, especially from melatonin, since supraphysiological melatonin doses were known to cause hypothermia, and it was even earlier hypothesized in 2000 that temperature may be a 3rd signalling pathway and potential treatment approach after light therapy and melatonin, which is further strenghtened by later empirical evidence and warm bath therapies being investigated (water-based passive body heating). Since then, experiments with daytime administration of melatonin clearly demonstrated that melatonin magnifies circadian rhythm induced thermoregulation and this is what underlies the soporific and circadian rhythm shifting actions of melatonin (see also here). Melatonin starts rapidly increasing, simultaneously to distal skin temperature rapidly decreasing and subjective sleepiness increasing, about 110min before core body temperature starts decreasing.
Melatonin is mostly secreted (2 orders of magnitude more) by the digestive tract, and to a lower extent by the pineal gland regulated by the suprachiasmatic nucleus in the brain, and to an even lesser extent by various structures such as the eyes themselves (including in the retina, lens and ciliary body), where melatonin "acts directly on ocular structures to mediate a variety of diurnal rhythms and physiological processes within the eye". The pineal gland can be considered a "vestigial eye". The melatonin secretion controlled by the retinohypothalamic-pineal (RHP) axis's responses to light is highly conserved throughout evolution in mammals, but melatonin secretion is present in almost all living organisms, "including bacteria, unicellular eukaryotes, algae, plants invertebrates and vertebrates" where it may also be the key player regulating the plants circadian rhythm despite the lack of a pineal gland. Virtually all biological structures in the body can produce melatonin thanks to the mitochondria (including skin - the largest human organ - but also the "brain, retina, Harderian gland, ciliary body, lens, thymus, airway epithelium, bone marrow, immune cells, gonads, placenta, gastrointestinal tract"), and they are themselves a primary target of the melatonin indole, as melatonin can be degraded via indolic and kynuric pathways with melatonin receptors widely distributed throughout the body, although melatonin has a wide spectrum of activities such as antioxydative and cytoprotective which are receptor-independent. Hence melatoninergic systems should be seen as decentralized, with a multitude of local melatoninergic systems at organs level and some global melatoninergic systems such as the chronobiotic regulation. Melatonin blood profile is primarily influenced by light exposure, and to a lesser extent by body position, physical activity, sleep, caffeine and drugs like beta-blockers such as atenolol, as these drugs increase core body temperature and hence affect the circadian rhythm and increase alertness. Melatonin is efficiently absorbed by the digestive tract, although its bioavailability remains low at around 15%.
Since melatonin is strongly associated with the circadian rhythm, and the DLMO marking the time of the beginning of an individual's biological night, melatonin profile is often used as a biomarker to measure the circadian rhythm, through salivary samples in a dim lit environment (dim-light melatonin onset). It was demonstrated that both non-24 (25h or 24.8h to 25.8h) and DSPD (between 24.5h and 25h) have a delayed and longer melatonin profile, confirming one of Czeisler CA et al's hypotheses. Although some studies suggested that a longer melatonin profile and hence a longer circadian period (tau) may be a hallmark of non-24 compared to other circadian rhythm disorders such as DSPD, the low sample size (less than 10 subjects in each studies, sometimes as low as 2 non-24) and the negligible difference particularly between studies does not allow to confirm this hypothesis.
About the receptor-independent extracellular actions of melatonin, a recent landmark 2020 study (and its awesome video abstract) have shed light on its likely major purpose. Indeed, this study is the first to investigate why sleep is necessary and how exactly it can cause death. Contrary to what was assumed before, it's not the brain, but the accumulation of reactive oxidative species (ROS) in the guts that cause death. By supplementing orally with melatonin to flies and rats who were prevented from sleeping, they could live a full life with no behavioral sign of brain injuries. Hence, this shows that melatonin, in addition to its receptor-dependent effect of inducing and consolidating sleep, is also used biologically to clean up oxidative stress and avoid death by cellular stress.
Due to these extracellular capabilities, melatonin is also be beneficial for a wealth of other health issues beside sleep, and melatonin deficiency can have life-threatening consequences. As an anecdote: everybody knows about the famous longevity experiment where mice that are restricted from eating, so that they eat a lot less, live a lot longer than mice who can feed anytime and as much as they want. This reproducible result is often interpreted in various ways: effect of fasting, calorie restriction, autophagy, etc. But in fact, it's known since at least the 90s that if melatonin is injected into the mice who can eat anytime they want, they will live longer, as long as the ones who are restricted (see this review for an explanation). Furthermore, if a pinealectomy (ie, remove the pineal gland which regulates melatonin secretion) is done on the mice who are restricted, they will die a lot younger, hence losing all the benefits of calorie restriction (TODO: check ref). In summary: instead of restricting eating, supplying melatonin was sufficient to extend the lifespan of the mice, and removing the organs regulating melatonin also removed all benefits from calorie restriction.
Melatonin was further shown in humans to substantially reduce risks of dying due to cancer according to a systematic review (see also this review, here and here) and from sepsis/severe inflammations, including in neonates (who do not produce melatonin yet), and even liver damage (potentially can help with the consequences of metabolic disorders such as NAFLD/NASH?). Melatonin is also hypothesized as being the reason why blind individuals have a much lower rate of cancers, as their melatonin levels are ever inhibited by light. Low melatonin levels are associated with endometrial cancer and breast cancer and is suggested to be used as a screening indicator of these cancers. A statistics study found that participants with higher melatonin levels had a lower likelihood of being diagnosed with COVID-19 and made an online calculator to predict those more at risk, and hence melatonin is part of the Marik's cocktail protocol for COVID-19 critical care. Melatonin supplementation may reduce delirium and symptoms of dementia such as sundowning and potentially increases brown adipose tissue which may help with diabetes. A review found that melatonin deficiency is associated with a "plethora of effects".
But melatonin activities go beyond the circadian rhythm regulation and antioxydative activity, it also regulates inflammation and melatonin supplementation has anti-inflammatory effects according to a 2021 systematic review of clinical trials, hence with applications for wound healing and tumor necrosis, and it also regulates a lot of other processes (such as hair growth and skin damage protection against sun's UVs), hence why melatonin is qualified as having pleiotropic actions (pleiotropic meaning "many") (see also this PhD thesis), including on the brain which makes it a candidate for the treatment of diverse neuropsychiatric disorders including epilepsy, schizophrenia, depression and anxiety disorders:
> A gathering body of evidence has shown that besides strong antioxidant activities, melatonin is a pleiotropic regulator molecule which orchestrates multiple functions through all the three melatonin receptors, i.e. MT1, MT2, and MT3. For example, MT2 receptor agonistic activity is attributed to neuroprotective, hypnotic and anxiolytic properties while MT1 and MT2 agonistic activity is associated with the clinical efficacy of agomelatine. The third melatonin receptor has been identified as quinone reductase (QR) 2, an enzyme involved in detoxification. MT3 agonist has been linked to strong hypotensive effects in preclinical study.
Ref: from this review.
There are also some preliminary results in animals that may indicate a neuroprotective effect against ischaemic strokes, although human trials are required. Idem for metabolic regulation in diabetes mellitus.
Note however that to obtain these extracellular protective effects of melatonin, the dosage required is much beyond what is provided in melatonin pills for circadian rhythm disorders (more details in the dosage section below). Interestingly, although melatonin is primarily used in skin for its extracellular, antioxydative properties, skin fibroblasts also make use of its secondary, circadian rhythm synchronization function simultaneously.
Melatonin's circadian rhythm phase shifting effect is at least are additive (see also here and here) with light therapy and maybe even more
Endogenous melatonin secretion has a seasonal cycle in humans, with longer secretions during winter and less during summer, unless artificial lights inhibit these seasonal variations.
Overview of the factors for optimal therapeutic effectiveness of melatonin pills
Therapeutic effectiveness of melatonin is influenced by several factors, including the following:
1- Immediate release formulation vs prolonged release: instant release form likely works better than prolonged release to treat circadian rhythm disorders. Instant release melatonin does not cause morning/wake-up drowsiness/brainfog whereas prolonged melatonin does. If unsure, just crush the pills into a powder, this will make it into an instant release form in any case because it's only the coating that can make melatonin into a prolonged release form. To crush tiny melatonin tablets, buy a mini-grater with tiny holes, such as those used to grate spices, such as this one (if the link is dead, see this picture for what it looks like).
2- Timing: intake should be before DLMO (ie, before the body starts producing melatonin), hence about 3-7h before your natural sleep onset (ie, when falling asleep), not the target one. However, the exact DLMO timing is highly variable between individuals, hence this requires some trial-and-error.
3- Dosage and overlap: bloodstream melatonin circulation from exogenous melatonin pills need to overlap with the endogenous melatonin secretion. Since higher dosages (1-3mg) remain longer in the bloodstream, they are easier for beginners. Furthermore, since melatonin's hypothermic effect and hence circadian rhythm shifting and sleep inducing effects, higher dosages should in theory produce more effect.
4- Bright light exposure (either by sunlight or bright room light) after taking melatonin will inhibit melatonin. Even a computer screen at medium or full brightness can inhibit melatonin (because the effect is stronger when you stare at the light source and humans are more sensitive to light at night than at day). Hence, it's crucial to avoid any bright light exposure and to remain in dim lighting conditions for melatonin to be effective. This was demonstrated in a study on using melatonin for typical sleepers to manage their jetlag, and which shown that indeed exogenous melatonin can shift the circadian rhythm faster but only in dim light conditions, demonstrating that light remains the strongest zeitgeber superceding any other, melatonin included. Indeed, it was demonstrated that a late evening administration of melatonin does not prevent the phase delay induced by concurrent bright light exposure. See the next section about light therapy for more details.
5- High variability in the quality control of over-the-counter melatonin pills, sometimes with a dosage 5x higher or smaller than what is labelled on the package, even for the same brand and product but between lots. This variability may be the root cause for why some people report too much effect, while others report no effect at all for the same dosage. It may simply be that under the hood they didn't have the same dosage at all. If you experienced too much effect with melatonin, at least this is a good hint this works, so you may try another brand with the same or different dosage to adjust and maybe find a brand with a good enough quality control, or ask a pharmacy to make a preparation in a lab for you. Personally, after trying lots of melatonin formulations including from pharmacies and labs, I found the Valdispert 1.9mg instant release melatonin works well and stably, but I can't guarantee the stability nor purity. Also, a tip as the authors note: "the least variable products were those that contained the simplest mix of ingredients, generally oral or sublingual tablets with melatonin added to a filler of silica or cellulose derivatives and were the most reproducible", which is the case of Valdispert 1.9mg instant release melatonin, where melatonin is the sole active component (no camomille or other "sleeping inducing natural herbs" stuff).
6- Optionally: Reduce/avoid food and sweet drinks after taking melatonin. Some people have a genetic mutation of the melatonin type 2 (MT2) receptor that does weird things when both glucose and melatonin are present in the blood stream.
Anecdotally, the author used melatonin for more than 10 years without any significant entrainment success, before finding the adequate parameters to increase the effectiveness of melatoninergic therapies.
Optimal timing of exogenous melatonin pills
To shift the circadian rhythm, exogenous melatonin needs to be taken before the body starts to produce endogenous melatonin, which is called the dim-light melatonin onset (DLMO). Technically, the DLMO is the tipping point of the melatonin PRC curve. If taken before the DLMO, exogenous melatonin advances the circadian rhythm phase by advancing the start of the endogenous melatonin secretion (DLMO). If taken more than 1h later than the DLMO, the phase is delayed according to some authors (see also here), whereas others state that melatonin's dead zone (where there is no effect) is during the biological night when endogenous melatonin levels are high in the blood, and the delays only start when residual melatonin is found after the endogenous melatonin offset:
> The dead zone of the PRC to light is during the day. The dead zone of the melatonin PRC, however, occurs during the “biological night,” that is, the time when endogenous levels of melatonin are usually high. Responses to melatonin are greatest when it is given exogenously at times when endogenous levels are not normally present, that is, during the day; when given in the morning, melatonin causes phase delays (shifts to a later time), and when given in the afternoon/evening it causes phase advances (shifts to an earlier time). Bright light causes phase shifts opposite to those caused by melatonin; that is, light exposure in the morning causes phase advances, and in the evening causes phase delays (responses are greatest during the night).
Ref: this letter.
It is now known that melatonin is most effective several hours (3-5h) before natural bedtime (not target bedtime), by assuming that on average DLMO happens 2-3h before the natural bedtime as in Lewy's PRC (see also here). This is in contrast with previous medical misconceptions, which often prescribed melatonin 1h before target bedtime which is ineffective:
> Although pharmacopoeias and the European food safety authority (EFSA) recommend administering melatonin 1–2 h before desired bedtime, several studies have shown that melatonin is not always effective if administered according to that recommendation. Crucial for optimal treatment of CRSD, melatonin and other treatments should be administered at a time related to individual circadian timing (typically assessed using the dim light melatonin onset (DLMO)). If not administered according to the individual patient's circadian timing, melatonin and other treatments may not only be ineffective, they may even result in contrary effects. Endogenous melatonin levels can be measured reliably in saliva collected at the patient's home. A clinically reliably DLMO can be calculated using a fixed threshold. Diary and polysomnographic sleep-onset time do not reliably predict DLMO or circadian timing in patients with CRSD. Knowing the patient's individual circadian timing by assessing DLMO can improve diagnosis and treatment of CRSD with melatonin as well as other therapies such as light or chronotherapy, and optimizing treatment timing will shorten the time required to achieve results.
Ref: this review. See also the figure 3 of this review for the appropriate timing for DSPD and non-24.
This usual recommendation is ineffective because of two points: the too late administration (1-2h before bedtime), and the uncoordination with the patient's circadian rhythm (by prescribing melatonin pills intake relative to the desired bedtime instead of the patient's current or natural bedtime).
This is because exogenous melatonin phase advances the circadian rhythm when taken before the body starts producing endogenous melatonin, which is called the DLMO point. In other words, exogenous melatonin is more effective when the body's endogenous melatonin level is low (ie, daytime levels): "phase shifts diminish around the time that endogenous melatonin appears in the circulation and remain minimal until melatonin levels start to decrease" (quote from this PRC study). Indeed, the DLMO point is the tipping point of the melatonin's PRC curve. By taking melatonin pills before the DLMO, the body is "tricked" into starting melatonin secretion earlier. However, if melatonin pills are taken after DLMO (1-2h before bedtime) as is usually advised, this will maximally delay the circadian rhythm, hence worsening non-24.
The importance of planning melatonin intake in the phase advance part of the melatonin PRC curve is known since at least 2003 with the work of Skene on optimizing melatonin and bright light therapies:
> Current research is directed towards optimizing these therapies with respect to time of administration, dose and formulation of melatonin, intensity, duration and spectral composition of light. [...] The ability of melatonin to entrain free-running rhythms depends, in part, on the time of melatonin administration relative to the subject's circadian phase. Subjects who were entrained by melatonin began their treatment in the phase advance portion (CT 6-18) of the published melatonin phase-response curves (PRCs), whereas those who failed to entrain began their melatonin treatment in the delay portion of the PRC.
Due to inter-individual variability in DLMO (DLMO-to-bedtime is highly variable between individuals, 60% have a DLMO bigger or smaller than 2-3h before bedtime (range: -0.3h to 5.8h)) and variable sensitivity to melatonin, there is no way to tell apriori what time and dosage of melatonin will be ideal for everyone. Hence, it's why prior assessment of the current circadian rhythm phase is crucial, as otherwise there will be less or no benefit or even unwanted phase delays. The timing chronobiotic therapies is not only crucial for the effectiveness of circadian interventions, but also for the treatment of other ailments, as demonstrated by a 2006 study by Lewy et al, leaders in chronobiotic research for depression, which found that the same circadian phase based timing of administration for melatonin also improved depression, and depression symptoms were improved in proportion with the degree of circadian realignment. These results are further supported by a 2019 systematic review finding light therapy to be as effective as antidepressants for the treatment of major and seasonal depressions. Hence, circadian alignment of therapies with the individual's phase, and circadian alignment of the individual's circadian phase with their social obligations are paramount for clinical improvements.
The most accurate and only way to find the optimal timing and dosage of melatonin is by sampling melatonin throuhout the day, usually by saliva, although there are urinary tests too. Although this works to define the DLMO point at one point in time, since by definition the DLMO is constantly moving for individuals with non-24, this would require repeated salivary melatonin sampling everyday, which is unrealistic in practice as it must be done in the clinical setting and is costly. Urine melatonin sampling may provide a solution at home, but is currently unavailable on the consumer's market. Since melatonin concentration can be detected in urine, urinary bands with a reactive agent for melatonin could likely be mass produced for a low cost, as is done for other reactive agents bands like Siemens Multistix, but unfortunately it seems no industrial actor decided to produce these yet.
Hence, in practice as of 2021, since methods of assessment of the circadian rhythm are lacking for at-home settings, the currently only available method to optimally time melatonin for individuals with non-24 is unfortunately by trial-and-error, using the time windows given above as a reference (ie, 3-5h before natural bedtime). Maybe future real-time circadian rhythm monitoring methods will allow to avoid trial-and-error by directly estimating the current state of the circadian rhythm and adapt precisely the timing of melatonin intake.
The hypnotic, sedative effect of melatonin kicks in 1h after intake of instant release sublingual melatonin. Hence, that's why melatonin is often taken close to bedtime as a sedative (and sleep consolidator for elders who lack endogenous melatonin), but with no circadian rhythm shifting effect.
Optimal dosage of exogenous melatonin pills
Summary: What dosage is optimal for melatonin? Opinions currently diverge. But evidence suggests that higher doses (>1mg) are more effective in shifting the circadian rhythm as they induce more hypothermia, but the optimal dose balancing optimal circadian rhythm shifting and reduced next-morning drowsiness can vary by at least 10x between individuals so that trial and error is necessary and lower doses (<1mg or even <0.1mg) can be sufficient for some individuals. But testing such low doses should be done from starting from a higher dose showing efficacy and then reducing progressively down until finding the lowest dose to maintain the circadian rhythm shifting effects.
As explained earlier, melatonin modulates the circadian clock by modulating the temperature, more precisely by decreasing core body temperature and increasing limbs temperature. However, although higher dosage proportionally increases the hypothermic effect of melatonin and hence its circadian rhythm shifting effect, the melatoninergic receptors are exquisitively sensitive, as very low doses ("nanomolar or lower concentrations") of melatonin are sufficient to activate the receptors and hence their circadian rhythm shifting and sleep induction effects. This is probably what contributed to the confusion around the effect of low to very low melatonin doses, with some authors suggesting that very low melatonin doses of 0.3mg per pill could be sufficient, which is likely correct, but this does not preclude that higher dosages cannot be more beneficial. A study on the PRC curve of melatonin in humans observed bigger phase advances with bigger melatonin doses (5mg versus 0.5mg), and that lower doses of melatonin (0.5mg) produce a delayed PRC of slightly decreased magnitude compared to higher dosages (3mg) or moderately according to other studies. So indeed, low doses can produce a circadian rhythm shifting effect, but higher doses can produce bigger shifts according to the current knowledge.
Another factor is that the exact optimal timing for melatonin pills intake may depend on the dosage. A PRC study on melatonin in humans found (see also this figure from this report) that lower dosages of melatonin were optimally taken later (ie, closer to DLMO) for maximal phase advance compared to higher dosages. This can be explained by Lewy's theory of overlapping, which proposed that the largest phase shifts in humans occur when exogenous melatonin in the bloodstream overlaps with the start of endogenous melatonin secretion (DLMO), so as to simulate an early dusk, and hence lower dosages such as 0.5mg need to be taken closer to DLMO to remain in blood circulation at DLMO compared to higher dosages such as 3mg. For an estimation, instant-release melatonin is eliminated within 3-4h of intake, although the blood levels may remain high up to the next morning when using supraphysiological doses. This hence suggests that higher dosages provide more leeway to get the therapeutic effects from melatonin, and hence higher doses of melatonin may be easier to time optimally for beginners. However, another study found an "inverse relationship between the timing of melatonin administration (irrespective of dose) and the magnitude of DLMO phase advance, such that earlier timing of the former (in relation to DLMO) resulted in greater phase advances", which suggests that the influence of timing is mostly independent from dosage, so that dosage does not need to be accounted for systematically when timing melatonin intake.
Furthermore, there is a high variability of melatonin sensitivity between individuals, partly due to different melatonin receptors density, and hence some individuals are insensitive with the common dosages. Other studies have estimated a 10-fold inter-individual variability in melatonin bioavailability or even up to 35-fold, in other words, some individuals require 10x to 35x the dosage that others use to get the same effect. For example, in a study of children with autism and insomnia, and hence likely misdiagnosed circadian rhythm disorders since they are very comorbid with neuroatypism, dosage varied from 2mg to 10mg after careful progressive stepwise increases depending on the child's needs as assessed under medical supervision. Anecdotally, there are several reports from adults on reddit who mention they tried 300mcg but found they required much higher dosage about 10mg daily.
Although higher dosages of exogenous melatonin may ease the proper timing and overlapping with the endogenous melatonin profile, the longer bioavailability increases the risk of exogenous melatonin profile overlasting endogenous melatonin and hence spilling onto the phase delay portion of the melatonin PRC curve, in other words to keep residual exogenous melatonin the next morning, not only causing the dreaded morning drowsiness typical of a too high melatonin dosage but also phase delaying the circadian rhythm. As such, "care must be taken to avoid unnecessarily high doses that would cause trailing levels that spill over onto the wrong zone of the melatonin PRC". The risk of "phase spilling" is not present in light therapy and this may be one of the factors explaining the more robust phase shifting effects of light therapy.
Finally, melatonin levels varies with age, as melatonin levels are the highest for children (except neonates under the first 3 months of life), and then they decrease over time with age, with elders often having a deficiency of melatonin leading to age-caused insomnia. The progressive increase in melatonin in neonates can be explained by the pineal gland maturation as evidenced by the fact that its size stabilizes at around 1 year of age which coincides with a stabilization in melatonin levels, whereas the pituitary gland continues to grow afterward, explaining the progressive decrease with age. Hence, melatoning is more effective with age, with older adults needing lower dosages of melatonin to get the same effect as younger adults. See this figure from this review and this figure about "normal melatonin peaks" by age.
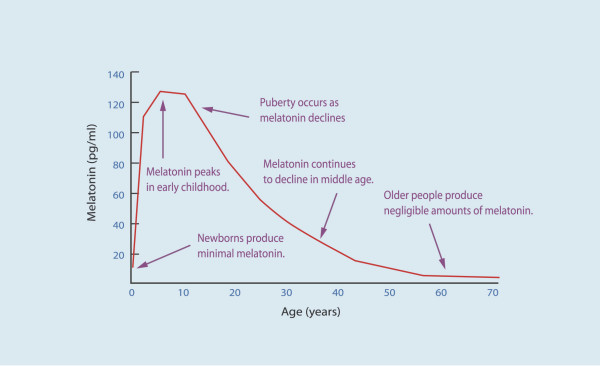
Variations in endogenous melatonin secretion by age. Figure from this review and licensed under CC-BY 2.0 Generic.
Accordingly, a year-long RCT study found that children with insomnia and autism needed between 2mg to 10mg melatonin for efficacy. Indeed, "children require a relative-to-bodyweight higher dose to induce sleep than adults". This shows that the dosage of melatonin need to be at least equal or higher than what the body naturally secretes to be effective.
To find the optimal (ie, minimal but effective) dosage of melatonin for children, a 2020 review on children with DSPD provide the following algorithm:
> We would advise starting melatonin treatment 3–5 h before bedtime. Try to find the lowest effective melatonin dose, starting with 1 mg in children between 6 and 18 years. If after one week no change occurs, increase the dose by 1 mg weekly until an effect occurs. When a 1 mg melatonin dose is already effective, try to lower the dose until a minimal effective dose is reached. If there is no effect using a 3–6 mg dose, stop melatonin treatment and try to measure DLMO, or reconsider the diagnosis [48] (clinical experience, MS).
Given all these information, why is there a common belief that very low doses of melatonin are better than higher doses? Very low doses of melatonin such as 0.05mg were indeed experimented, such as In this study, however only one blind non-24 entrained with 0.05mg, all the others had to use 0.5mg. Keep in mind they are blind, hence have lower melatonin levels than sighted individuals. Although these subjects could be entrained, the article makes no claim that this dosage is better than higher doses, simply that they may be sufficient, but by following a precise protocol of starting with a higher dose to first get an effect, and then progressively reduce until the subject reaches the lowest dosage they can use while still having the effect of melatonin.
To trace back where the belief that low dose melatonin is better than high dose comes from, we may find the following study, a follow-up study by the same authors as the above cited study stems from this single-case study, titled: "Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period". This result was hence only on one subject, and who was blind, hence with lower endogenous melatonin levels again... This is hence a preliminary result that cannot be generalized yet, and given there is at least a 10-fold inter-individual variability in melatonin bioavailability in typical sleepers, with this estimated variability not even accounting for extreme cases where the melatonin profile is modified as with circadian rhythm disorders such as non-24, it's unlikely to hold in the general case. Hence, it's safe to assume that low doses do indeed work for non-24, but not necessarily very low doses and some individuals may very well need dosages of up to 10mg/day depending on their sensitivity (melatonin bioavailability and receptors density). See also this interesting informal review about low dose vs high dose melatonin in 2001 and with a sales review showing that consumers preferred higher dosages (up to 10mg!).
Another study that certainly participated to the confusion as it is often cited to back up the claim of low doses being more effective is a 2001 study by the MIT of elders who commonly suffer from age-related insomnia due to reduced endogenous melatonin production with inxreasing age. The study found that low dose melatonin 0.3mg was as effective as 3mg but with reduced next morning drowsiness and hypothermia. The very low dose of 0.1mg was not as effective. This study is often used as evidence that low dose melatonin is always preferable for circadian rhythm disorders, which is misleading: first because the study was on elders who have very low engodenous melatonin production and hecne require lower doses of exogenous melatonin, so this result does not apply to younger adults and certainly not on kids, 2) this was a treatment on age-related insomnia, which is fundamentally different from circadian rhythm circadian rhythm disorders, as the aim of the former is to improve sleep consolidation as is the major metric used to claim efficacy in this study, whereas for circadian rhythm disorders the goal is to shift the circadian rhythm disorder, which was not studied here. Nevertheless, the study notes that only the 3mg dose induced a hypothermic effect, which suggests that 3mg was more effective at shifting the circadian rhythm, since core body temperature modulation is how the SCN in the brain synchronizes cells clocks throughout the body. As a side-note, the authors indeed patented low dose melatonin in USA but as of 2018 this patent expired so that other producers can manufacture low dose melatonin.
There is however some cases where low to very low doses of melatonin are always better indicated, such as restless legs syndrome (RLS) or periodic limbs movement disorder (PLMD). Indeed, the acute triggering of RLS symptoms shows a circadian pattern. This was also demonstrated for Periodic Limb Movements in Sleep (PLMS/PLMD). This is because melatonin can trigger the symptoms of RLS through an interaction with the dopaminergic system. Hence, low doses of melatonin should be preferred for people with RLS or PLMD, or alternatives not involving melatonin such as light therapy should be explored as a therapeutic option. There are also reports that bright light therapy may indirectly through photic history increase melatonin levels during subsequent nights, with a similar risk of increased triggering frequency of PLMD/RLS symptoms. Of note, sleep deprivation through adenosine buildup can downregulate dopamine receptors:
> Increased adenosine load associated with sleep deprivation triggers downregulation of dopamine (DA) D2 and D3 receptors (D2/3Rs), resulting in decreased receptor membrane expression within the striatum (internalized receptors; grey). Consequently, there is a greater ratio of D1R to D2/3R availability, and the relative increase in striatal D1R activation by DA.
Inversely to receptor-dependent effects which can be activated with very very low doses, receptor-independent cellular protective actions of melatonin, such as antioxydative effects, only appear with much higher doses of melatonin : "These receptor-independent protective actions of melatonin and its metabolites would require high intracellular levels of the molecules, which can only be met by melatonin in situ production in the relevant tissue, since cellular melatonin uptake is very limited because only 0.1% of extracellular melatonin can enter the cell (Fischer et al., 2006a)." This is not surprising, since the digestive system, which is by far the largest producer of melatonin, produces 2 orders of magnitude more than the brain, with the melatonin secreted by the digestive system likely for its antioxydative properties to repair oxydative damage following food consumption, whereas for the brain much smaller doses are sufficient to activate the melatoninergic receptors and induce the sleep-related actions of melatonin. In practice, as shown by the usage of melatonin on septic patients, the dosage necessary to produce the cellular protective actions would be about 8mg/kg/day in humans. Until more optimal delivery methods of huge doses of melatonin are found, dark therapy is the only way to preserve the bulk of endogenous melatonin secretions and its antioxydative action.
To test lower dosages of melatonin, below 1mg, which are usually very hard to find in both pharmaceutical products or over-the-counter dietary supplement products, it's possible to either ask for a magistrale preparation at the pharmacy (extemporaneous preparation) who can dose very precisely for you but will be more expensive, or get melatonin in liquid form, although note that in the latter case the degradation of melatonin is much faster, so once the liquid bottle is opened, expect the contained melatonin to be inactivated after a few days. It's also possible to try to cut solid tablets, although this leads to a great variability in the dosage between 50% and 150% even with specialized cutters, and it will break the coating and hence make the melatonin instant release (which can be an issue if you seek prolonged release melatonin, this is in fact contraindicated for any "slow or modified release" drug). For more details including a thorough evaluation of the efficacy of the various types of extemporaneous preparations (ie, methods of pharmacologists to prepare individually tailored drugs, and also various at-home transformations of drugs such as cutting tablets or grinding and dispersing in water the powder to drink), read here, here and here. Magistral preparations can only be done under some specific circumstances as regulated in the European Union (see also here). Extemporaneous preparations have fallen out of favor in some countries such as United Kingdom in favor of specials instead, which are manufactured by industrials specifically for a set of patients. If OTC products are chosen, always ensure to choose products that only contain pure synthetic melatonin, not any other compounds such as valerian as combined products usually have much lesser quality controls and hence more variable dosage, up to 10x less or more than what is claimed on the label.
Side-note: this blog post is often cited online to justify the use of very low melatonin dosage. However, the inference drawn by this source is incorrect, as it draws on studies on age-related insomnia, which is fundamentally different as the goal was not to treat a circadian misalignment but sleep fragmentation, and furthermore elders are a different population with very low endogenous melatonin secretion. A better resource is this one instead, from a medical doctor who did a more comprehensive and accurate literature review, with a clear distinction between the indication to treat insomnia and sleep fragmentation (lower dosage) versus circadian rhythm disorders (higher dosage may be preferable), or this set of recommendations by the Circadian Sleep Disorders Network.
Split-dosed melatonin
There is also an experimental "melatonin split-dosing" scheme, where a low dose of melatonin is taken before the DLMO (pill taken 3-7h before natural bedtime) to maximally shift the circadian rhythm, and a higher dose 1-2h before natural bedtime to maximally induce sleepiness (similarly to a sleeping pill). This approach is not yet validated and was only used for one published sighted non-24 case. The idea with split-dosed melatonin is to target both types of melatonin receptors with an optimally timed and dosed melatonin for each separately, instead of trying to target both with a single pill. In addition, this also doubles the likelihood of getting at least one timing right, and hence reduce the risk of disentrainment. However, given that exogenous melatonin needs to overlap with endogenous melatonin for maximal circadian rhythm shifting, it may be more effective to also use a high melatonin dose before DLMO (and hence high dose for both intakes). Thus, the author would recommend to test 1-3mg before DLMO (3-7h before bedtime), and 2-3mg 1-4h before bedtime. An individual with DSPD tried this scheme and reported great results, greater than with any other method they tried, although the dosage require some experimentation.
See also this discussion for some practical feedbacks: https://archive.is/FrkFN
Long-release versus instant-release melatonin
Unfortunately, there is a lack of clinical trials comparing instant-release melatonin versus long-release melatonin. Historically, long-release melatonin was devised for age-related insomnia, for which there is a biologically plausible reason, as the issue of age-related insomnia is a characteristic sleep fragmentation potentially caused by an irregular or too low endogenous secretion of melatonin. Hence, supplementing with melatonin during the whole sleep period makes sense to consolidate sleep.
In the case of circadian rhythm disorders, "most clinical studies in children with DSPD have been performed with immediate-release melatonin preparations".
It's worth noting that although some authors suggested cutting or crushing long-release melatonin tablets to transform them into an instant-release form, as it's only the special coating that makes melatonin in a long release form, recommending this is considered illegal:
> A paper by Chua et al. published in 2016 in the journal Pharmaceutics recommended the use of divided or crushed Circadin tablets (as a licensed product) where an immediate release melatonin was required, in preference to a manufactured immediate release tablet (unlicensed in the UK) [76]. However what the authors had not stated was that this represents off-license use of a licensed medicine, promotion of which is illegal [77]. Clinical studies to compare the efficacy of immediate- and controlled-release melatonin preparations have not yet been published [78].
Recently, in 2021, clinical guidelines are starting to differenciate the use of prolonged-release melatonin for insomnia symptoms or comorbid insomnia to mood disorders (such as depression), schizophrenia and autism, whereas instant-release melatonin should be preferred for circadian rhythm disorders.
Safety and contra-indications of exogenous melatonin
Is melatonin safe for kids? Melatonin should be safe for kids, as it was used in multiple trials for autistic children, including a long-term one (> 1 year) with doses up to 10mg/day, and is even commercially available as a medically regulated drug under the name of Circadin or PedPRM. There was a concern about the potential adverse effect of melatonin on growth hormone regulation and on reproductive function and development, but studies found no difference in adolescents using melatonin and a placebo, and melatonin was shown to be a reproductive organs protector and a potential candidate to treat diseases of male reproduction and also female reproduction (see also here, here and here with not only melatonin but circadian cycle impact on fetal and placenta physiology). The AASM guidelines issued a WEAK FOR in favor of melatonin treatment for children with DSPD. Melatonin is even considered safe for use in hospitalized neonates, to treat sleep issues and severe inflammations and tumor necrosis including in neonates. Reviews concluded chronic melatonin intake is effective to improve glucose metabolism and potentially manage diabetes type 2, as well as lower blood pressure in patients with essential hypertension.
Melatonin has very few side effects and no serious (dangerous) ones. It is non genotoxic, non mutagenic, non carcinogenic and there is no withdrawal nor dependency and hence not addictive. Both the EFSA (european commission for food safety) and the ANSES (french commission for drugs safety) considered melatonin effective for circadian rhythm adjustements if one day dose contains at least 0.5mg and generally safe except for individuals with neurological comorbidities and pregnant women. Apparently, the FDA also considered melatonin safe and decided not to regulate it as a drug. It's impossible in practice to overdose on melatonin, as the toxic threshold of >400mg/kg is only reached when taking tens of thousands more than what is provided in 3-6mg formulations. Even this figure is subject to debate, with some studies stating that no maximal safe dose (lethal dose) could be established yet, as "even enormous doses such as 800 mg/kg are not lethal" in animals, and furthermore "in a study of 11 patients, doses up to a massive 6600 mg/day for 35 days were given with no severe toxicities occurring". What about the long-term use of melatonin? According to the AASM 2015 guidelines, current evidence shows that melatonin is safe for long-term use, as was assessed on children with DSPD and ADHD for a mean follow-up time of 4 years with doses up to 10 mg with no serious adverse event, and an open-label follow-up study of adult patients with DSPD and neurodevelopmental disabilities who received prolonged-release melatonin up to 15 mg for up to 3.8 years found no adverse effects. A 2020 review of clinicial trials also found melatonin safe and effective for use in children and adolescents with DSPD from age 2 years and more, including long-term studies involving the use of melatonin in children and adolescents for 1 to 10 years, with "no substantial deviation of the development of children with respect to sleep quality, puberty development and mental health scores [...] provided that it is administered at the correct time (3–5 h before endogenous melatonin starts to rise in dim light (DLMO)), and in the correct (minimal effective) dose". Since the circadian rhythm may change with age, especially during adolescence, potentially making DSPD only a temporary issue, the authors further recommend in practice to "to stop melatonin treatment at least once a year (preferably during the summer holidays)".
There is a common minor side-effect at the root of most complaints, and is that melatonin can produce more vivid dreams. More vivid dreams are not necessarily nightmares, but they often are. Hence, melatonin increases the likelihood of experiencing nightmares. Are nightmares bad for the circadian rhythm or sleep? According to a study (the only one on this question, there is surprisingly very few scientists who investigated this question), nightmares affect subjective feelings of sleep quality but not sleep quality per se, as there is no objective change in the sleep structure whether or not the dream is good or bad. This finding can also be put into perspective with the slightly more extensive research about lucid dreams, which shows that being conscious during dreams does not impact the sleep structure or quality either. So this indicates that the dreams content is irrelevant to the sleep structure or quality. This matches what I observed experimentally, that nightmares do not impact the circadian rhythm nor mood nor energy levels during wakefulness periods, it's rather the sleep duration and timing (relative to the circadian rhythm) that matters.
Another potential minor side-effect is that melatonin may have a temporarily negative effect on mood, since melatonin is an antagonist to serotonin.
Another common complaint is the next morning drowsiness/brainfog, which is likely due to using a too high dosage (>2mg) of prolonged release melatonin, with a lower dosage or using instant release melatonin often fixing the issue, but this is only a temporary side effect that will disappear after reducing the dosage since there is no tolerance:
> In full agreement with numerous findings on immediate-release melatonin, all studies on the prolonged-release formulation unanimously show that the recommended dose does not cause next-day hangover, but rather favors morning alertness – although some exceptions have been described in other investigations using different doses. It does not lead to dependence, early or late withdrawal effects after discontinuation.51,77–79 The development of tolerance is usually absent with melatonin, although a few exceptions have been reported, especially in some children with neurological disorders.91–94 Should the development of tolerance turn out to be a consequence of altered metabolism, which remains to be demonstrated, other melatoninergic agonists might be tested. A recent randomized, double-blind, placebo-controlled crossover study on prolonged-release melatonin confirmed the absence of next-day impairments of psychomotor functions, driving skills and memory recall, in contrast to 10 mg zolpidem.109 Controlled-release melatonin (2 mg) was successfully used even for facilitating benzodiazepine discontinuation.110 Like melatonin, ramelteon did not cause next-day hangover (as revealed by subjective feeling, psychomotor and cognitive tests, and ability to concentrate),105 rebound insomnia or other withdrawal effects, or development of tolerance or addiction.20–22,105 Under these conditions, both prolonged-release melatonin and ramelteon appear safe in short-term treatment, as may be assumed for other exclusively melatoninergic drugs in general.
Melatonin can interact with the dopaminergic system as shown by its detrimental interaction with restless legs syndrome (RLS) (see also here and here), while sometimes producing a positive side-effect on mood and motivation (a user reported both effects simultaneously), but this effect of melatonin on the dopaminergic system is not well studied at the moment. Hence unfortunately for people with both RLS and a circadian rhythm disorder, melatonin is contra-indicated unless in very small doses if the individual tolerates it, but this should be done under a medical doctor's supervision.
Interestingly, in a mice study, dopamine was shown to cause phase delays (lengthening the freerunning circadian rhythm period).
Although all the currently published research tend to demonstrate a lack of addiction and tolerance to melatonin, some reddit members anecdotally (but after extensive self-experimentation) reported a fast build up of addiction and tolerance to melatonin, with a withdrawal period of 2 months after 6 months of use.
There are anecdotal reports on peer communities (see here and here) of a potential paradoxical effect in some users, for whom melatonin does indeed help in inducing sleep earlier, but cause a sleep fragmentation with a premature wake up after just a few hours of sleep. This detrimental side effect is for the moment undocumented in the academic and medical literature. It may involve the interaction between the melatoninergic system and the dopaminergic system, as observed with RLS and PLMD patients, by causing a sort of forbidden sleep zone / second wind effect during sleep, especially in individuals with (potentially undiagnosed) RLS/PLMD or another disorder caused or triggered by an impaired dopaminergic system. Here is how an individual with non24 described this paradoxical effect, which interestingly mentions that this effect appeared after stopping bright light therapy while continuing melatonin supplementation (source: private communication):
> When I go to bed, I feel the “melatonin” coming out and I start to doze off, then I enter this state where I am 50% conscious 50% unconscious. I have “thoughts” that are very logical, and related to my every day activity. Not entirely dreams but close to dreams and close to reality at the same time. And this keeps going on for about 1 sleep cycle (~1h30?). I do NOT feel rested from this “weird state”, and then when I finally do sleep, I sleep for 4 sleep cycles directly (5.5-6h), and wake up not being able to go back to sleep. Usually, I never sleep 4 cycles without interruption, and I can go back to sleep. Usually I get 5-6 sleep cycles per night which is great. But with this thing happening, I am always getting 4 cycles and now I’m so tired. It has been happening all week this week and it started the day I stopped doing light therapy to free-run…
Some clinicians with 25 years of experience with DSPD in children report additional rare side effects, such as melatonin-induced diarrhoea, headache and enuresis. In case they appear, the authors recommend to discontinue melatonin and instead try other therapies such as bright light therapy, as in their experience there is no other treatment that can relieve these symptoms except melatonin discontinuation.
Difference between melatonin and hypnotics sleeping pills (benzodiazepines and Z drugs)
Melatonin is not a sleeping pill, it's different, because it does not affect GABA receptors, and hence does not affect REM sleep nor the distribution of sleep stages. Hence, melatonin is much safer than sleeping pills such as benzodiazepines. Furthermore, "melatoninergic agonists do not cause next-day hangover and withdrawal effects, or dependence [and] do not induce behavioral changes, as sometimes observed with z-drugs [ndlr: benzodiazepines sleeping pills]." Sleep researchers disadvised against the use of sleeping pills to treat sleeping disorders since at least the 1975s, and particularly for circadian rhythm disorders such as non24. Benzodiazepines analogs such as zolpidem or zopiclone can produce paradoxical insomnia (ie, long but unreparative sleep, in other words a superficial sleep) and in clinical trials worsened sleep parameters compared to age-matched insomniac but untreated patients, and interestingly most of them were also diagnosed with untreated sleep apnea. An observational study also found they increase the risk of developing dementia. Melatonin can be used to facilitate benzodiazepine discontinuation.
As several sources state, melatoninergic agonists are not addictive (see here, here and here). Although the melatonin receptors type 2 can be desensitized at the molecular level, just like all G-protein-coupled receptors, this desensitization is quickly reversible and hence there is no observed addiction nor tolerance in practice. Another piece of evidence is that the melatonin receptors density varies over the day but mostly in parallel with the circadian rhythm, so that there is a parallel increase in both melatonin receptors density and melatonin concentration (which should be the opposite if there was a desensitization), allowing receptor sensitivity to be sustained since it was demonstrated that "long-term melatonin exposure produces microtubule rearrangements that enhances protein kinase C activation (which modulates melatonin receptor function through its action on G-proteins)". Hence, it's not surprising that longitudinal studies shown that melatonin could be used with no loss of efficacy over a whole year in children at dosages from 2mg to 10mg/day.
Actually, melatoninergic agents, including melatonin, are nowadays under active investigation to reduce or eliminate the addiction to other substances: alcohol, cocaine and even benzodiazepines and non-benzodiazepine Z drugs. Indeed, in some countries such as France, sleeping pills are used over the indicated maximum 4 weeks, which prompted authorities to implement health strategies to reduce their use. Between 2008 and 2013, the launch of prolonged release melatonin in the market led to a huge decrease of the BZD/Z drugs consumption, with 4x less BZD/Z drug consumption for each single unit of melatonin consumed, also showing that the dosage of melatonin required by the patients is less than what they needed with BZD/Z drug, likely because the latter build up a tolerance and hence require higher and higher doses to maintain their effect, whereas melatonin does not and the same dose will elicit the same response for most users. Several scientists decry the "abusive" prescription of sleeping pills and support this call for switch from benzodiazepines and BZD/Z drugs to melatonin for all insomniac patients (see here and here), with a successful switch being associated with a reduction of co-morbid major depression disorder, whereas the type of antidepressant was not.
Indeed, hypnotics aka sleeping pills should not be prescribed for more than a few weeks at most because of the rapid development of tolerance, which progressively makes sleep issues reappear despite continued use of sleeping pills. But then, since the patient's neural networks have "habituated" to the sleeping pill, are because of changes to the GABA receptors (see also here) which are known to underlie addiction and tolerance buildups, they will experience a withdrawal syndrome when discontinuing use of hypnotics (since they would have no benefit anymore due to tolerance), insomnia will get drastically much worse. And it may take years without the drug to be able to just get to lessened insomnia symptoms as they were before starting the use of hypnotics. Hence, hypnotics tolerance not only guarantees the disappearance of the benefit after just a few weeks of use, tolerance also then worsen the patient's insomnia, and is thus the reason why hypnotics are now considered to have little if any benefit for treating insomnia. And the withdrawal side effects can remain for a very long time after discontinuation, for example up to 8.4 weeks after discontinuation of Alprazolam (Xanax). There is even a peer support group on Reddit focused on recovery from benzodiazepines withdrawal with quite a lot of horror stories about how fast the tolerance and addiction build up: r/benzorecovery. Consult this subreddit if you have doubts about whether a specific molecule is addictive or not (but you will likely find that all benzodiazepines and z-pills are).
In line with these observations, despite benzodiazepines and Z drugs being still widely prescribed for insomnia, a meta-analysis found that hypnotics capacity to induce sleep is so small compared to placebo that it may not even be clinically significant according to regulatory bodies, which, given the tolerance issue, prompted these bodies to disadvise prescription unless for a short term period, and instead explore alternative medications such as melatonin, as the UK NICE states (updated in 2019):
> As long ago as 1988, in the January issue of Current Problems in Pharmacovigilance, the committee on safety of medicines advised that benzodiazepine hypnotics should be used only if insomnia is severe, disabling or causing the person extreme distress. The lowest dose that controls symptoms should be used, for a maximum of 4 weeks and intermittently if possible.
> NICE's technology appraisal guidance on zolpidem and zopiclone recommends that when, after due consideration of the use of non-pharmacological measures, hypnotic drug therapy is considered appropriate for the management of severe insomnia interfering with normal daily life, hypnotics should be prescribed for short periods of time only, in strict accordance with their licensed indications. A meta-analysis discussed in NICE's eyes on evidence commentary on small benefits of Z drugs over placebo for insomnia found that 'Z drugs' reduce the time taken to fall asleep by 22 minutes compared with placebo but this may not be clinically significant. NICE's technology appraisal guidance states that there is no compelling evidence of a clinically useful difference between the 'Z drugs' and shorter-acting benzodiazepine hypnotics from the point of view of their effectiveness, adverse effects, or potential for dependence or abuse. There is no evidence to suggest that if people do not respond to one of these hypnotic drugs, they are likely to respond to another.
Interestingly, the meta-analysis notes that it was "found that both Z drugs and placebo statistically significantly reduced sleep latency", demonstrating that an intervention reducing sleep latency is not sufficient to prove efficacy if not properly controlled against a placebo.
Note how the NICE states in the above excerpt that the common practice of switching a hypnotic class for another is not supported by evidence: if a patient does not see an improvement to their sleep issues using one class of hypnotics, it's unlikely to improve with another class. This does not apply to melatonin since it is not a hypnotic.
A 2017 American Family Physician guidelines statement goes further, by recommending melatonin as a first-line treatment for insomnia:
> Controlled-release melatonin and doxepin are recommended as first-line agents in older adults; the so-called z-drugs (zolpidem, eszopiclone, and zaleplon) should be reserved for use if the first-line agents are ineffective. For the general population with difficulty falling asleep, controlled-release melatonin and the z-drugs can be considered. For those who have difficulty staying asleep, low-dose doxepin and the z-drugs should be considered. Benzodiazepines are not recommended because of their high abuse potential and the availability of better alternatives. Although the orexin receptor antagonist suvorexant appears to be relatively effective, it is no more effective than the z-drugs and much more expensive. Sedating antihistamines, antiepileptics, and atypical antipsychotics are not recommended unless they are used primarily to treat another condition. Persons with sleep apnea or chronic lung disease with nocturnal hypoxia should be evaluated by a sleep specialist before sedating medications are prescribed.
A study on children with sleep-onset insomnia found that the combination of melatonin and bright light therapy was effective to improve their sleep parameters, with melatonin having the most important effect.
A common claim in defence of benzodiazepines use, even in the long-term, is that anything that can help sleeping is valuable to treat insomnia, especially in suicidal individuals, since insomnia can cause suicidal thoughts. There are however two issues to this claim: firstly, as explained above, the long-term use of benzodiazepines causes addiction and tolerance, which means that continuous use will cause the beneficial sleep-inducing effects to progressively wear off despite continued use of medication, and any withdrawal attempt will face a withdrawal syndrome that in this instance consists in experiencing an even poorer sleep than the individual had before starting benzodiazepines medication, and this can last for weeks to years. Secondly, there is no basis to assert that benzodiazepines use for insomniacs can reduce suicide risk. On the contrary, there is ample evidence from a majority of studies on humans and animals that benzodiazepines increase the risk of suicide, especially "for nonantidepressant users, for the young, and for males" (see also here, here, here and here). Even when the suicide attempt failed, there are lasting neurological changes in the density of benzodiazepine receptors (see also here) and anterograde amnesia. What insomniac individuals with suicidal ideation need is an antidepressive treatment, in addition to an appropriate treatment for their insomnia such as melatonin.
To help withdrawal from hypnotics, in addition to melatonin, antihistaminics such as doxylamine can be used, although the AASM 2008 guidelines on the clinical management of insomnia could not recommend antihistaminics due to the lack of trials on their safety and efficacy to treat insomnia.
See also these anecdotal experiences from people with sighted non-24 about various sleeping pills.
TODO: "Benzodiazepines (eg, valium) increase Stage 2 sleep, while decreasing the other Stages, including Slow Wave Sleep and REM sleep [JOURNAL OF SLEEP RESEARCH; Perlis,L; 6(3):179-188 (1997)]. Unlike sleep induced by benzodiazepine drugs, melatonin-induced sleep does not suppress Rapid Eye Movement (REM) sleep and slow-wave sleep — and does not result in "hangover" feelings the next day [CLINICAL PHARMACOLOGY AND THERAPEUTICS; Zhdanova,IV; 57(5):552-558 (1995)]. Nonsteroidal anti-inflammatory drugs such as aspirin (which disturbs sleep), decrease plasma melatonin levels [PHYSIOLOGY & BEHAVIOR; Murphy,PJ; 55(6):1063-1066 (1994)]." https://benbest.com/nutrceut/melatonin.html
Interactions between melatonin and other compounds
Over supplementation in vitamin D may inhibit melatonin which is hypothesized to result from a cross side effect of vitamin D production, which is triggered by skin exposure to UV, so the eyes are often also concurrently exposed to bright light, so a inhibiting pathway between vitamin D and the circadian rhythm may have hence developed as a byproduct of light exposure, although other individuals with non-24 and DSPD reported that treating their vitamin D deficiency significantly improved their sleep and circadian rhythm stability, so it seems there needs to be not too little but not too much vitamin D to avoid impairing sleep.
Food timing, diet composition and metabolic disorders
The digestive system is the main peripheral (body) clock. It is also exquisitively linked bidirectionally with the circadian rhythm and the central (brain) clock. Indeed, the suprachiasmatic nucleus, better known as the "master clock" processing photic signal to entrain the body's circadian rhythm by signalling the pineal gland when to release or inhibit melatonin, also includes leptin and ghrelin receptors, the hormones regulating appetite and hunger respectively, which makes sense since they also follow a 24h circadian rhythm. The gastrointestinal tract is the biggest producer of melatonin 2 orders of magnitude (by far) and in response to food, the pineal gland being unnecessary for the body to produce most melatonin. Melatonin is then able to influence the brain by readily crossing the blood-brain barrier. Perhaps there is no better illustration of the intimate relationship between the digestive system and sleep than the recent finding that accumulation of oxydants (ROS) in the guts is the cause of death by sleep deprivation. The parasympathetic system, also called "digest and rest system", is a well-known mode of the autonomic nervous system with strong interactions with the central nervous system (brain). Each organ has its own clock, and for the digestive system, its clock is reset by food intake. (See also this talk).
Here is an excerpt from a scientific statement of the American Heart Association:
> The magnitude of circadian regulation of metabolism is underscored by recent appreciation that metabolism is an integral component in the mammalian circadian network.55,63 More specifically, the molecular timekeeping mechanism within individual cells, known as the circadian clock, directly modulates multiple metabolic processes in a time-of-day-dependent manner, whereas fluctuations in metabolism act in a feedback manner, thereby modulating the clock mechanism (through changes in energy charge, ROS, redox status, acetylation, O-GlcNAcylation, etc).55,63 An added layer of complexity stems from appreciation that the responsiveness of the heart to a variety of factors known to modulate metabolism is similarly subject to circadian regulation (eg, insulin responsiveness).64,65 In many ways, metabolism can be considered a “moving target.”
For a quick introduction/summary of some of the infos that are in the following sub-sections, here is an excellent video by Dr. Rhonda Patrick:
VIDEO: Late-night eating and melatonin may impair insulin response by Dr. Rhonda Patrick and Pr. Satchin Panda (extended talk here and a TEDx talk here).
The interactions between the digestive system and the circadian rhythm are highly interesting but complex and still under active research, hence we will split this section into multiple sub-sections for clarity.
Meal timing and melatonin
The circadian rhythm and glucose metabolism: mutual inhibition between melatonin and insulin
Food timing is crucial, both for health and to shift the circadian rhythm. A whole body of work emerged in the last decade demonstrating the crucial importance of food timing in metabolic regulation, and it's worsened by a genetic mutation.
In 1997, a study shown that for an identical meal given to participants at 3 different time of the day (8am, 2pm and 8pm), the glucose response was much higher for the evening meal than when the exact same meal was provided earlier. This demonstrated that, in humans, glucose processing is modulated by the circadian rhythm.
Since then, other studies have since demonstrated under constant routine that glucose metabolism and leptin are indeed both regulated by the circadian rhythm. In addition, several studies found at least one of the causes: high levels of endogenous melatonin in the blood reduced glucose and insulin tolerances (ie, increase insulin resistance) at night for all participants (without a circadian rhythm disorder), and this effect could be reproduced during daytime with exogenous melatonin pills. This interaction happens regardless of the time of the day, as supplying melatonin pills during the day increased insulin resistance just like at night. In line with these studies on the melatonin-insulin interaction, a study found that eating meals during night shifts led to an insulinoresistant-like state, with "postprandial insulin, glucose, and triacyglycerol (TAG) levels were significantly elevated during night shifts compared to the day time shift", and with triacyglycerol levels remaining elevated even after returing to a day shift schedule.
Melatonin was found to be a key element of this interaction between the circadian rhythm and the glucose and insulin metabolism. As Panda et al state in a review:
> Human studies in young adults found that eating close to when levels of the sleep hormone melatonin start to rise (i.e., close to bedtime), is associated with having more body fat. In a randomized weight-loss study, women with obesity who ate earlier in the day lost more weight. A small study in adults found that late-night eating increases blood sugar levels after the meal and the following day. Observational studies in people have also found that late-night eating is associated with obesity and greater risk of poor cardiometabolic health.
>
> The circadian system prepares the body to be more efficient at digesting, absorbing, and metabolizing food earlier in the day (active phase). For instance, insulin sensitivity (needed to regulate blood sugar) is greater in the morning. Thus, larger meals are processed better when eaten in the first half of the day. Conversely, since melatonin (released at night) reduces insulin release, the body is not able to process glucose properly when you eat late at night or very early in the morning, when melatonin is high. Therefore, eating larger meals earlier in the day and avoiding food for a few hours prior to bedtime may have health benefits.
This interaction between the circadian rhythm and insulin was found to be mediated by the pancreas. Indeed, the pancreas expresses receptors of both melatonin and insulin (see also here and here). It was further discovered that melatonin levels are reversely correlated with insulin levels: not only does melatonin inhibit insulin, but also insulin inhibits melatonin, so that melatonin and insulin appear to be functionally antagonistic. In fact, the pancreatic secretion of insulin shows circadian rhythmicity, and this plays a key role in the proper functioning of beta cells and for its survival, as out of phase exposure to bright light caused beta cells loss in rats. In addition to melatonin interacting with insulin through the melatoninergic receptors on the pancreas, it was hypothesized in 2014 that melatonin may interact with insulin directly at the protein level, and hence that melatonin type 2 receptors could be a promising target for new treatments of diabetes. Indeed, melatonin-induced inhibition of insulin causes an artificial temporary insulinoresistant state. Some animals results suggest this antagonism exists to adapt the metabolism to different needs during diurnal or nocturnal periods, or more precisely to act as failsafe mechanism to avoid the occurrence of the potentially detrimental interaction between glucose tolerance, insulin resistance and melatonin when possible. For example, one hypothesis, initially developed by La Fleur from results on mice, is that the body expects that no caloric input will happen during the circadian night, since the individual should be asleep, and hence to avoid the dangerous risk of hypoglycemia during such a long period (1/3rd of lifespan on average), the pancreas switches insulin off and hence the processing of glucose when melatonin is received, which theoretically signals the start of the circadian night, so that the user remains in an artificial insulinoresistant state with a constant glycemic levels throughout the circadian night. Eating during the night hence throws off this mechanism as the body is not expecting to have to process glucose, and hence any new glycemic input "overloads" the system. There is some evidence from animals studies that this is the case, including the metabolic overload when eating in circadian misalignment. Inversely, during the circadian day, the body expects loads of glucose and lipids to fuel its efforts, and so the body "turns on" insulin to process nutrients. This circadian rhythmicity of the glucose metabolism was demonstrated to be independent from meals timing, in a study with rats being fed 6 meals regularly throughout day and night, the rhythm was still maintained, showing it was independent from the feeding/fasting cycle. There is also some evidence from human studies, with night shift workers systematically displaying increased levels of glucose and triglycerides than on day shifts, which resorb to normal levels when they resume a day shift schedule. A rigorous forced desynchrony protocol demonstrated in humans that acute circadian misalignment (here a 12h phase reversal) per se increases postprandial glucose and insulin responses, suggesting increased insulinoresistance, as well as increased blood pressure and decreased sleep efficiency and leptin levels (hunger). The liver likely also contributes to this whole mechanism, since the liver consumes (metabolizes) about 70% of all the melatonin secreted by the body, which is also the organ that majorly regulates insulin. On top of the interaction between melatonin and insulin and glucose, the circadian rhythm also modulates lipid metabolism, with a higher absorption of cholesterol and lipids during the circadian day and lower during the circadian night as observed in mice (see also here), which can hamper sleep, given that cholesterol is synthesized into cortisol, the wakefulness/vigilance hormone. Furthermore, as can be expected since melatonin is tightly coupled to photic inputs, bright light exposure during the circadian night can also cause decreased insulin secretion and overall failure of pancreatic islet cells as shown in a rats study. Some researchers such as Dr. Aleksey Matveyenko are convinced that there is a direct causal link between circadian disruptions, including potentially mediated by MTNR1B melatoninergic receptors and CRY2, and diabetes type 2.
This demonstrates that the circadian rhythm, and especially (but not only) melatonin, directly affects and is affected by glucose metabolism through insulin, causing an artificial insulinoresistant-resistant state during the circadian night.
This detrimental interaction is especially pertinent with metabolic and hormonal disorders, as adolescent girls with PCOS having a misaligned circadian rhythm phase as evidenced by a delayed melatonin profile also have a worse insulin resistance and obesity. It's worth noting that even lean women and normal-weight adolescent girls with PCOS also have an insulin resistance and increased liver fat.
Note that beyond the interaction with the glucose metabolism, melatonin also has a wide spectrum of antioxydant activities useful for the digestive system, including in the upper tract where melatonin not only regulate circadian entrainment, but also does "free radicals scavenging activity, protection of mucosa against various irritants and healing of various GIT lesions such as stomatitis, esophagitis, gastritis and peptic ulcer". This may also be a strong explanation as to the necessity of melatonin and expectation of no caloric/food input, as melatonin is necessary for sleep to be restorative, otherwise reactive oxydant species accumulate in the digestive tract and kill the host, since food is the biggest point of entry for oxydative species. It thus makes sense for the body to assume the digestive tract should not be used while this restorative process led by melatonin is undergoing.
A genetic mutation simultaneously worsening circadian rhythm disorders and glucose metabolism
Since the glucose metabolism and the circadian rhythm are directly inter-related, it is conceivable that some specific genetic mutations may concomittantly cause disorders in both kinds of systems (eg, DSPD and diabetes). And indeed, there are.
The artificial insulinoresistant state during the circadian night is further worsened for carriers of a mutation in melatonin type 2 receptors called MNTR1B rs10830963-G allele, as this allele explains 26% of the variance of glucose profile, and is estimated to be quite highly prevalent, as an estimated 30% of the world population have it, and with 51% of the carriers being of European ancestry. A meta-analysis found that the MTNR1B rs10830963 mutation could be a marker of diabetes type 2 in South Asia populations, and an analysis of the UK Biobank found the G allele and late chronotype increase the risk of diabetes. Another analysis of the UK Biobank found that the individuals with the G risk allele, apriori with a delayed sleep phase, but who shift worked (ie, worked later according to their circadian rhythm) may not have increased risks of diabetes. However, the effect size of the associations between metabolic issues and MTNR1B mutations usually remain small due to not accounting for the time of assessment and hence the melatonin levels (see also here). Indeed, the culprit is not the genetic mutation, but melatonin itself, of which the genetic mutation is only a magnifier: a cross-over study found that late dinner when melatonin levels are high in the blood impaired glucose tolerance, and it was even worse for the individuals with a genetic mutation MTNR1B in melatonin receptors type 2, in particular the G allele although the C allele also increased risks. Note also this study was only on CG carriers, meaning they only had one G mutated allele, and on female rugby professional players, hence this effect is not due to sedentarity, and it can be even worse for the double GG alleles carriers, as the Figure 1 of this study on DSPD shows.
But metabolic issues such as diabetes are not the only association of the MTNR1B mutations: they are also highly frequently associated with circadian rhythm disorders. Although it was already known that diabetes risks were increased by sleep disorders including "short, low-quality or mistimed sleep", it was found in a study on cohorts that carriers of the MTNR1B rs10830963-G mutation have a significantly delayed melatonin profile (1.37h) and longer duration of elevated melatonin profile (41 min). This is exactly in the range of non-24 (25h or 24.8h to 25.8h) and DSPD (between 24.5h and 25h) circadian rhythm disorders. In other words, carriers of the MTNR1B rs10830963-G mutation had at least a DSPD circadian rhythm disorder. Interestingly, the study further found that early risers among these carriers, in other words individuals with DSPD who constrained their sleep to wake up earlier than their circadian rhythm, had increased risk of diabetes. The authors further tested whether sleep deprivation was a factor, but they found that "the relationship between rs10830963 and melatonin duration is not mediated by sleep duration" — in other words, circadian misalignment is sufficient for increased diabetes risk for carriers of this mutation and who eat when melatonin is high in their blood. They note that not only eating and melatonin has a detrimental interaction, but also the reduction of night-time melatonin signaling has the same effect, increasing risks of diabetes. The study concludes that "MTNR1B rs10830963G extends the duration of melatonin secretion later into the morning, and waking up earlier in the morning magnifies the diabetes risk with MTNR1B genotype", and hence that the carriers of these mutations should avoid waking up too early, or at least eating too early to avoid "food intake to coincide with elevated melatonin levels in the morning", to avoid this detrimental interaction between melatonin and glucose-insulin intolerance. A study on mice further showed direct evidence that meal mistiming and circadian misalignment can directly cause diabetes, in wild mice that were otherwise fed the same quantity and diet as other mice, but just slept and ate out of phase with their circadian rhythm. Another mice study testing both glucose, circadian misalignment and aberrant light exposure as a model of shift working found that "shift working" mice had a higher glucose area under the curve, indicative of a lower glucose tolerance, and it was correlated with the circadian misalignment to the light exposure as demonstrated by the phasor analysis, which provides a strong link between aberrant light induced circadian misalignment and glucose intolerance. As a side-note, since bright light therapy efficiently inhibits melatonin, it could be a potentially helpful venue to reduce the risks of DSPD who are early risers due to work constraints. This study is of extreme importance for individuals with circadian rhythm disorders, as it demonstrates that the usual sleep hygiene predicaments, beyond their ineffectiveness, can also cause serious metabolic diseases such as diabetes.
The evidence of a strong association between the MTNR1B mutations and circadian rhythm disorders is only emerging but is very compelling. The cohorts study above studied DSPD, but another study found a similar association not only with DSPD, but also with 4 individuals with non-24 who were misdiagnosed, as their analysis shown they were carriers of the MTNR1B rs10830962 mutation, but the authors unfortunately did not discuss this association as they clearly were unaware of the link between MTNR1B and circadian rhythm disorders and metabolic disorders. Furthermore, we mentioned above that individuals carrying the double GG alleles have a higher risk of diabetes than CG carriers, but also interesting to note is that they also have a more delayed melatonin profile (see the DLMOff in Figure 1). The author of the current document highly suspects than individuals with sighted non-24 may commonly have the GG mutation, which unfortunately remains to be tested.
To summarize these findings, there is now a whole body of work demonstrating that melatonin type 2 receptors mutations MTNR1B in various alleles (MTNR1B rs10830963 and rs10830962 and rs1387153) are strongly associated or even predictive (causing?) both metabolic disorders including diabetes and obesity (see also this systematic review and this review), as well as circadian rhythm disorders including DSPD and non-24.
A new molecule, Nocturnin, which also show circadian fluctuations, has been shown to mediate fat metabolism. This may be an additional piece to the melatonin-insulin interaction puzzle.
Internal desynchronizations between peripheral organs and the central (brain) clock
Until 1990s, it was thought that the circadian rhythmicity was solely maintained by the central (brain) clock. It has since then been well established that independent, peripheral clocks exist in most cells throughout the body beyond the brain, and it is well accepted that all mammals possess cells that include the whole circadian machinery (CLOCK, PER), as shown by the figure 2 of this review. This suggests that metabolic dysregulation may happen with any kind of internal desynchronization between the central clock and any of the peripheral clocks, not just the digestive system, as summarized in the figure 4 of the same review. The two figures are reproduced below:
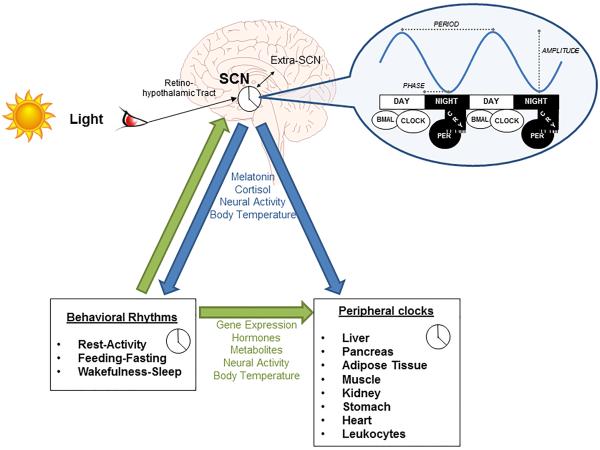
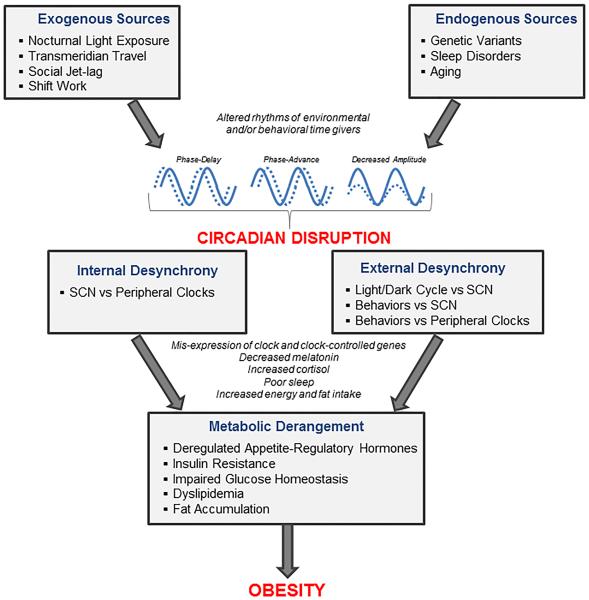
The hierarchy of influence of the various clocks is concisely summarized in this quote from a 2020 review:
> The master clock in SCN regulates energy expenditure, food intake, and whole-body insulin sensitivity, and these actions are further fine-tuned by peripheral oscillators in organs such as the liver, muscle, pancreas, and adipose tissue.
In addition to circadian disruptions causing metabolic dysregulations, it was shown that metabolic dysregulations, through meals mistiming, could shift and disrupt the peripheral molecular clocks especially in some organs involved in metabolic regulation such as the liver, even when bright light exposure was controlled (see also here). Another study shown that >80% of rhythmically cycling transcripts in the liver were blunted in rats under prolonged fasting, and another estimated that "in the liver, 14% of rhythmic transcripts are dependent on systemic signals, whereas 86% are dependent on local oscillators." Several mice studies shown that several organs, including the liver, pancreas, kidney and heart, peripheral clocks could be phase shifted by feeding, while the central clock remained unaffected.
Beyond meals mistiming, some neurodisruptive edibles such as alcohol can cause a desynchronization between the central (brain) clock and the peripheral (body/digestive) clock, as suspected already since the earliest works on circadian rhythm science in 1966. Indeed, alcohol use, even with a single dose, is strongly associated with circadian rhythm and melatonin disruptions, with more alcohol causing more disruptions. Furthermore, a rat study shown that, targeting improvements of the circadian rhythm and sleep by using melatonin on alcohol-dependent rats yielded improvements or even remission of alcohol use disorder.
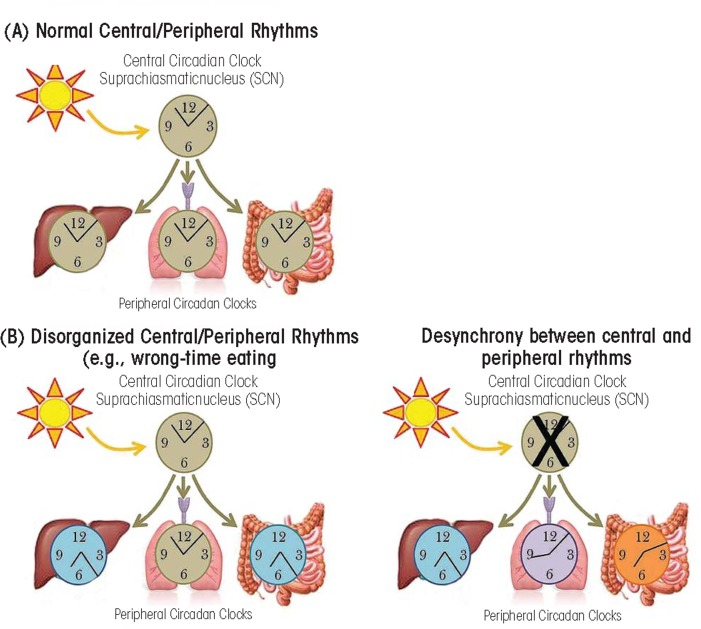
Central (brain) and peripheral (body/digestive) clocks can become desynchronized by mistimed meals and neurodisruptive edibles such as alcohol. Figure extracted from this study under Public Domain.
How this relates to metabolic syndromes such as diabetes and obesity
Metabolic syndromes such as diabetes and obesity are often associated with circadian dysregulations (see also here), with for example diabetic individuals displaying an abnormal pattern of melatonin secretions. Current evidence further suggest that circadian dysregulations can cause metabolic syndromes such as diabetes and obesity (see also here), with for example shift workers having a greater risk of developing a metabolic syndrome. One identified pathway for this increased risk is the destruction of pancreatic beta cells and impairment of the the insulin-regulated glucose metabolism in hepatocytes (liver cells) because of circadian misalignment. This prompted researchers to call for renaming both metabolic disorders as circadian syndromes given the strong links between metabolic syndromes and circadian rhythm disorders, with others even arguing that the increase in incidence of circadian rhythm disorders to be a significant contributor to the global epidemics of diabetes type 2 and obesity (see also here). Circadian disruption can also affect metabolism by cascade the circannual (seasonal) rhythm, as observed in the animal kingdom, and is an additional evidence to suspect circadian misalignment contributes to the epidemic of obesity in US children.
Metabolic syndromes are defined by the development of a resistance to insulin (ie, metabolic syndrome is equivalent with the insulin-resistant phenotype), which manifests as carbohydrate/glucose intolerance. Metabolic syndromes includes various diseases such as diabetes, obesity, non-alcoholic fatty liver disease (NAFLD) (see also here) and more recently Alzheimer. Indeed, there is accumulating evidences that brain insulin resistance (as opposed to a body insulin intolerance for diabetes) is central to Alzheimer disease, to the point some call it a type-3 diabetes, it also often co-occurs with other metabolic syndromes and it can be primarily treated by diet and lifestyle changes (see also here), just like other metabolic syndromes. A pathway was recently identified, with evidence that the hypothalamus can regulate insulin in peripheral tissues without affecting the liver, and hence lending credence to the hypothesis that there may be brain insulinoresistance without hepatoinsulinoresistance. Circadian dysregulation is emerging as a potentially major factor of insulin resistance development, and it's suggested that the interaction between insulin and melatonin may be a previously underestimated major factor causing metabolic disorders. Even the liver may be affected and potentially improved by circadian realignment therapies, since a 2019 review about NAFLD states that "NAFLD and NASH are increasingly prevalent and may be largely mitigated with effective lifestyle modification and, potentially, circadian rhythm stabilization."
Another piece of the puzzle is the evidence that not only circadian misalignment can impair metabolism, but circadian realignment can improve it. Indeed, a systematic review concluded that evidence indicate that melatonin deficiency is associated with metabolic dysregulations, but also that "melatonin treatment successfully improved glucose homeostasis, energy balance, and overall health" in diabetes type 2 as well as reduce complications due to melatonin antioxydative properties and hence may be a promising new therapy for diabetes type 2 according to a 2020 systematic review in animals, but human clinicial trials are needed to confirm. Interestingly, diabetic individuals also have decreased levels of melatonin and increased pancreatic receptors density, and studies on animals demonstrated that low melatonin levels may increase the risk of developing diabetes. Melatonin was also found by several studies to improve blood pressure levels in patients with essential hypertension, but only after 3 weeks of daily intake, but with no influence after one acute dose at nighttime, indicating that it's not the drug's acute vasodilatory effect but rather its circadian realignment effect that mediated the decrease in blood pressure.
A breakthrough was done by Ahmad Kobiita for his 2016 PhD thesis, in which he elucidated the molecular cascade from zeitgebers perturbations down to circadian alterations, and found that mice eating in circadian misalignment (ie, during the rest period instead of the active period) altered the metabolism by immediately causing a hypoinsulinaemia during the active phase, inducing PPARalpha, which reprograms metabolism and gene expression of RevErbalpha, which in turns causes a 12h phase shift in peripheral tissues, and shifts the SCN central clock too. Inhibition of PPARalpha prevents the central clock from being phase shifted by peripheral clocks, causing a circadian misalignment between the central clock and peripheral clocks. Finally, this circadian misalignment between central and peripheral clocks causes a metabolic syndrome similar to that observed in shift workers.
In the past, it was theorized with the "carbohydrate-insulin model" (CIM) that the effect of insulin on metabolic disorders was mediated through the consumption of carbohydrates: if no carbohydrates were consumed, this hypothesis predicted that the metabolic disorders would be regulated. Recent experiments disprove this theory, suggesting that although insulin plays a key role in body fat regulation, the effect of insulin on metabolism is through a variety of organs, not just through carbohydrates. The interaction between melatonin and insulin is one example of the pleiotropic roles of both insulin and melatonin, that will need to be elucidated in the upcoming decades. See also this interesting discussion on reddit about this paper.
In conclusion, due to the higher susceptibility of individuals with chronic circadian disruption, such as with a circadian rhythm disorder, to develop diabetes type 2, it may be wise to lower the risk to adapt both food timing to follow the endogenous circadian rhythm and food content to be in line with diabetic diets recommendations.
What to do in practice? How to plan meals?
What can be done in practice? It is advised to plan meals at times when melatonin levels are low (see also here and here), as the timing of food intake predicts weight loss or weight gain (see also this systematic review) independently from other factors. This is of crucial importance when a sleep disorder is present, as both sleep deprivation and circadian misalignment independently impair metabolic control and body weight regulation. Indeed, another study shown that circadian misalignment independently from sleep duration predicts an increased risk of cardiometabolic diseases. To reduce this risk, it is reasonable to follow a rule of no meal past melatonin pills intake, during the biological night and not too early in the biological morning (eg, delay breakfast). The absolute time does not matter, what matters is that you eat outside of your biological night (eg, if you currently ideally sleep during the day, you can safely eat at night). A 2017 systematic review on obesity prevention concluded that "meal timing appears as a new potential target in weight control strategies" that may even counteract genetic predispositions to obesity according to another review. Indeed, there is some evidence suggesting that individuals who sleep sufficiently long and sleeping (and hence eating) in phase with their circadian rhythm may be better protected against weight gain, and even experience weight and fat loss. For this reason, some forms of meal timing scheduling such as intermittent fasting are now recommended as an option by the American Heart Association, as they may reduce or even reverse metabolic diseases and cardiovascular diseases (see also this talk and this animal experiment). Intermittent fasting, more precisely time-restricted feeding, can also regulate the gut microbiome, and was shown in animals to reduce cognitive impairments such as anxiety and depression symptoms in mice following a night shift work schedule. But contrary to the currently practiced forms of intermittent fasting, it is crucial to schedule meals relative to the individual's circadian rhythm and the melatonin levels variations, as a study on mice observed that timed feeding did not have any beneficial effect unless timed in phase with the circadian rhythm. In another rodent study, time-restricted feeding was found to prevent cardiometabolic diseases such as obesity induced by chronic phase advances by light-dark therapy in jet-lagged mice. According to a review by Panda et al, despite lacking data in humans, evidence points to restricting meals under less than a 12h window per day to be likely healthier.
> WHAT IS THE BEST EATING PATTERN?
> There are almost no studies in humans comparing different meal-timing schedules to determine if one meal-timing strategy is better than the others. From what we know, our best available research suggests that 3 meal-timing habits are likely important for good health:
>
> * a consistent daily eating duration of fewer than 12 h per day,
> * eating most calories in the earlier part of the day, and
> * avoiding food intake close to bedtime, while sleeping, or very early morning, when melatonin levels are high.
However, meal timing can be challenging for these populations (circadian rhythm disorders and metabolic disorders). Indeed, it was shown in animals with a lesioned SCN (destructed central clock) that they completely lose their daily rhythm in food intake: they just eat at seemingly random times. This suggests that we can expect humans with a disrupted circadian rhythm to also demonstrate a disordered eating pattern. In diabetic individuals, melatonin levels are decreased and its secretion pattern is abnormal, so that it is more challenging for them to know what is the ideal time for their meals since the circadian rhythm is disrupted by their metabolic disorder, which in turns also worsen the metabolic disorder in a vicious cycle. Hence, although in theory it is quite clear that avoiding meals during melatonin secretion is a reasonable approach to health, in practice it can be very challenging for populations with a metabolic or circadian rhythm disorders since there is a lack of consumer-grade tool to monitor melatonin levels in real-time. Until then, guess work is the only approach for these patients, and this is obviously fraught with inaccuracies and hence mistimings, even when the patient is rigorous with their meals timing, as their abnormal patterns of secretions may be unpredictable.
Although we mostly discuss circadian misalignment above, sleep deprivation has even worse metabolic effects. Shorter sleep has been linked with metabolic syndromes development (diabetes and obesity) as acknowledged by the American Heart Association 2016 guidelines. Short sleepers who extend their sleep duration may lose weight and see a reduction in risks of diabetes. Voluntary bedtime restriction to fit in the modern 24h society leads to chronic sleep loss, which dysregulates the neuroendocrine regulation of appetite and hunger, potentially via the suprachiasmatic nucleus's leptin and ghrelin receptors, which may lead to weight gain. Indeed, a single night of sleep deprivation is sufficient to increase ghrelin levels, although there some evidence that ghrelin may promote slow-wave deep sleep in addition to regulating hunger. Combined with the body of evidence strongly associating short sleep and obesity, this suggest that short sleep increases the risk of obesity primarily by increasing hunger, as well as some metabolic changes such as increasing abdominal adiposity. Those predisposed to metabolic syndromes have a majorly higher risk of developing obesity and cardiovascular diseases when sleep deprived, including children and teenagers. Sleep deprivation can majorly curb the benefits of diet or lifestyle changes, since humans under a calorie restriction diet maintained 55% more fat when sleep deprived compared to those who were not, which is logical given a biological study identified that the mechanism through which calorie restriction improves life expectancy may be through restoring the hepatic circadian rhythm.
In fact, researchers at the interplay between sleep and metabolic disorders argue, such as in this review, that "traditional risk factors such as over-eating, poor nutritional choices and lack of exercise cannot fully account for the high prevalence of metabolic disease", with sleep disruption and circadian misalignment emerging as two novel risk factors:
> Millions of individuals worldwide do not obtain sufficient sleep for healthy metabolic function, and many participate in shift work and social activities at times when the internal physiological clock is promoting sleep. These behaviours predispose an individual for poor metabolic health by promoting excess caloric intake in response to reduced sleep, food intake at internal biological times when metabolic physiology is not prepared, decreased energy expenditure when wakefulness and sleep are initiated at incorrect internal biological times, and disrupted glucose metabolism during short sleep and circadian misalignment. In addition to the traditional risk factors of poor diet and exercise, disturbed sleep and circadian rhythms represent modifiable risk factors for prevention and treatment of metabolic disease and for promotion of healthy metabolism.
A study on healthy humans allowed to derive quantitative estimations of the impact of eating in circadian misalignment, such as in the morning when forcefully waking up earlier than the natural wake up time. Eating in circadian misalignment and sleep deprived reduced by 20% the oral and intravenous insulin sensitivity after just 5 days with a sleep duration restricted to 5h! This could be partially reversed by allowing to sleep up to 9h per night for 3 days, but this only restored oral insulin sensitivity to baseline, not intravenous insulin sensitivity:
> Imposed short sleep duration resulted in morning wakefulness occurring during the biological night (i.e., circadian misalignment)-a time when endogenous melatonin levels were still high indicating the internal circadian clock was still promoting sleep and related functions. We show the longer melatonin levels remained high after wake time, insulin sensitivity worsened. Overall, we find a simulated 5-day work week of 5-hr-per-night sleep opportunities and ad libitum food intake resulted in ∼20% reduced oral and intravenous insulin sensitivity in otherwise healthy men and women. Reduced insulin sensitivity was compensated by an increased insulin response to glucose, which may reflect an initial physiological adaptation to maintain normal blood sugar levels during sleep loss. Furthermore, we find that transitioning from the imposed short sleep schedule to 9-hr sleep opportunities for 3 days restored oral insulin sensitivity to baseline, but 5 days with 9-hr sleep opportunities was insufficient to restore intravenous insulin sensitivity to baseline. These findings indicate morning wakefulness and eating during the biological night is a novel mechanism by which short sleep duration contributes to metabolic dysregulation and suggests food intake during the biological night may contribute to other health problems associated with short sleep duration.
There is a wide agreement in the metabolic disorders and sleep disorders scientific communities that these two kinds of disorders are inter-related and should be systematically monitored (eg, diabetes studies should systematically monitor the participants sleep and screen them for sleep disorders) (see here, here and here). Indeed, not only was there a parallel increase in metabolic disorders incidence and sleep disorders incidence, but there are now well established biological pathways linking the two systems. It's hence unsurprising that sleep extension, in other words sleep disorders treatment, is suggested to be a potential new approach to treat obesity and other metabolic disorders.
Theoretic pathways from sleep disorders to metabolic disorders, figure excerpt from Depner et al, 2014.
Since humans display a seasonal rhythm in melatonin secretion, with longer melatonin secretion that stops later in the morning during winter compared to summer due to later sunrise, it may be healthier to eat later during winter than summer to adapt to the longer melatonin secretion profile and avoid insulin inhibition (eg, skip breakfast if waking up earlier than sunrise).
So to summarize: research shows that eating or drinking caloric beverages should be avoided when melatonin blood levels are high, which is either during the circadian night, or when melatonin pills medication is taken, which is exactly what Panda et al also recommend in a review. This applies for everyone, although some people with a genetic mutation have it worse. When waking up much earlier than the natural wake up time, or when being jet lagged after travelling, it may be wiser to delay food intake to a later time more in phase with the individual's circadian rhythm. As Pr. Satchin Panda said: "when we eat is as important as what or how much we eat".
It is worth noting that some studies found no effect of food timing on the timing of the circadian siesta, although the timing of the dip varied greatly on its own from day to day. Since the siesta is part of the circadian rhythm phase and allows to predict the circadian night, this is a strong suggestion that food timing scheduling may not affect the circadian rhyhm that much. Rather, the focus should be to avoid ingesting food when the body produces melatonin, to avoid the interaction between melatonin and insulin.
A 2019 study found that sleep deprivation and non-24h cycles were independently associated with changes in morning saliva insulin levels as well as the microbiota bacterial composition and concentration, supporting further the results from a previous RCT study on the saliva microbiota.
TODO: "Changes in insulin secretion, clearance and/or action across the day have been demonstrated. Studies in subjects receiving continuous intravenous glucose infusion have shown that major alterations of glucose tolerance occur during sleep and that sleep quality markedly influences glucose utilization. Diurnal variations in glucose tolerance result from the alternation of wake and sleep states as well as from intrinsic effects of circadian rhythmicity. The important roles of physiological variations in levels of counterregulatory hormones which are markedly dependent on sleep (i.e. growth hormone) or circadian rhythmicity (i.e. cortisol) have only begun to be appreciated." Scheen AJ, Van Cauter E. The roles of time of day and sleep quality in modulating glucose regulation: clinical implications. Horm Res. 1998;49(3-4):191-201. doi: 10.1159/000023170. PMID: 9550124.
Bigger meals have a stronger resetting effect
Studies on rodent observed that timed feeding (ie, feeding at a predefined time) could shift the circadian rhythm. Indeed, by modifying the time of feeding, the animals started to sleep and wake up at a different time to adjust with the time they would get fed. Furthermore, bigger meals had a bigger circadian shifting effect than smaller meals. Timed feeding could even allow for entrainment of mice under constant light (ie, no light zeitgeber). Entrainment to food cues was observed even in mice without a SCN (ie, no entrainment to light), which shows that there are different circadian clocks managing these two kinds of entrainments.
In fact, other rodents basic studies found that the clocks of peripheral organs and some brain regions are preferentially reset by meal timing and not the SCN (bright light exposure), as "lesions of specific hypothalamic, corticolimbic and brainstem structures do not eliminate all food anticipatory rhythms, suggesting control by a distributed, decentralized system of oscillators, or the existence of a critical oscillator at an unknown location".
A study on mice clarified the mechanism, by finding that timed feeding of dense food (ie, a big timed meal) can change the circadian rhythm by modulating the dopamine signaling in the suprachiasmatic nucleus (SCN), the same region regulating melatonin secretion. This resulted in overconsumption of food. The mice without the D1 dopamine receptor (Drd1-null) were resistant to diet-induced obesity, diabetes and circadian disruption due to energy-dense diets.
Inversely, sleep deprivation also increases (unhealthy) dense food cravings, and the digestive system is majorly dysregulated by sleep deprivation.
However, big meals, particularly if rich in carbohydrates, can cause postprandial sleepiness (also called reactive hypoglycemia), and digestion also produces melatonin (see also here). Although the siesta timing is independent from the feeding timing, its amplitude may be affected by diet composition and quantity since the digestive lower tract produces melatonin during digestion. Hence, a balance in terms of quantity and timing are critical parameters for effects of big meals on the circadian rhythm.
Diet composition and ketogenic diet
Reducing the quantity of consumed carbohydrates can be highly beneficial, as each 1% reduction improves the metabolism and reduces risks of obesity and metabolic disorders and sleep issues for carriers of the MTNR1B-rs1387153-T allele according to a meta-analysis. It's also an advised treatment to deal with postprandial sleepiness and particularly reactive hypoglycemia (see below). This shows that there is a dose-dependent effect of carbohydrates intake on the metabolic dysregulation: any reduction of carbs improves health.
The importance of the diet composition, along with meal timing, for the treatment of circadian rhythm disorders was likely pioneered by the Argonne Anti-Jet-Lag diet and publicized in the 1980s, with use by the military personnel. A review suggests that specific macronutrients may have specific effects on the circadian rhythm (TODO: read and extend + read this).
Lipids (triglycerides) modulate up to 30% of the temperature profile's amplitude and stability, and since body temperature is strongly tied with the circadian rhythm, lipids can likely significantly impact the circadian rhythm.
Low glycemic index carbohydrates (ie, prolonged-release carbs) such as pasta has been shown to require more energy to process and hence increase body temperature and hence impair the circadian rhythm, up to 24h after ingestion (hence well into the next day even if eaten at lunch the previous day!) ; and contrary to a widespread misconception, systematic reviews could not find a significant effect of low glycemic index foods on diabetes nor glucose level management.
There exists a type of diet called ketogenic diet, where most intake is from lipids, some protein, and little carbohydrates. This diet was initially conceived and used (up to this day) for the treatment of treatment-resistant epilepsy. Given the findings described aboved, it makes sense to investigate whether the ketogenic diet may have an impact on the circadian rhythm. And indeed, some studies have done just that.
Only one study has studied the ketogenic diet effects on humans sleep so far, more precisely with epileptic children. It was found that the strict ketogenic diet shortened sleep but without reducing sleep quality (only stage 2 is decreased), decreased daytime sleep (naps/drowsiness), increased attention and increased REM sleep. In other words, the ketogenic diet decreased the total sleep time but actually improved sleep quality. Anecdotally, the current document's author also observed the same reduction is sleep duration without loss of sleep quality.
How does that work and what does it mean for the circadian rhythm? For the moment, only rodents studies are available on this aspect, but they are quite enlightening. A study on mice found that the ketogenic diet produced a phase advance and shortened the circadian rhythm period (tau), which can explain the finding with the epileptic children of a reduced sleep duration without loss of sleep quality. Another rodent study (summary here) goes further by showing a differential effect of the ketogenic diet on the liver and the intestines: the liver clock genes are inhibited (ie, the liver clock is frozen), whereas the intestines clock genes are overexpressed (ie, boosted). In other words, the ketogenic diet makes the body clock more reliant on food intake, and hence magnifies food intake effect on the circadian rhythm, hence it may facilitate entrainment by meal timing by reducing the liver's contribution to the whole-body circadian rhythm. We may hypothesize that the insulin and melatonin interaction that may happen primarily in the liver can be at the root of this observation (the liver is known to metabolize both insulin and melatonin). Interestingly, the circadian clock also regulates liver functions.
To summarize, the ketogenic diet both reduces the circadian rhythm period and hence the sleep duration without loss of sleep quality, and nudge the circadian rhythm to be more easily reset by the timing of food intake. Hence, the ketogenic diet may facilitate entrainment.
Furthermore, the ketogenic diet may also improve sleep indirectly by weight loss, as weight surplus is associated with obstructive sleep apnea and snoring, which may resolve with weight loss; and by reducing digestive issues for individuals with irritable bowel syndrome disorder as it reduces or eliminates the intake of FODMAP, since they are specific kinds of carbohydrates, which are avoided in the ketogenic diet. In other words, there are no FODMAPs in lipids nor proteins, so the ketogenic diet is a good option for those with FODMAP allergy.
Is the ketogenic diet healthy? In 2018, both the National Lipid Association, the American Diabetes Association (see also here) jointly with the European Association for the Study of Diabetes published statements which recommend the low-carb diets, including high-fat (ketogenic) diet, as an option for diabetic or obese individuals that can reduce the need for diabetes medication, and they listed the factors for a healthy low-carb diet, such as avoidance of saturated fats, increased protein ratio to reduce muscle loss and increased fruits and vegetables serving (see also here and here). Indeed, with such adaptations, the ketogenic diet was found to reduce risks compared to a typical western diet (see also here). Other experts even claim that saturated fats are not the primary macronutrient to avoid for diabetic individuals, whereas carbohydrates are a major factor, and hence that metabolic syndrome "is most effectively managed by a low carbohydrate diet" such as the ketogenic diet. Interestingly, the NLA notes that "the amount of carbohydrate intake required for optimal health in humans is unknown". The ketogenic diet is also recommended by experts to treat familial hypercholesterolaemia. Even the previous consensus that saturated fat should be avoided is now questioned. Coincidentally, this pivoting on low-carb diets coincides with the appointment in 2018 of a new CEO for the American Diabetes Association who has diabetes, which is unexplicably a first in the 80-years history of the institution, and who furthermore declared in 2020 being using a low-carb diet to manage her own diabetes without drug.
The main drawback to following a strict ketogenic diet is that it is highly inconvenient to follow, especially when trying to eat a healthy ketogenic diet (ie, with a reduced amount of saturated fat). Hence, a healthy ketogenic diet requires a lot of planification beforehand and particularly before social events to ensure to take the food needed as it is unlikely that a meal obtained outside will be adequate (ie, no to little carbohydrates). This is colloquially termed as the "keto struggle".
If you are a fast metabolizer and need to eat not too long before sleeping, then try to eat a smaller meal and reduce its carbohydrates content, particularly slow carbs/starchy food such as pasta, rice and floor, as well as sugars and sweet food and drinks. See this ADA guide for a classification of carbohydrates. Indeed, both carbohydrates and proteins cause increases in glucose levels, especially when insulin is low, but it seems fat does not. In line with these observations, an observational cohort study found increased risks of cardiovascular diseases when animal proteins and low-quality carbohydrates were consumed at dinner compared to breakfast, but found reduced risks when unsaturated fat foods were consumed at dinner instead.
To monitor if the ketogenic diet is done properly, use ketostix, an urinary measument bands that will color depending on the amount of ketone bodies in urine. A strict ketogenic diet should consistently produce a color between the 2 highest grades (0.8 g/L to 1.6 g/L). A reusable alternative may be the electronic breath ketone meters, but the author did not test this kind of product yet.
Interestingly, some authors draw an analogy between the importance of a healthy food diet and a healthy spectral (light) diet, suggesting that light composition (eg, quantity of blue light, light intensity) matters just as much as food diet composition.
Some ketogenic diet users report an easier ability to formulate clear and focused thoughts, which may hold some truth given the recent trial that observed improved cognitive performance, daily function and quality of life in people with Alzheimer's disease undergoing a modified ketogenic diet over 12 weeks.
Anecdotally, the author of the present document tried the ketogenic diet for several months on two occasions at 1 year interval. The first time, it seemed like the ketogenic diet affected sleep duration and quality, but in the second run, with new tools and a refined entrainment therapy at his disposal, when sleeping in circadian alignment, the author could sleep a full 8h night and a bit more. Hence, the ketogenic diet did not show any significant effect on sleep nor on dreams during the 2nd run, which means that the ketogenic diet does not appear to improve, nor impair, sleep. It can hence be used by individuals with the non-24 disorder for other purposes in parallel to an entrainment therapy (eg, the ketogenic diet may be part of a diabetes management therapy).
Nevertheless, a recent body of scientific evidence suggest that the ketogenic diet may be beneficial to health, and especially reduce some risks that sleep disorders increase. For instance, this diet can lead to a significant weight loss, and in the author's experience, this consistently and greatly reduces the occurrences of sinus issues such as snoring, which may indirectly improve sleep quality. In the author's experience, the most effective for weight loss is a combination of ketogenic diet and timed big meal which is a form of time-restricted feeding (ie, skip dinner). A re-analysis of a national cohort found that low-fat diets prescribed as "heart healthy" diets to postmenopausal women found that there is no evidence supporting low-fat diets as heart healthy since they actually increase the risk of cardiovascular diseases by about 25%. Hyperglycemia (high levels of glucose) have been demonstrated to promote atherosclerosis (veins rigidity). And of course high-carbohydrate food and drinks promote the risk of metabolic disorders such as diabetes and obesity (TODO: add refs), with an increased risk when consuming food in circadian misalignment due to the melatonin-insulin interaction. Since the risk of cardiometabolic diseases is also increased by sleep disorders, changing the diet/lifestyle to one that reduces these risks can help in counterbalancing and normalize these risks.
Recent evidence from animal studies on carbohydrates quality suggest that an alternative to carbohydrates elimination may be to reduce simple carbohydrates (sugar) intake and increase intake of complex, less digestible sugars such as fibers and resistant starch, with a low protein intake.
Another interesting avenue is the composition in micronutrients, as "micronutrients like amino acids, glucose, and free fatty acids can modulate clock gene expression and function in the SCN master clock and peripheral tissues. "
If you really need to eat in the evenings, prefer to consume tropical fruits such as bananas, which were shown to cause an uptick of melatonin in the blood 2h after consumption, although note that it is likely only the receptor-independent antioxydative activity that is promoted, it is unlikely to help with sleep, but this hypothesis was not tested so we don't know. But at least, fruits are a healthy kind of meal and stack if you need to eat, especially when late in the evenings.
On the other hand, if you need to stay awake, sufficient intakes of cholesterol should be considered, as cholesterol is necessary to secrete cortisol, the daytime wakefulness/vigilance hormone.
Postprandial sleepiness may be a sign of (pre-)diabetes
Feeling sleepy after lunch and irresistible naps are likely a common occurrence for people with circadian rhythm disorders, because of the natural postprandial response and the sleep deprivation on top, which can be very inconvenient when trying to follow a new sleep schedule. Indeed, eating produces melatonin and serotonin, both having an effect on the circadian clock, although the circadian rhythm seems to remain mostly independent from feeding time. Indeed, the digestive tract does produce melatonin after lunch as a "postprandial response", strongly contributing to the magnitude of the postprandial sleepiness (but not its timing). Thanks to the PRC, for normal sleepers who eat during the day their meals, eating causes slight drowsiness only but does not impact their circadian rhythm because melatonin then gets produced at the "dead zone" of the melatonin PRC, where it has little to no effect, but for non24 and their difficulty in knowing their circadian rhythm, a mistimed meal can offset their circadian rhythm one way or the other, and even just the availability of food can shift the circadian rhythm. Postprandial sleepiness is hence normal, but its frequency and severity can be increased by meals high in carbohydrates, which is then called a reactive hypoglycemia (also called postprandial hypoglycemia or "sugar crash"), which shows a greater contribution of carbohydrates as a cause of postprandial sleepiness. In case of experienced consistently a postprandial sleepiness frequently after lunches, the treatments for reactive hypoglycemia can help whether or not it's reactive hypoglycemia or a simple postprandial sleepiness, which includes the reduction of carbohydrates. For this reason, some ketogenic dieters report a reduction of postprandial sleepiness, and the author of the present document also observed a clear reduction in post-lunch sleepiness with the strict ketogenic diet, which included the inability to nap (ie, to fall asleep) after lunch even when trying to forcefully nap.
Reactive hypoglycemia (sleepiness under 4h after a big carbohydrates meal) and the dumping syndrome (digestive issues under the same timeframe) can both be signs of (pre-)diabetes, which shows another link between sleep and diabetes. If these signs are experienced, it can be a good idea to get screened for (pre-)diabetes, as this can allow to potentially optimize treatments not only for diabetes and the digestive issues but also for the circadian rhythm disorders such as non-24.
In the author's experience, the ketogenic diet also eliminates postprandial sleepiness. If postprandial sleepiness disappears under a ketogenic diet, this can reinforce the diagnosis of postprandial sleepiness caused by glucose intolerance/hypersensitivity.
Circadian rhythm and gastric issues
Circadian dysregulation is associated with alterations in colonic motility and disruption of clock gene expressions in the intestinal tract's cells. Circadian misalignment is associated with digestive pathologies such as constipation and irritable bowel syndrome. There is strong evidence that the gastrointestinal system (guts) is governed by a circadian rhythm with both peripheral and central (brain) inputs.
Since melatonin modulates gastrointestinal motility by being an antagonist of serotonin, and also serving as a major antioxydant, melatonin supplementation is being researched as a potential treatment or adjuvant for gastric ulcers, colitis, irritable bowel syndrome, Crohn's disease, necrotizing enterocolitis and children’s colic, with "low dose melatonin treatment accelerating intestinal transit time whereas high doses may decrease gut motility".
A case study of sighted individuals with non-24 include a case where entrainment was associated with a proportional improvement in abdominal discomfort, with a more stable and earlier entrainment associated with further improvements.
A review also concluded that sleep disruptions can worsen gastrointestinal issues.
Other factors that can affect feeding behaviors
TODO: This section is a work-in-progress!
Given the links between the suprachiasmatic nucleus (which has leptin and ghrelin receptions) with the digestive system, it may be possible that light therapy can directly improve "abdominal discomfort" and digestive issues as observed in one study and by the author of the present document. Indeed, it was shown that the suprachiasmatic nucleus (SCN) also modulates feeding behaviors, and can promote the consumption of dense food (ie, weight gain and obesity). Hence, light therapy may modulate feeding behaviors (ie, hunger) through the SCN.
Circadian entrainment may hence reduce metabolic issues such as binge eating for people with non-24, as a few discord members and the author of this document experienced.
- REF TOADD: ultraviolet A light may play a role in circadian rhythm resetting, so it's not only light density but also radiations: Negelspach, D. C., Kaladchibachi, S., & Fernandez, F. (2018). The circadian activity rhythm is reset by nanowatt pulses of ultraviolet light. Proceedings of the Royal Society B: Biological Sciences, 285(1884), 20181288.
- Skin has its own rhythm: https://www.sciencedaily.com/releases/2019/10/191016133015.htm
- And skin circadian rhythm is synchronized with melatonin, so melatonin is not only used for antioxydative properties in skin but also circadian rhythm synchronization! https://scanr.enseignementsup-recherche.gouv.fr/publication/tel-01220534
- BESTREFS: Insulin resistance and circadian clocks: for the moment, there is evidence that circadian rhythm misalignment can influence/worsen metabolic syndrome, but not the other way around (limited evidence in 2nd paper). But if ketogenic diet works, it's some evidence of metabolic syndrome -> circadian rhythm misalignment! Best would be to confirm prediabetes in non24 subjects for whom ketogenic diet helps.
- Circadian clocks and insulin resistance, Nature reviews, https://www.nature.com/articles/s41574-018-0122-1
- The Role of Circadian Rhythms in the Hypertension of Diabetes Mellitus and the Metabolic Syndrome https://link.springer.com/article/10.1007/s11906-018-0843-5 - that's evidence in humans
- The endogenous circadian clock programs animals to eat at certain times of the 24-hour day: What if we ignore the clock? https://www.sciencedirect.com/science/article/abs/pii/S0031938418301938
- Pharmacotherapies for sleep disturbances in dementia. Cochrane systematic review, 2016. "From the studies we identified for this review, we found no evidence that melatonin (up to 10mg) helped sleep problems in patients with moderate to severe dementia due to Alzheimer Disease."
- [29]: «Advanced circadian timing was associated with a number of subjective memory complaints and symptoms. By contrast, sleep fragmentation was linked to lowered perceptions of cognitive decline, and less concern about memory failures. As circadian disruption is apparent in both MCI and Alzheimer's disease, and plays a key role in cognitive function, our findings further support a circadian intervention as a potential therapeutic tool for cognitive decline.» → it might not be simply age that is responsible for cognitive decline, but more sleep deprivation, fragmentation etc due to misregulated circadian rhythm. https://www.ncbi.nlm.nih.gov/pubmed/30320584
- https://www.washingtonpost.com/national/health-science/why-crossing-time-zones-makes-you-feel-bad-and-what-you-can-do-about-it/2018/07/20/39417254-6a88-11e8-9e38-24e693b38637_story.html
- Interesting: digestive issues and sleep and autism: Are Gastrointestinal and Sleep Problems Associated With Behavioral Symptoms of Autism Spectrum Disorder? 2018 https://pubmed.ncbi.nlm.nih.gov/29091821/
- "GI and sleep problems were prevalent in Chinese ASD children. Moreover, ASD children with GI symptoms reported more severe ASD core symptoms than others. Autistic children's GI symptoms were associated with maternal sleep problems during pregnancy, child's 0-6 month food sources and picky eating. ASD children with sleep disturbances had lower performance in daily living skills, social cognition, social communication and intellectual development than ASD children without sleep disturbances."
- BEST TOCHECK: too much carbs can increase depression and mood swings? https://www.cheatsheet.com/health-fitness/this-terrifying-side-effect-is-a-sign-youre-eating-too-many-carbs.html/
- bloating possible with both carbs: https://www.livestrong.com/article/444370-why-do-i-get-bloated-when-i-eat-carbs/ and keto: https://ketogenicdiet.reviews/keto-bloating/
- Today, 75% of the world’s food originates from only twelve plants and five animals. https://www.sciencedirect.com/science/article/pii/S2212877816000387 and https://joinzoe.com/2019/07/23/improve-microbiome-diversity-gut-health
- Carbs craving during coronavirus crisis: https://www.inverse.com/mind-body/carb-loading-in-quarantine-why-do-we-crave-bread-pasta-in-a-crisis
- Intriguingly, genetics seems to play only a minor role – we’ve found that identical twins only share 37% of their gut bacteria,only slightly more than two unrelated people. https://joinzoe.com/2019/07/23/improve-microbiome-diversity-gut-health
- BEST: 1000 healthy people given the same meal, had large variations in biometrics: https://joinzoe.com/2019/06/21/we-reveal-the-first-results-from-the-largest-nutritional-study-of-its-kind-in-the-world
- BEST: dietary diversity is important for healthy microbiome (review 2016): https://www.sciencedirect.com/science/article/pii/S221287781600038[[https://www.sciencedirect.com/science/article/pii/S2212877816000387|7]]
review nature 2012: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3577372/
- Ketogenic diet: Do Lower-Carbohydrate Diets Increase Total Energy Expenditure? An Updated and Reanalyzed Meta-Analysis of 29 Controlled-Feeding Studies - Ludwig - December 2020 - "Calories are not metabolically alike, physiological adaptation to lower carbohydrate intake may require 2 to 3 wk" https://doi.org/10.1093/jn/nxaa350 and https://www.reddit.com/r/ScientificNutrition/comments/k6nafg/do_lowercarbohydrate_diets_increase_total_energy/
- Histamines, and hence anti-histaminics drugs, may affect hunger and the feeding timing: "However, it has not yet been clarified how important a role the brain histaminergic system plays in the regulation of the circadian rhythmicity of feeding. FMH and H1 receptor blockers have been found to stimulate food intake in rats during the light, but not during the dark period and thus to shift the feeding behaviour from night to day [7,59]. It may be speculated that during the daytime the histaminergic inhibition of feeding dominates and therefore histamine blockade has a clear effect. On the other hand, histamine infusion directly into the rat SCN induces a similar change in eating pattern by suppressing the nocturnal food intake [14]. However, the increase of available histamine may [31] or may not [25] change the circadian rhythm of food intake. It is important to point out that the periodicity of feeding is regulated not only by circadian cues but also by the subject’s energy balance [66]. Histaminergic neurotransmission has been implicated in the homeostatic maintenance of energy balance and in the adaptive behaviour to environmental temperature [58,60]. Finally, a clear circadian rhythm in histamine release has been shown in fasted animals [82], suggesting that feeding behaviour cannot be a major factor influencing the rhythmicity of histamine release [46]."
- Gut microbiota may for some reason be lacking essential bacteria to process some kinds of food, this is what is called a gut microbiota imbalance. Unfortunately, research is far from being able to define what is a balanced gut microbiota, given the tremendous complexity of studying the very many strands of bacteria in our guts (with different bacteria colonies in different people). But such digestive issues caused by gut microbiota imbalance can sometimes be improved upon using medical probiotics such as PiLeJe's C10M during a course of 30 days treatment. In the present document's author's experience, this worked wonders. PiLeJe also offers other formulations with various strands of bacteria to solve different kinds of digestive issues.
Conclusion about food, metabolic disorders and circadian rhythm
All the factors laid above interact together, and likely share at least some of the same causes. For example, we saw that food intake when melatonin is high increases glucose insulin resistance, and in parallel that each 1% reduction in carbohydrate intake reduces diabetic risk. Obviously, these two phenomena combine together, and hence both are targets of improvements: by both reducing the carbohydrates intake and avoiding food intake when melatonin is high (particularly carbohydrates!) can significantly reduce the risks, particularly for the carriers of the MTNR1B risk alleles.
Furthermore, a vicious cycle can be created, since the diet composition and timing affects the gut microbiota, which in turns also affects the body/peripherial circadian clocks. The gut microbiota is so important that sequencing the microbiota's organisms DNAs allows to better predict if someone is more likely to die within the next 15 years than the individual's own DNA.
Hence the necessity of timing meals accordingly to the circadian rhythm and carefully design the composition of one's diet, as it appears food is a "powerful environmental cue with the potential to destroy or restore the synchrony of circadian rhythms in metabolism".
Given the strong links between non-24 and diabetes, it is the author's conviction that individuals with non-24 should be systematically screened for (pre-)diabetes, as this can lead to a significant improvement in quality of life and major reduction of long-term metabolic (including cardiovascular) risks by treating both diabetes and non-24. It is also conceivable that a family history of metabolic syndromes (obesity, diabetes, potentially alzheimer disease) may predispose some individuals to develop a circadian rhythm disorder such as non-24.
For future studies, insulin and glucose profiles should be systematically monitored in individuals with circadian rhythm disorders, as this would likely lead to interesting discoveries in this blind spot where data is lacking despite the clear interactions between insulin and the circadian rhythm, and we may draw a parallel with Alzheimer where the central role of insulin is still poorly understood due to a lack of data because of the oversight in insulin monitoring in most studies. It is however not surprising, as it was thought before that the brain was insensitive to insulin, just like it was incorrectly assumed that the circadian rhythm and sleep was solely governed by the brain.
It further makes sense in light of these links that insulin regulating treatments for diabetes and metabolic syndromes may help individuals with a circadian rhythm disorders carrying the MTNR1B genetic mutations. Insulin resistance may manifest as hunger craves, especially of dense (carbohydrate) foods, late into the biological evening or night, which should not happen since the liver and adipose tissues are there to ensure that humans can fast for some period of time, at least during 1/3rd of the day during sleep, and up to several days in case of food unavailability. In these cases, intermittent fasting and the ketogenic diet are effective methods to reduce insulin resistance and improve glucose profiles and reduce hunger, as well as manage weight. Interestingly, a biological study found that calorie restriction can regulate the liver's circadian rhythm (not the central one). The author of the current document tried both primary for entrainment (which did not work) and observed incidentally these positive results on the metabolism. Pharmacological treatments such as Saxenda may also help as some users reported. Contrary to therapies for circadian rhythm disorders, these interventions do not necessarily need to be continued lifelong, the current recommendations advise to use them (under medical supervision) for up to 6 months to see a significant reduction in insulin resistance and hence in hunger cravings, before transitionning to a more diverse healthy diet such as the scientifically designed DASH diet. Note however that in author's experience, although these interventions may ease entrainment, they are not sufficient to maintain it and hence need to be combined with at least light therapy.
If you suspect you may have (pre-)diabetes, you can get screened either at some pharmacies who offer free screening using a glycemic reader , or you can buy a glycemic blood reader by yourself as it cost less than 50 euros. In the latter case, the author recommend the Ascensia Contour Next One reader, as it is inexpensive, require little blood and can connect in Bluetooth to a smartphone to store and graph past measurements and hence better track the evolution of glycemia over time. If the app or reader gives you a red alert, it's an indication you should do an OGTT test for diabetes at a clinic.
Sleep disruptions and sleep fragmentation
This section presents other factors that can impact various sleep parameters besides the circadian rhythm, along with tools to either reduce the impact of these factors or leverage it to improve the management of various aspects of circadian rhythm disorders. The most commonly affected parameter of sleep quality besides the circadian rhythm in practice appears to be sleep fragmentation (also called sleep disruptions), with sleep fragmentation affecting all aspects of health independently from other factors, and so this is the parameter that will be most discussed here.
More specifically, some researchers suspect that (cognitive) arousals are the primary mechanisms by which sleep fragmentation affect health. Indeed, "arousals, irrespective of the underlying mechanism, impact heart rate, blood pressure, and cardiac hemodynamics acutely, but, when frequent, may also disrupt the circadian rhythm of the CV system, which is associated with unfavorable metabolic profiles, such as higher blood pressure, dysregulated blood lipids, and insulin resistance,” according to the researchers, who found that sleep arousal burden was associated with all-cause and cardiovascular deaths in a demographic study over 8001 people with overnight polysomnograms (PSG) (see here and here). This study is notable as the authors had access to the PSG data for all the studied individuals, so that their assessment of night-time arousals / sleep fragmentation can be assumed to be very reliable.
Environmental sleep disturbances: bright light and auditory noise (use an eye mask, ear plugs and sleep earmuffs)
Environmental sleep disturbances can have a very strong detrimental effect on sleep efficiency, sleep quality and the circadian rhythm. Two major sources of sleep disruptions are unwanted exposure to bright light during the circadian night, and audio noise.
Unwanted exposure to bright light is obviously the worst offender, as it directly affects the circadian rhythm and releases cortisal, and its effects carrIes over several days after the disturbances happened due to photic history. This leads to an increased sleep fragmentation, with more periods of unconscious wakefulness, also called cortical arousal, which increases the risk of premature death and cardiometabolic diseases (see also here). This increase in risks is likely mediated by the increase of cortisol, the hormone of wakefulness and stress, which is released when exposed to bright light, and which explains why it is very difficult to continue sleeping or to fall back asleep after being exposed to bright light (but only after being in darkness, since it's the contrast/transition from darkness to bright light that triggers the neuronal circadian machinery). Delirium is a common multi-factorial disorder that can appear in critically ill patients of any age in hospitals and is associated with worse outcomes, hence studies try to determine what factors increase or decrease its likelihood (this should not be confused with age-related dementia). Sleep and circadian disruption is one of the primary factors increasing the likelihood of delirium in hospitals' intensive care units' (ICU) patients, along with aberrant bright light exposure (see also here and here), with some scientists even suggesting that the expression of delirium is very similar to sleep deprivation, although the results of interventions remain mixed. Interestingly, an interventional study found that hospitals' rooms with a window did not improve delirium rates compared to rooms without a window, however exposure to natural sunlight was associated with a significant improvement. Sleeping with room lights on is associated with a reduction in deep sleep stages duration, more sleep fragmentation and a persistent effect on brain oscillations that are implicated in sleep depth and stability.
The other offender, audio noise, is less investigated but is a very prevalent environmental pollution, especially in urban areas, just like artificial light pollution. An inverventional study found that a nocturnal sound-reduction protocol significantly reduced dementia rates and the use of sleeping drugs, even though sleep quality remained unaffected, but it's always worth keeping in mind that sleep quality is subjectively measured from questionnaires, this is different from sleep efficiency that is assessed with actigraphy. Another study found that there was a "striking correlation" between the rate of nocturnal announcements in a hospital ward and cardiac arrests and ventricular ectopy, in line with other studies on atrial fibrillation finding an increased risk of cardiac events for individuals with frequent night awakenings regardless of whether they had sleep apnea or not. A systematic review on the effect of noise on ICU patients was inconclusive since studies protocols have been widely varying and hence incomparable, although they found that most of the sleep disturbances remained unexplained by any of the studied factors. An earlier prospective cohort study found several factors that can increase delirium rates in ICUs, including the absence of visible daylight. In a mice study, aircraft noise was found to increase vascular and cerebral oxydative stress. Finally, the biggest amount of data available on the impact of noise on human health comes from studies of traffic, trains and airports activity impact on residents. In 2018, the WHO released environmental noise guidelines for the European region based on their systematic review and meta-analysis that states the following:
> Sufficient information was deemed available to quantify the burden of disease from environmental noise for cardiovascular disease, cognitive impairment in children, sleep disturbance, tinnitus and annoyance. The report, based on a limited set of data, estimated that DALYs lost from environmental noise in western European countries are equivalent to 61 000 years for ischaemic heart disease (IHD), 45 000 years for cognitive impairment in children, 903 000 years for sleep disturbance, 22 000 years for tinnitus and 654 000 years for annoyance (WHO Regional Office for Europe & JRC, 2011). These results indicate that at least one million healthy years of life are lost every year from traffic-related environmental noise in western Europe. Sleep disturbance and annoyance, mostly related to road traffic noise, constitute the bulk of this burden. Available assessments place the burden of disease from environmental noise as the second highest after air pollution (WHO Regional Office for Europe & JRC, 2011; Hänninen et al., 2014; WHO 2014b).
An update to the WHO guidelines found that "transportation noise is negatively associated with self-reported sleep". Nevertheless, the evidence for the causal impact of transportation noise on cardiovascular events including strokes and all-causes mortality remains weak, and a review for the UK concurs. However, UK's IGCB(N) found that there were enough new evidence in 2020 to warrant a new systematic review and potential guidelines update. There is some evidence of an effect of environmental noise on cognitive abilities in children and in 45+ years old aged adults.
Since this is a frequent issue in hospitals' ICUs, where the patients are often interrupted by the staff to provide medical treatments regardless of the time of the day or night, several studies looked into potential strategies to reduce the impact of external sleep disturbances.
The two most effective strategies involve wearing earplugs (to reduce auditory noise) and an eye mask (to reduce unwanted bright light exposure). The combination of both was found to be more effective at improving sleep than any alone, and they increased the amount of deep sleep and hence sleep efficiency. A systematic review found that using earplugs even reduces the gravity and occurrences of delirium in ICU (a concrete real-life example of the adage that noise can drive anyone crazy). A controlled clinical trial found earplugs to be effective at reducing delirium already after 48h of admission in ICU. Interestingly, a study found that melatonin is more effective to improve ICU patients' sleep than using an eye mask and ear plugs, but obviously combining would only be better.
While most studies found sound is not a zeitgeber per se, and hence has no strong influence on the circadian rhythm, there is one small sample study of 10 humans which found that auditory stimuli could phase delay the circadian rhythm, as monitored via core body temperature and melatonin sampling, in line with a previous study on primates finding that auditory stimuli were a weak zeitgeber (zeitnehmer) unless the SCN was damaged, then entrainment by sound became more effective (read also this blog from which these references were found). Note however that the participants could watch TV and there was no control group with no auditory stimuli presentation, to assess whether the laboratory conditions may have been inducing a relative coordination effect. In addition, although the circadian rhythm of drosophilia (flies) may theoretically be synchronized by sound, the findings are of this study are quite surprising and hence require further confirmation on a bigger sample and better controlled trial, as there is no evidence apart from this study that the core body temperature of endothermic animals such as humans, whom core body temperature is internally regulated and shielded from environmental factors, could be influenced by sound, one of the most variable environmental factors. This would defy the purpose of endothermic regulation.
Although an eye mask and an earplugs can't directly entrain your circadian rhythm, they are essential to avoid disturbances from messing with your sleep and hence your entrainment. Indeed, as a rule of thumb: you can't stay entrained if you are sleep deprived.
What eye mask to choose? A silk black eye mask is inexpensive ($10) and very comfortable, use that in most cases. For those who have no curtains whatsoever or who are super sensitive to light and don't mind being less comfortable (or if you are on a road trip for example and you have no way but to be directly exposed to sunlight in the morning), the 3D black eye masks can be better suited, as they are rigid and hence completely opaque, but they are less comfortable and also a bit more expensive (but still very affordable, like $40).
Here is a simple silk eye mask:

Having an eye mask is an easy and portable solution, but not sufficient to completely shut down light during the day. Hence, it must be complemented with opaque, so-called "blackout" curtains.
Eye masks filtration efficacy (ie, their ability to blackout sunlight) is drastically improved by simply cutting in the middle to make a DIY nose hole. This improves comfort by leaving the nose unpressured by the mask and also reduces or even eliminate the opening around the nose from which sunlight can still pass through. This also improves the fit as the eyemask can then be placed lower, with the upper side resting above the eyes, as indeed the eyemask should not apply any pressure on the eyes but only on the forehead. Furthermore, the attaching band can be tightened below the occiput of the back of the head, which prevents the eyemask from slipping away during sleep. This was tested on cheap $3 AliExpress sleep masks, and this simple modification made them into the second most effective and most comfortable blackout tool the author of the present document could test after the Hibermate (see below).


A more expensive alternative is the Manta Sleep Mask, but the attaching band is uncomfortable. A cheap and potentially as effective alternative is to use "whole-head" eyemasks such as Medcosa Cotton Sleeping Mask, that wrap around the whole head and on top of ears, with the added benefit of maintaining in-ear plugs in place:

The efficacy of blackout curtains can be improved. But do NOT put cardboard or black plastic behind your window to curtain it, because this will accumulate temperature in the window's glass and ultimately break it! Prefer to use an eye mask. If you really want to curtain your window, you can try a light reflector / windscreen shade reflector or using aluminium foil on outwards to reflect the light and temperature, and then add a black tape on the inside layer, which is a technique reported to be effective by a Discord member, but be warned the author did not test this and there are risks this may break your window: https://www.amazon.co.uk/SILVER-FOLDING-WINDSCREEN-SHADE-REFLECTOR/dp/B004VMORXE
What type of ear plugs to choose? There are essentially two types of ear plugs: inner ear plugs and outer ear plugs. The issue with ear plugs is that they need to be comfortable enough to stay the whole duration of your sleep. In my experience, it seems most of the time when an ear plug falls, it's because I unconsciously removed it because of itchy ears.
How to avoid itchy ears with ear plugs? Inner ear plugs block the inside of the ear canal and this often causes a proliferation of bacteria or fungus, which likely produces itchy ears. If you don't want itchy ears, then the only way is to avoid that is not put anything in the inner ear. Luckily, that's exactly the purpose of outer earplugs. They are more difficult to handle so you have to learn how to use them, and they reduce a bit less the noise, but they are much more comfortable for sleeping. Plus, if they are in silicon, they are washable with soap and water, so they are reusable for about a week. Just make sure to NOT put the outer ear plugs inside your ears, always keep them mostly on the outside. Examples of outer ear plugs in silicon that I tested: Mack's silicon ear plugs or Medigrade silicon ear plugs.
![]()
Also, if you use outer earplugs, don't worry about accidental events: they don't mask alarms or very loud noises, they just tone down, so you will hear your alarm clock in the morning and hear the siren if there is a fire, it's just so toned down that you can choose to ignore it and go back to sleep if you want to. So the sound is less filtered than with a typical inner ear plug, but the advantage is that they do not get itchy and it's highly comfortable to sleep with outer earplugs compared to inner earplugs.
Unfortunately, outer ear plugs work by warming them via friction to stick them to the outer ear. But during winter, the cold temperature prevent effective sticking. In this situation, prefer to use inner ear plugs, especially the Howard Leight Laser-Lite (Honeywell 3301105) soft foam earplugs which are extremely comfortable, although not as much as outer ear plugs, since unfortunately any inner ear plug is bound to cause itchiness at some point, but out of all the inner earplugs the author tried, the Howard Leight Laser-Lite are by far the most comfortable, 2nd to the Medigrade outer ear plugs.
 earplugs.jpg)
Worth noting, bone conduction headphones can be used in combination with ear plugs, since the sound pass through the skin and bones directly without involving the ear canal.
Avoid using in-earplugs during the day to listen to your computer or phone, as obstructing your ear canals during the day increases the likelihood of bacterial proliferation and humidity in your ear canal which in turns will increase the likelihood that night-time earplugs will feel very itchy. Instead, use a bone conduction headphone, which will allow your ear canal to be free of any obstruction during the day, so that you can use in-earplugs during sleep. The leader in bone conduction headphones is Shokz (ex-AirShockz), but there are cheaper but qualitative alternatives such as WANFEI BS01. Good quality bone conduction headphones are completely silent to the surroundings and hence can be used at night time without bothering others. They are also very hygienic, usually long battery (8h of continuous use), very light (easy to forget we are wearing them) and comfortable (no ear canal blockage so there is absolutely no discomfort in wearing them for very extended periods of time). Pro tip: when storing, keep the legs crossed to preserve a good grip around the head over time. Pro tip2: Good quality bone conduction headphones should provide sound quality similar to other types of headphones, not less. In practice, sound quality depends on: 1) choosing stereo quality input on the computer or phone instead of the hands-free mode, 2) good skin contact (ie, avoid a single ear headbhone, prefer headphones with an arch that goes around the head and goes over both ears), 3) good placement either close to the ear (ie, on the tragus) or a little further to superpose above the ear-drums (with experience we get the habit of placing them instantly on the correct spots). Pro tip3: properly working bone conduction headphones should transmit audio to your ears even when your ear canal is closed (eg, by fingers or earplugs), fake ones won't (ie, pseudo bone conduction headphones that do not offer proper skin contact or uses normal speakers instead of vibrations optimized speakers).

To improve the stability and hence efficacy of the ear plugs, it is possible to also wear a sleep headband. The sleep headband, worn on top of in-ear plugs, provides significant benefits, 1) greater stability of ear plugs by preventing friction between the ears and the bed sheets while tumbling around during sleep, which is the most common cause for ear plugs unwillingly detaching during sleep, 2) if the sleep headband has a Bluetooth module and integrated speakers, it can also double as a white noise machine and hence increasing the auditory noise isolution, by using smartphone apps such as the opensource Noice on Android. Sleep headbands are inexpensive, the current document's author highly recommend getting one, as it unexpectedly provides an impressive improvement in stimuli isolation and hence sleep quality when combined with a sleep mask and ear plugs.
Warning: Avoid overlapping the sleep headband with an eye mask, as although it can increase a bit the "blackout" and stability of the eye mask, it also increases the chances of both of them slipping away while sleeping. Indeed, since the sleep headband sits on the top of the head and usually partially on hair, and since the sleep headband has a lot of surface all around the head which can come in contact with bedsheets, the sleep headband can easily slip away while sleeping, and when it overlaps the sleep mask, it also drags the sleep mask away too; prefer instead to cut a nose hole for the sleep mask to be able to wear the sleep mask lower on your face and avoid overlapping both). Here is what should not be done (overlapping a sleep headband over a sleep mask):
Alternatively, a tight covering cap ("bonnet" in french) can be used as a sleep headband, although less comfortable than a dedicated sleep headband.
For traveling, it's possible to drastically improve sleep quality by wearing just ear plugs and a bonnet, which are weightless and small enough to be carried anywhere in a vest pocket.
Very low profile sleep earmuffs are another portable noise filtering solution. As of 2021, only the Hibermate brand, which started in 2014 as a kickstarter crowdfunded project, manufacture such earmuffs with a low enough profile to sleep. In particular, we recommend the Hibermate gen 4, with rigid earmuffs casings, as they are more durable and allow to replace earpads and fit bigger ears, contrary to gen 5's smaller and malleable casings. They can be used as alternatives to in-ear plugs for increased comfort, or in combination for increased sound filtering, with the advantage that the earmuffs maintain the in-ear plugs in place. Since they are a small company, during the pandemic, they are only available from their own website, shipping from USA, although before there were imports available on Amazon in other countries. The cost is about 50 euros without shipping, whch roughly equals 6 months of daily use of in-earplugs, with the advantage that the Hibermate can be reused for potentially longer. There are sometimes copycat products such as GH Dynamics sleep earmuffs.
After systematic daily use of the Hibermate Gen 6, the author of the current document highly recommends the Hibermate sleep earmuffs, either as standalone or in combination with in-earplugs, as they effectively filter noise while being comfortable enough for side sleeping. The author consider the Hibermate a necessary tool to sleep a long duration, along with in-earplugs and an eyemask (included in the Hibermate).
In practice, the effects of sleeping with a Hibermate are:
2) even if the filtering is less than in-ear plugs, the sound is "bottled" and sounds like it is from further away than it really is and it significantly dampens explosive sounds, which makes sound noises much less likely to impact sleep,
3) in combination with in-earplugs, it greatly helps keep them in place, as otherwise they have a tendency to inadvertently slip away while sleeping due to friction with bed sheets.
However, there are a few aspects that are uncomfortable with the Hibermate ear muffs:
2) the earmuffs are so effective at isolating that they also block air movement, which causes the same uncomfortable air pressure feeling in the ears as in planes. A simple solution is to lift them up again and lowering them down until they get in full contact with the skin very slowly, as to expel as much air as possible. This may require a few retries at first, but over time with experience this becomes automatic. An alternative technique is to apply a slight pressure on the earmuffs plastic exterior with the index, while gently and slowly donning them, so that the skin contact is progressive and a maximum of air is gently expelled, and the slight pressure with fingers on the earmuffs allow to counterbalance the pressure by releasing this slight pressure at the end (which creates more room for the air). This technique takes a bit of training to get right, and often requires a few tries before getting it right, but it often eliminates the issue. Another solution is to wear in-ear plugs simultaneously as the pressure then does not reach the eardrums due to ear canal blockage.
3) again due to the earmuffs isolation from outside air, the ears still accumulate biological liquid even when the earmuffs are used alone, just like when in-ear plugs are used, although with much less itchiness. There is no workaround for this last issue.
4) the attachment system fits very tightly, even when opened at its maximum, which is necessary to ensure that the earmuffs tightly fit around the ears and hence isolate properly. There is no workaround since a tight fit is necessary for this tool to work, but the advantage is that neither the earmuffs nor the eyemask are likely to come off unexpectedly while sleeping, which ensures isolation from both sound and bright light during the whole sleep. Update: after 1 year of continuous, daily use, sometimes twice a day (for naps too), the attachment loosen a lot, so now it's actually necessary to turn one of the earmuff on itself to make the strings cross to tighten the fit. It is still very useful and still isolate quite well, but not as well as in the past.
It is worth noting that the filtering efficacy of Hibermate can be increased by replacing the cushions, as per the commenter Carl Bowen described:
> Product very comfortable but needs improvement with sound reduction using the same tech that Peltor safety ear muffs use for cancelling out sound .
> No ear muffs will ever cancel out sound completely but the materials that are used by the Peltor company for both industrial ear safety and fire arm ear safety
would improve on your design . Not to slam your product because it is a ingenious design and stands apart from any other sleep mask products on the market . A customer on a youtube video changed out your earmuffs cushions and replaced them with the Peltor ear muff cushions and foam padding , which fit perfectly together on your soft plastic ear cups and this improved your product ten fold in canceling out even more sound . I have placed an order from Peltor for there 3M PELTOR x5 Ear Cushions & Pads thru amazon in order to try and
prove if one of your customers on youtube is correct for improving on your product . With al do respect to you and your hard working team your product is very good but could be great once your design is improved upon .
> Best Regards To You And Yours , Thank You Carl p. Bowen .( California Usa )
The comment above likely refers to the three videos (see also here) by Butch Holladay, and especially this last video where he can be seen testing gels-based earpads (3M HY80) which apparently impressive noise reduction capabilities, although he does not advise sleeping on them as they can be uncomfortable, but Peltor standard earpads should be a good fit for sleeping. To find these replacement earpads, search for 3M Peltor Hygiene Kit x5a or Optime: 3M is the manufacturer, Peltor the products line/brand, Hygiene Kits are the kits with just the earpads, and x5a or Optime are the models that are compatible with Hibermate gen 4. He recommends Hibermate gen 4, as they are more comfortable and durable than gen 5, and to use gels-based Peltor earpads with Shannon Cuddle Fleece or sherpa fleece in the interior (the bottom of the earmuffs, where the ears can be in contact with the foam) to make them more comfortable. Both the dampening foam and the gel-based cushion (3M HY80) can help reduce noise. A potential (untested) alternative to 3M HY80 can be the Sightlines Noisefighters gel-based ear cushions and compatible copycats (see also here). After one year of continuous use, it is necessary to change the ear pads anyway, either with the Peltor, or Hibermate offers to buy them separately.
For those who cannot get access to Hibermate products, there are whitebranded copycats, but of course the quality can be expected to be a lot lower.
An alternative solution for traveling to both the sleepmask and the sleep earmuffs is the Wrap-A-Nap travel pillow, but the sound filtering is much less effective and it requires a precise positioning to achieve the sound filtering effect that not everyone seem to achieve. Ostrich pillows are another similar alternative but they are known to leak easily and hence have a very short durability.
For additional noise isolation, a white noise machine can be used. A free Android app, Noice, with a preset, can be freely used to make any phone or tablet or even computer using an Android emulator into an effective white noise machine for free.
Finally, a last alternative is to soundproof the home walls, which is more comfortable, but is more expensive and not portable (ie, not a solution for travels).
Is it worth investing in these tools if you don't know whether noise is actually a factor hindering your sleep quality? Yes, definitely, but first test with the cheapest yet very effective tools available. In general, acquiring a cheap eyemask (cut a nose piece yourself if the eyemask does not have one, as this drastically improve comfort and effectiveness) and in-ear plugs is very inexpensive and they are available worldwide, and they are very effective basic tools to reduce environmentally caused sleep disruptions. After using these two tools for a few weeks, if you notice a reduction in your sleep fragmentation and reduced daytime sleepiness (ie, increased energy levels), then you can consider getting additional tools, such as the Hibermate very-low profile sleep earmuffs.
Beyond sound and light isolation, there can be other nuisances that can cause sleep disturbances, such as floor vibrations due to indelicate neighbors or building works. These other factors should also be attended to, to try to improve them, such as by using anti-vibration carpets and better beds suspensions. However, in some cases, there is nothing that can be done to reduce environmental factors causing sleep disturbances to a satisfactory level. In these cases, moving out to another place may be the only viable solution.
Health conditions, including benign chronic skin conditions such as atopic dermatitis, can also cause chronic sleep disturbances, and adequate management of these conditions for themselves and on sleep is indicated.
Endogenous sleep disturbances: digestive issues
TODO: WIP (need to add refs)
Besides the risk of circadian misalignment, which can be managed using scheduled meals timing / (natural) intermittent fasting during the circadian night, there is also the risk of sleep fragmentation that may be caused by various digestive issues, including even seemingly innocuous ones such as gases due to allergies. At circadian night, the immune system and digestive motility are decreased, as the digestive system and immune system follow their own circadian rhythms synchronized with the central clock (unless there is a pathology, see the section about food timing for more info). This is why digestive issues are more usually likely to happen at night, or when the digestive system restarts early in the circadian morning.
There are lots of different causes for digestive issues, but most are treatable or at least can see improvements and most likely can be mitigated fully for the night, for example by avoiding the triggers close to bedtime, or at worst concentrating meals earlier in the circadian day to allow for digestion to fully complete before going to sleep.
There are however a few usual suspects to investigate, the primary one is food allergy or intolerance, which is often the cause of irritable bowel disease (IBS). A common issue is lactose intolerance, which concerns only 1/4th of kids, but 3/4th of adults, which shows that most humans are genetically programmed to lose the ability to digest cow milk with age.
Less known but likely as common if not more, is intolerance the group of FODMAPs, which is an acronym that stands for fermentable oligosaccharides, disaccharides, monosaccharides and polyols. FODMAPs include lactose intolerance, and is hence a generalization. Often, people who are intolerance or allergic to one category of FODMAP - such as lactose - are also intolerance or allergic to at least one or several other categories of FODMAPs to various degrees. It is however rare to be intolerant/allergic to all categories, but this does happen for some unfortunate people.
To identify allergies/intolerance to FODMAPs, a rigorous elimination diet is the recommended approach, by choosing for a week food that contain no FODMAPs (which can be very difficult, especially since conditioning can change the concentration of FODMAPs - ie, there can be more FODMAPs in a can than in the same food served fresh, and cooking can again change concentration by increasing or decreasing). After one week, digestive issues should have greatly improved if the individual has at least one intolerance or allergy to FODMAPs. Then for one week, introduce food containing if possible only one category of FODMAP and minimal levels of the others, and at the end evaluate how often you had digestive issues. Cycle like this for each category of FODMAPs to identify which categories the individual is allergic or intolerant to.
Once an intolerance or allergy to FODMAPs is identified, the only thing that can be done is avoidance. There is unfortunately no reliable curative treatment available currently to reduce this issue. There are a few studies investigating the role of an imbalance in the guts microbiota, and with some interventions such as external fecal matter introduction to rebalance the guts microbiota, with promising results but this is very experimental and can lead to catastrophical results if not done in a very rigorously controlled clinical environment. The more commonly available alternative being probiotics such as Pileje's but they only contain a few strands (a droplet in an ocean of bacteria, viruses and yeast already present in the microbiota), so it rarely works, but it is safe to try usually so the benefits-risk ratio it very positive even if rare.
Finally, trying another diet, such as a ketogenic diet, may sometimes help alleviate some digestive issues, or reveal some, which can then be better handled.
In general, when it comes to digestive issues, the first and most important step is to identify the issue and the triggering conditions, then the usual solution is to avoid these triggers, especially in the hours before bedtime. After we eat, it takes about six to eight hours for food to pass through your stomach and small intestine, so it is safe to set as a rule of thumb that we want to avoid eating triggering foods at least 8 hours before sleep.
Wakefulness promoters such as long-release caffeine
TODO: WIP
Sleep disorders are also wakefulness disorders as the efficiency of both states are affected in parallel. It is hence common for patients with sleep disorders to get an off-label prescription of wakefulness promoting medication to improve wakefulness, mainly by "turning off" the sleep homeostat (the adenosinergic system, sleep pressure), with the logic that by medicating both the sleep and the wakefulness, the clinician may be able to "guide" the sleep and wakefulness of their patient onto a more healthy pattern. Unfortunately, in practice, this does not work as well as intended.
A commonly prescribed wakefulness agent, usually indicated for narcolepsy, is Modafinil. Modafinil is a drug initially created for narcolepsy, as it has been found to be as effective as amphetamines in promoting alertness but without the addictive high. However, there were initial concerns about it potentially causing overconfidence, although more recent studies have not confirmed this, but it remains forbidden permitted under certain policies such as for air force staff.
Later studies found that Modafinil only produces effects of a similar magnitude to caffeine, which prompted researchers to question the usefulness of this agent, especially given it's much higher cost and risks of side effects compared to the very safe caffeine. In addition, surveying the current scientific and medical literature on modafinil suggest that it has no advantageous effect on the sleep processes beyond affecting the sleep homeostat, just like caffeine (see the chapter on Adenosinergic agents). Hence, it is the opinion of this document's author that there is generally no need to try modafinil over caffeine except in some circumstances (ie, some patients reported more response from modafinil than caffeine, but there are more reports of a similar effect).
Caffeine in coffee usually takes about 15 to 30 minutes to enhance alertness when consumed on an empty stomach, but this can extend to over an hour if taken after eating. In contrast, caffeinated chewing gum, initially developed by the Walter Reid Army Institute of Research, delivers caffeine more rapidly. This gum, containing the same amount of caffeine as a cup of coffee, works by releasing caffeine through the mouth's mucous membranes, leading to increased alertness within 5 to 10 minutes.
Besides these instant release forms of caffeine, there also exist long-release tablets forms for caffeine, which allows to provide a continuous small dosage of caffeine but all day long. These are usually not under prescription and can be bought over the counter as dietary supplements. There is hence very little incentive to study them, so what follows is mostly the author's own experience.
Optionally, and only when freerunning and NOT using bright light therapy, long release caffeine tablets can be useful to promote wakefulness and replace a bit the wakefulness promotion effect of light therapy that is missing when freerunning. In theory, caffeine long release can have 2 benefits: reduce sleep pressure during the circadian day (even if it happens at night) without affecting the circadian rhythm (contray to bright light or melatonin), and increase sleep pressure during the circadian evening and night due to the coffee crash/adenosine buildup and sudden release.
The brand used was Lucovitaal, with 200mg of caffeine per tablet. Usage was to just take one tablet (200mg) during the circadian morning, usually at natural wake-up. The long-release form ensures a constant inhibition of adhenosine and hence of sleep pressure, and the tablet form avoids the diuretic effect of hot liquid coffee. The long release tablet form makes caffeine a pharmacological compound with more stable pharmacodynamic properties, more reproducible and dosable. However, the same usual issue with caffeine are also present here: caffeine tends to delay the circadian rhythm, and for slow caffeine metabolizers (this is defined genetically), then it can stay in the bloodstream too long and carry over into the night and next day.
In practice, this should only be used while freerunning (when phase delays are not an issue). When using both light therapy and caffeine, caffeine can often cause a weird insomnia the night just after intake, with a wide sleep fragmentation gap during the circadian night sleep session, that resorbs after discontinuation of either light therapy (but hence causing freerunning resuming), or long-release caffeine. Nevertheless, it can be a great tool to ensure being able to perform for a whole day, even under sleep deprivation, and reduce the likelihood of random wakefulness drowsiness bouts that is intrinsic to non-24, but it can only be used as a "wildcard" for a single day, with a necessary rest day the next day, it certainly cannot be used on a daily basis (except if freerunning).
How to find adequate dosage for you: if when you take the tablet, your sleep starts alternating one day being able to sleep, but the other hyperactive and weird insomnia-like fragmented sleep, then it means the dosage is too high, and the dosage should be lowered such as by breaking the tablet (but then it may break the long-release, because usually the long-release is done via a special coating, if it gets broken this transforms the drug into instant-release).
Other sleep-wake issues and tips
This section covers various sleep-wake related phenomena, their scientific basis and potential ways to mitigate them if necessary. These are phenomena related to sleep, not sleep parameters per se, and hence manipulating these phenomena will not improve sleep nor circadian rhythm complaints, but can improve other areas in terms of quality of life, or just provide a better understanding of some sleep-wake deviation patterns (biphasic, relative coordination, etc).
Why individuals with non-24 cannot stay reliably entrained? Relative coordination and transient entrainment
Summary: the circadian rhythm freeruns faster when out of phase with the day-night cycle and more slowly, almost frozen, when in phase. This is called "relative coordination" and is due to the effect of sunlight, providing a "free" light therapy. This is unfortunately temporary, and can be both helpful by causing temporary (transient) entrainment but can also cause the opposite, disentrainment for already entrained individuals. But the good news is that a pattern of relative coordination strongly suggests that light therapy is effective for the individual, and so investing into a light therapy device (especially glasses) will be worthwhile.
Even with an effective entrainment therapy, no individual with non-24 can forever stay reliably entrained to a 24h cycle. Why? Because the circadian rhythm period/length changes dynamically all the time.
Indeed, a 2013 landmark study has evidenced two new interlinked phenomena in blind non-24 participants:
- relative coordination, which is the effect of uncontrolled exposure to zeitgebers such as sunlight, which partially and imperfectly synchronize the participants' circadian rhythms, hence the term of "relative coordination".
- transient (dis-)entrainment, which is the observation of temporary slowed down freerunning, sometimes even temporary entrainment (stable sleep and wake up times), followed by periods of temporarily accelerated freerunning. Transient (dis-)entrainment is likely caused by both intrinsic factors (ie, due to the non-24 disorder) and extrinsic such as the relative coordination factor (ie, uncontrolled exposure to zeitgebers).
A common example experienced by individuals with non-24 is to see their circadian rhythm delay faster when they are awake at night ("night-walking") than when they are awake during the day ("day-walking"). The figure below shows that that the daily phase delay of freerunning be more than 2x faster during night-walking ("Fast Zone" in C) than the average at all time and more than 10x faster compared to than during day-walking (the "Slow Zone")!
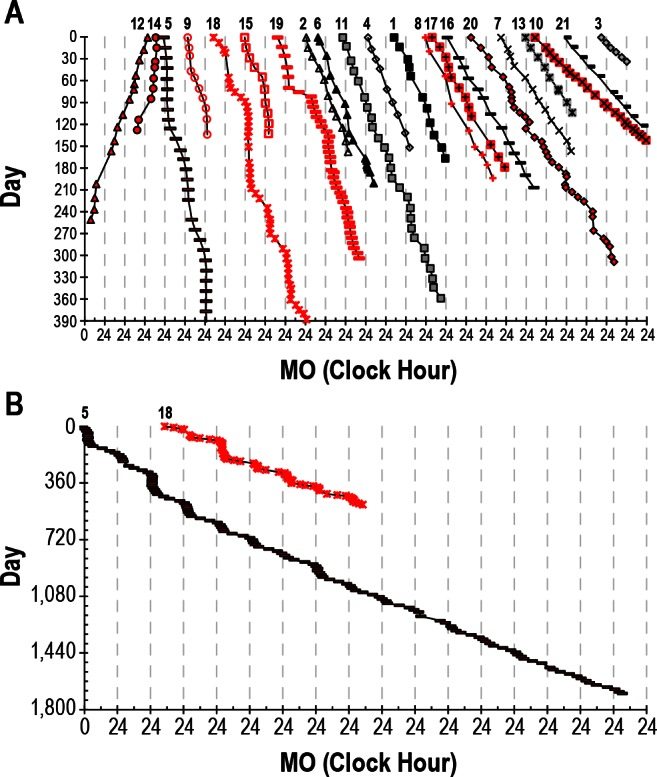
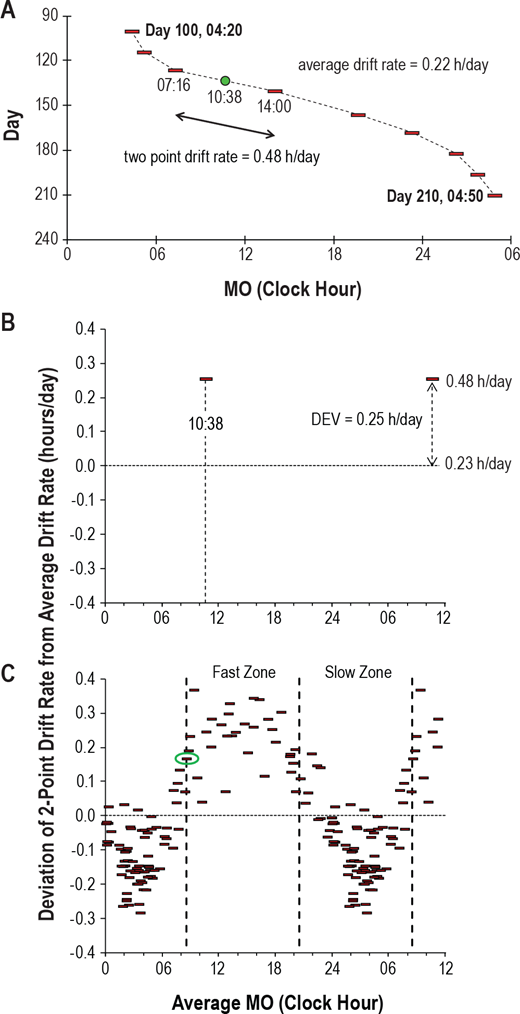
Daily phase delay relative to the DLMO (melatonin onset MO). Figures reproduced from Figure 1 and 2 of this study.
On an individual basis, here is what relative coordination looks like on a sleep diary:
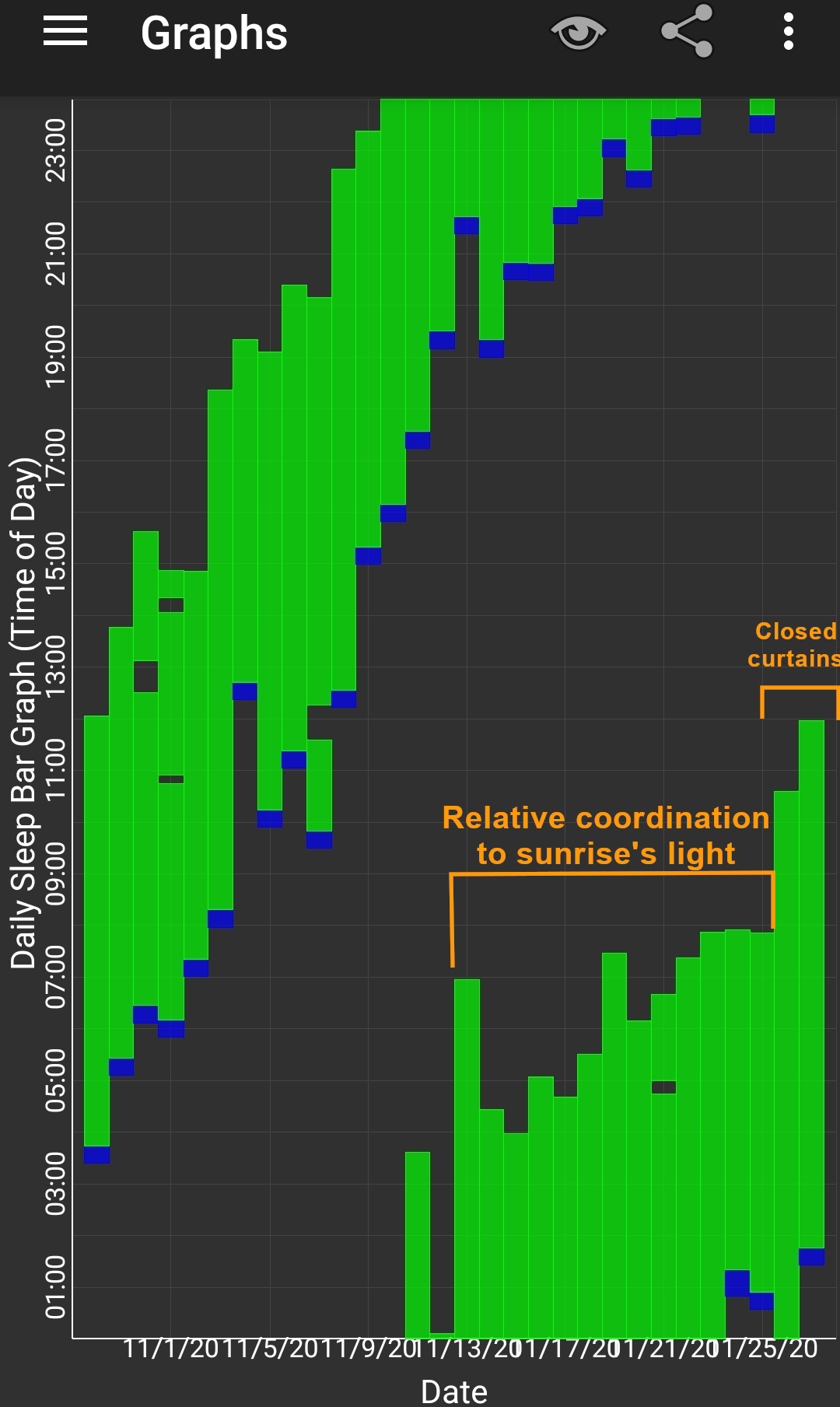
Relative coordination in the sleep diary of a freerunning sighted non-24 individual (kindly provided by our Discord member Pillar, licensed under Creative Commons CC-BY v4). A faster entrainment circadian period and phase delay can be observed in the left of the graph, when the individual was sleeping during the day and awake during the night, and afterward the freerunning period was reduced and nearly entrained to a 24h cycle in the middle of the graph when the individual's circadian rhythm was more in phase with the day-night cycle due to the influence of sunlight. The freerunning restarted with the usual freerunning period when the individual closed the curtains, confirming the transient entrainment was due to relative coordination to morning sunlight. Without closing the curtains, the freerunning would have restarted anyway, as although the wake up time was seemingly entrained, the fall asleep time was not and was still freerunning, as evidenced by the diminishing sleeping duration, which shows the necessity for a more controlled artificial therapy. Relative coordination was similarly observed in several other freerunning sighted non24 individuals on the discord server, showing that relative coordination is a common phenomenon to all non24, regardless of whether the individual is blind or sighted. Update in 2023: since the popularization of the concept of relative coordination, this effect was observed by all members of the online non-24 community on reddit.
This relative coordination to natural zeitgebers such as sunlight explains why individuals with non-24 have a slower freerunning period when daywalking than when they are nightwalking (ie, awake at night), and explains away a lot of false hypotheses, as individuals with non-24 can be misled into thinking that something in their habits (such as sleep hygiene) changed their freerunning period, when it's in fact a naturally reoccurring phenomenon provoked mostly by the phase alignment between the individual's circadian rhythm and sunlight (and other natural zeitgebers to a lesser extents).
This also explains why individuals with non-24 or another circadian rhythm disorder such as DSPD typically find their circadian rhythm more manageable (waking up earlier) during spring and summer than during winter, as the sunrise happens earlier and with more intensity. Indeed, relative coordination is not specific to non-24, but also happens to typical sleepers as evidenced by the seasonal variations in the wake up time (see the section about seasonal variation above).
Note however that in practice, an individual may find themselves getting uncontrollably entrained when their circadian rhythm is in phase with the day-night cycle, but only partially: only the wake up is instantaneously entrained, but not the bedtime which continues to freerun, as previous studies have shown that DLMOff instantly synchronizes to the wake up time and phase advance induced by light exposure, but not DLMOn which takes several days to adjust accordingly, as illustrated by this sleep diary kindly provided by Pillar (look at the top 4 days):
To a smaller extent, the same relative coordination phenomenon is also observed in the seasonal variations of typical sleepers wake up time, but not bedtime which remain relatively constant.
Spontaneous transient entrainment, which means being entrained for no reason but only temporarily, can last not just a few days but more than 3 months in some cases!
> we found three subjects (5, 14, and 18) who demonstrated such “transient entrainment” for a total of 98, 42, and 71 days, respectively, with an average “entrained” linear regression drift rate of 0.00 ± 0.03 h.
Likewise, a non-24 participant who was stably entrained since almost 1 year spontaneously disentrained, despite no obvious changes in environmental nor biological factors!
> Furthermore, later study of subject 15 demonstrated almost 1 year (345 days) of entrainment with a linear regression drift rate of 0.00 ± 0.01 h per day before she lost entrainment with a linear regression drift rate of 0.33 ± 0.06 h per day (Figure 5). There were no known changes in medications, activity, eye status, or hormonal status that precipitated either the spontaneous entrainment or the abrupt resumption of a nonentrained pattern, and the subject drifted at a rate nearly identical to her baseline drift rate of 1.5 years prior."
This highlights that relative coordination and transient (dis-)entrainment is a natural part of the non-24 disorder, so that the patients should not feel ashamed (nor be ashamed by healthcare practitioners) when they restart to freerun after being entrained: this actually is entirely normal and is to be expected even with an effective entrainment therapy. Indeed, this shows that cyclical loss of entrainment for individuals with non-24 is not necessarily due to patient uncompliance nor lack of sleep hygiene (as is commonly assumed in the clinical practice), but that it's a natural part of the disorder.
This is why it is crucial to allow and educate the patient to modulate their therapy's dosage and timing, so that they can react and adapt their therapy according to the natural cyclical variations in their circadian period, such as by doing longer light therapy sessions and increasing melatonin dosage or earlier melatonin pill intake. Indeed, it is not sufficient for an entrainment therapy to be effective: an effective entrainment therapy needs to be flexible and easy enough to adapt by the patient to readapt according to the intrinsic and extrinsic changes in circadian rhythm period. A strict therapy can never be effective in the long-term for non-24 because of this uncontrolled dynamism of the circadian rhythm.
However, not everything is grim about relative coordination, as a sleep diary showing a pattern of relative coordination strongly suggests that the individual is likely responsive to light therapy (artificial and natural). Hence, the individuals that are indecisive due to the cost of artificial light therapy devices but who observe a pattern of relative coordination can wisely be counselled into acquiring a light therapy device.
Given the high variability in circadian period and entrainment status, the study's authors recommend that diagnosis (and therapeutic efficacy of new treatments) need to be assessed over at least 3 months of data to reduce the false positive rate due to transient (dis-)entrainment! In other words, relative coordination and transient entrainment explain why there are so many short-lived miracle cures, and it's hence necessary to test therapies over a longer timeframe to ensure it's neither a false positive (transient entrainment) nor false negative (relative coordination leading to transient disentrainment hiding therapeutically achieved entrainment):
> Perhaps the most important implication of relative coordination and transient entrainment is in the diagnosis of this disorder. Individuals who demonstrate transient entrainment might easily be misdiagnosed as entrained if circadian phase is not assessed for a sufficient period of time. Inspection of Figure 1 indicates that it may be necessary to assess observed circadian phase for more than 3 months in some cases before a conclusive diagnosis can be made.
The authors further state that transient entrainment needs to be accounted for in analyses, although given the difficulty of identifying the factors of relative coordination, it's unlikely that bias can be completely removed:
> It could also be argued that even an overall average drift rate in circadian phase cannot be calculated in the presence of relative coordination or that, at the very least, periods of transient entrainment should be excluded from the analysis. However, we do not think it is possible to edit the data in such a way as to remove the influence of the time cues that were causing the relative coordination because we were not measuring either the strength or timing of those cues and indeed have not even positively identified them.
The authors suggest that transient (dis-)entrainment is likely at least partly caused by environmental time cues (relative coordination):
> There is significant heterogeneity in the physiological presentations of non-24-h disorder in the blind. This variability occurs both between and within N-24s and this likely reflects differences in their exposure or response to environmental time cues. Notably, some individuals demonstrate periods of transient entrainment where the disorder may appear to remit for lengthy periods of time.
Transient (dis-)entrainment reinforces the necessity of the preparatory phase (ie, to wait to be in phase with the day-night cycle first) before starting the entrainment therapy, because this also allows to naturally reduce the daily phase delay, hence the therapy has less to compensate and hence the likelihood of entrainment is improved.
In practice, a clear sign of loss of entrainment due to circadian period lengthening (ie, daily phase delay getting faster) is the appearance of a upward staircase-like pattern in wake up times (ie, waking up later and later), which suggest the need for longer light therapy. Inversely, a downward staircase-like pattern in wake up times (ie, waking up earlier and earlier) is suggestive of circadian period shortening (ie, daily phase delay getting slower) and requires a shorter duration of light therapy and maybe lower melatonin dosage or later timed pill intake.
Transient entrainment may also explain DSPD misdiagnosis instead of non24: if the individual gets diagnosed during the "slow part" of the freerunning cycle, they will likely be diagnosed as DSPD instead of non-24 if the assessment is done over a too short timeframe (eg, a week). To differenciate real DSPD from misdiagnosed non24, we could chart the sleep over months to see if there are cyclical chaotic sleep periods lasting a few days to a few weeks, if correct then these periods are indicative of misdiagnosed non24.
Transient (dis-)entrainment, as in intrinsic issue to the non-24 disorder, compounds with extrinsic difficulties: very few treatments are currently available, no self monitoring tool are available for circadian rhythm, poor understanding of how sleep and the circadian rhythm works and hence the optimal conditions for the treatments (how long? how much? when?). This all makes this disorder very non trivial to treat.
Of interesting note, another study reused the concept of "relative coordination" but slightly differently: instead of hypothesizing a partial entrainment of the circadian rhythm to zeitgebers, the authors here qualify the chaoticity in the sleep patterns of non-24 individuals with a restricted sleep schedule which causes the homeostatic sleep pressure to continuously expand and contract and hence "relatively coordinate" in a chaotic fashion with the circadian rhythm.
When things go wrong
If the therapy works and your circadian rhythm gets entrained, in practice slip ups or external disturbances can still derail your therapy. What should you do then? This section covers a standard protocol to follow that was found by the author to be the most effective to get back on track quickly, and then some of the common scenarios and how to react optimally will be discussed.
Standard reentrainment protocol: this is the protocol that has shown the most effectiveness to reentrain the author's circadian rhythm in case of spontaneous or externally caused disentrainment. The reentrainment usually completes under a week and has been used to phase advance up to -6h.
- The top priority is to avoid and recover from further sleep deprivation and recover any sleep whenever you can, especially by long napping whenever you can (ie, for 2+h) and as early in the day as possible (ie, so as to leave some time for sleep pressure to build up before the next night), try to go to sleep whenever you feel tired the next night, etc. The only solution to the lack of sleep is to sleep, there is no replacement.
- But don't stay in bed all day either if you can't sleep! Wake up, do some stuff and allow yourself to come back to bed for a nap whenever you feel like it.
- Sleeping and napping is essential because sleep deprivation reduces the effect of the zeitgebers and hence of the entrainment therapies, as well as make sleep more chaotic by disrupting the sleep homeostasis.
- Light therapy and caffeine and others psychostimulants can boost your energy levels, but it can not replace sleep, so if you are sleep deprived, you will still feel sleepy, no matter what. Hence, it can happen that you feel the urge to take a nap during your light therapy session if you slept a short/half night prior, that's normal and if you can afford a nap then do it, this will not shift your circadian rhythm (just reduce your sleep pressure but there are ways around and napping allows to reduce dopamine levels which can prevent sleeping, so all in all napping does not impair the ability to sleep the next biological night, and it reduces sleep deprivation in any case so that's a win-win).
- Adapt the timing for melatonin to a later time relatively to your new (later) wake up time. The timing will be readjusted to an earlier time when reentrainment will be completed.
- Use light therapy for a longer time than usual (eg, if 3h were needed for entrainment, use 4h during reentrainment). This will allow to force the effect of light therapy to kick in faster, as well as allow for a greater phase advance that hopefully will exceed the endogenous phase delay (in other words: this should allow to wake up earlier and earlier). Use at wake up. If the sleep is biphasic (ie, sleep a half night then wake up for several hours before going to sleep/long nap again), then use light therapy after the last sleep session. Do not forget to use dark therapy too as usual.
- Try not to eat too late, especially not when you would like to be asleep, as otherwise food will reprogram your peripheral clocks (ie, your body clocks) that it should expect food at night and hence should be awake. If you really must eat, prefer to eat a ketogenic meal (ie, mostly lipids, moderate to low proteins, very low to no carbs).
For other issues, here are common scenarios and solutions:
- If for some reason you had to discontinue using light therapy for some time, let's say 30 min, can you resume after or should you stop? You can continue, this should have little impact on the light therapy. Indeed, pulsed and intermittent light therapy was shown to be as effective (if not more - see also here) than continuous light therapy, although less efficient at inhibiting melatonin. In fact, this was already observed before with bright light therapy for SAD. Hence, if you stop for some minutes and resume light therapy after, you can expect to still get the same phase advance, however the effect on vigilance may be reduced, particularly if you experience drowsiness (brain fog) due to melatonin leftovers (aka sleep inertia).
- If you wake up 1 or 2h earlier, you can do the light therapy. If wake up earlier than that, wait for the usual time to start the light therapy, don't do at wake-up. If the night was too short and you need to sleep again later in the morning (ie, weird insomnia aka biphasic sleep, see related section), then don't use light therapy in the middle, wait for your last wake up to start the light therapy.
- If you wake up later than usual, do the light therapy at wake up and as long as usual, or even longer to compensate. This is to ensure a long enough light exposure (photic history) to prevent biphasic sleep, as waking later reduces the amount of natural light exposure. Since you woke up later, your day will be shorter, so your sleep pressure (homeostatic process S) may not have enough time to build up sufficiently to make you feel sleepy enough to sleep at your usual time, but by maintaining light therapy duration and melatonin intake timing, your wake up time should remain stable and your sleep schedule will stabilize over the next days back to its previous state.
- What happens when you sleep outside of your biological night (eg too late, naps etc) for several days? If was entrained for at least 10 days, the photic history should keep your wake up time constant for several days. After, or without previous entrainment, your circadian rhythm will revert to its natural freerunning delay. Indeed, when the range of entrainment is exceeded, the circadian rhythm reverts back to its natural freerunning period, and sleeping outside of one's biological night (ie, circadian misalignment) is an instance of exceeding this range.
- What if you take melatonin later than you should have? In the author's experiments, it seems this may have a big impact on when you fall asleep, but not the wake up time. You should stay entrained even if you took melatonin later and slept later one or 2 days, but try to take melatonin at your entrained time the next days to go back on track.
- What if you slept too late for some reason but still woke up as usual, and hence are sleep deprived? If you feel like you can go back to sleep (and can do so), then it's actually better to go back to sleep if you feel it's needed. It's crucially important to reduce sleep deprivation to stay entrained (by avoiding dopamine buildup causing the "wake maintenance zones", see below), so sleep at long as you need and as early in the day as possible. This will actually help you fall asleep at the usual time this evening, if you take melatonin at the usual time. Do NOT move the melatonin intake time, i found that keeping it constant works best (when you are entrained of course).
- What if you did not sleep enough or at the wrong time for a few days? Then it will take a few days of sleep a full night and under your biological time (circadian alignment) to clear up the residual sleep deprivation and brain fog, these won't go away the first day you sleep sufficiently, there is some inertia due to the accumulation of sleep deprivation/debt.
- How many days of light therapy or melatonin can be missed? Although it takes about 10 days for freerunning to fully take effect again, missing on light therapy will already wear off entrainment stability and reduce vigilance under just a few days of missing light therapy. As a rule, 1 day is generally ok, 2 days is risky, more is asking for troubles, similarly to birth control pills (both playing on hormonal signaling).
How to wake up earlier and earlier
As a prerequisite, it's necessary to be entrained to avoid freerunning. Once your sleep, and primarily your wake up time, is stabilized and hence you are entrained, the following can be applied to adjust the sleep and wake times to an earlier time (instead of having to freerun all the way around the clock).
First, extend the duration of the light therapy session, by adding 1 or 2h compared to what you usually do for entrainment. For example, the author needs about 3-4h of light therapy every day to stay entrained, so that to wake up earlier, the author uses 4-5.5h of light therapy. If you do not use light therapy for your entrainment, then just use 1-2h of light therapy to wake up earlier (but this may take more than a couple of days due to the time necessary for the photic history to settle in, you may have to wait a week or more for the first effects).
Secondly, wait a few days, about 2-3 days. During this time, try to pay attention to when you feel sleepiness and when you wake up. After 2-3 days, your wake up time should suddenly move 1h30 earlier than usual, which is a sign the extended light therapy session was effective to phase advance more the circadian rhythm.
When this happens, you can take melatonin 1h30 earlier as well, to match your new wake up time. The author found that advancing melatonin administration in synchronization (or consecutively) with light therapy was necessary to maintain the new earlier wake up time (although the wake up time foremost happens because of extended light therapy exposure).
Finally, keep in mind that changes in your wake up and fall asleep times happen in ultradian cycles increment of about 1h30-2h, what is called "sleep gates". Hence, it is useless to try to progressively sleep 15min earlier everyday, because then you would try to sleep in-between 2 sleep gates that are 1h30-2h apart, which is not only ineffective but may develop insomnia as then you'll stay longer awake in bed until the next sleep gate comes. Thus, prefer to first use very long light therapy to induce a change in your circadian rhythm first, and then leverage this by paying attention to the sleep gates and try to sleep at the previous sleep gate before the one you usually sleep in. For example, if you usually sleep at 5am, after a few days of longer light therapy you should also feel sleepy at 3h30, and if so, just try to sleep, if it works you'll likely be able to stay entrained at this new time.
Also, pay attention to the 2 major sleep gate, the siesta one in the biological afternoon, about 12h after your usual fall asleep time of your biological night. For example if you usually sleep at 5am, you'll feel a dip in your energy at around 5pm, the time of the siesta. This is very informative as this indicates when your next biological night is likely to happen. For example, if you feel the energy dip at 3.30pm, while usually you sleep at 5am, then this is a good indication that tonight you'll likely be able to sleep at 3.30am instead of 5am (ie, sleeping at the previous sleep gate, since the siesta happened earlier than usually, a clue that your circadian rhythm has indeed advanced).
Once this process worked, you can continue it and repeat until you wake up as early as you wish.
To nap or not to nap?
If you need to restrict your sleep and cannot sleep during your biological night, then the most healthy strategy is to do long naps (shorter sleep than the biological night) but regularly (avoid all-nighters), as nothing can replace sleep.
If the healthier strategies such as following an entrainment therapy or freerunning are not possible in some circumstances, in other words if sleep restriction is required with the unavoidably associated sleep deprivation, the most healthy strategy seems to be to take long naps (ie, at least one ultradian cycle of 1h30-2h) whenever possible. There is some conflicting opinions on this matter, with some cohort studies showing a correlation between long daytime naps and increased cardiovascular diseases risk. However, these studies often fail to account for underlying undiagnosed sleep disorders, as daytime naps are a consequence of such disorders, hence what these studies did is to compare healthy sleepers with people with a sleep disorder, not the isolated effect of nap for healthy sleepers, and it's arguable that people with a sleep disorder would have an increased risk without naps. Indeed, lab controlled studies demonstrated to the contrary that long naps are the most effective way to eliminate detrimental cardiovascular and neurologic health issues due to prior sleep deprivation, whereas short naps or no naps do not. Furthermore, a systematic review found that "participants required approximately 50% of the total time they had been awake to recover from the deprivation period [e.g., 50 h of sleep to recover from 100 h of sleep deprivation]", but the residual cognitive impairments could last for days or even weeks for some individuals. Furthermore, sleep deprivation reduces light therapy effectiveness by reducing the magnitude of the light PRC, so napping can be beneficial contrary to commonly prescribed therapies requiring sleep restriction in combination with light therapy, sleep restriction should be avoided for maximal effectiveness of light therapy through adenosine buildup. Given these evidences, it seems clear that an effective therapy for circadian rhythm disorders should not require further sleep deprivation from these individuals who are already chronically sleep deprived, so naps should not be contra-indicated but instead recommended. Another study on 634 elders found that napping and bedtime were not implicated in chronic poor sleep, concluding that "many commonly held assumptions about sleep disruptions in older individuals are myth rather than reality".
An excellently well-designed lab study compared the effect of napping versus pulling an all-nighter for 40h on humans, and found that the circadian rhythm (estimated from the core body temperature and distal skin temperature) was left unchanged either way. The only difference was that after the all-nighter, the participants felt more subjective tiredness than when they could nap. In other words, napping only reduced the sleep homeostat (adenosine) build up, but had no effect on the circadian rhythm. This demonstrates that all-nighters are no long-term solution for circadian misalignment, as they only allow to more easily fall asleep once thanks to the sleep pressure (adenosine) over-buildup, but after the recovery night the individual will have to either re-do an all-nighter (and suffer from all the health complications of extreme chronic sleep deprivation) to restart the same process, or their sleep pattern will go back to their natural sleep-wake schedule as the body tries to realign it to be in phase with their circadian night. And in fact the results from the study show that the same sleep-wake schedule can be achieved whether the individual naps or not, while incurring less subjective tiredness than when pulling an all-nighter (complete sleep deprivation). This means that chronotherapy should not require sleep restriction. The results of this study contradicts previous research, which is explained, as the study's authors note, by the fact previous studies were less stringently controlled and hence were biased by uncontrolled factors such as posture. Keeping in mind that core body temperature is tightly coupled with the circadian rhythm, another study confirm these observations, finding that sleep deprivation did not demonstrate any incidence on core body temperature whether in a comfortable ambient temperature nor after cold air exposure (see also here and here). Note however that sleep deprivation does mask proximal skin temperature, but not core body temperature.
Some studies argued that long naps may cause sleep inertia (aka brainfog, the difficulty to wake up and with lower cognitive performance for 10 min to a hour after) and hence short naps should be preferred. But subsequent studies found that it's rather the prior sleep deprivation that exaggerates sleep inertia, not the nap duration. Indeed, other studies have shown that short naps can also produce sleep inertia, and furthermore sleep inertia is more frequent when the individual is awakened during a deep sleep stage (stage 3 or slow wave sleep stage SWS) (see a summary here), confirmed the findings of another study that night-time awakenings by disturbances caused much more sleep inertia than sleep deprivation. This can potentially be reduced by using chronobiological alarm clocks (also called "smart alarm clock"), which monitor the user's sleep stages and vibrate when it detects the user is in a light sleep stage, such as Sleep As Android, Sleeptracker Pro watches (discontinued) or FitBit. This study also suggests more precisely that to avoid sleep inertia, the strategy should be to avoid waking up in the middle of deep sleep, which happens usually 25 to 30min after falling asleep, hence either shorter naps (less than 25 minutes) or full naps (one ultradian cycle of 1h30) should be preferred.
Napping is a well known optimal strategy already for shift work (see also here). A 2020 systematic review found that most shift workers practiced daytime napping and caffeine consumption, "in line with best-practice fatigue-management strategies, but contrary to existing sleep hygiene recommendations". Naps allow to reduce sleepiness due to the sleep homeostat S, so that only the sleepiness due to the circadian rhythm is left, which is lesser. In a study of for rapidly rotating shift work schedules, long napping before shifts was found to be an effective strategy to reduce the sleep debt incurred by the necessarily shorter sleep after the shift due to circadian misalignment preventing full sleep. Given the significant reduction or elimination of health issues, cognitive impairements and work errors due to sleep deprivation, a 2020 review concluded that napping while on duty should be allowed for public safety shift workers, in line with a previous systematic review. Another review found that intra-shift naps improved sleep-related performance and reduced sleepiness, contrary to caffeine which improves vigilance at the expense of an worsened sleep quality and duration, which suggests that napping is likely preferable to caffeine both to reduce the error rates and the worker's health. Long napping can arguably be advantageously used for circadian rhythm disorders to improve management by reducing the interacting effect of the sleep homeostat so that only the circadian rhythm misalignment is left to be managed. In fact, the circadian rhythm is what affects more the subjective sleepiness than the sleep homeostat. In terms of productivity, several studies of "assessments of vigilance in monophasic versus polyphasic sleep schedules indicate that performance is comparable given equivalent time in bed (Nicholson et al., 1985; Mollicone et al., 2007; Mollicone, 2008)", which means that what matters is indeed the cumulated total sleep duration of all the sleep periods, including naps, under a circadian period (ie, about 24h), to assess sleep deprivation, which is ultimately what determines decreases of productivity.
Some clinicians argue that napping impairs sleep, especially in children, and hence that napping should be avoided. There is no evidence for this claim. Instead, it was found that children who take daytime naps actually have the same total sleep duration as children who don't. This incorrect information is likely based on improperly designed studies, as most studies on children napping actually did not use actigraphs that could reliably detect naps. Furthermore, it was demonstrated that napping toddlers retain learnt spatial information, whereas toddlers who remain awake forget. Memory consolidation is crucial in the child's development, as it precedes lexical development, and is mostly done during sleep. But sleep also affects semantic development, since infants who napped, but not those who remained awake, could remember 1.5h after the learning event what was the precise word meaning and moreover how to classify new category exemplars they did not see before, demonstrating a capacity for generalization that infants who avoided naps could not. Another study demonstrated that naps promoted abstraction in language learning of infants, another high level cognitive capacity. In fact, a study shown that 3-years-old infants could only remember a visual stimuli, a cartoon face, 1.5h-2h after presentation only if there was a period of sleep/nap in-between, even if short, hence the authors concluding that even short naps are beneficial for infants memory development (and likely other cognitive functions). Chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if from a young age. In summary, sleep including naps is necessary for children's neurocognitive development. Sleep and nap restriction should hence be avoided. It's worth noting that most of the studies promoting sleep restriction on children were using imprecise actigraphs or behavioral questionnaires, which were so imprecise they couldn't record daytime naps.
There are at least two kinds of naps: the skippable ones and the irresistible naps. Indeed, naps can be aligned or misaligned with the circadian rhythm, and this defines the duration of the sleep latency (ie, time to fall asleep) and restorativeness of the nap. If your issue is with feeling the irresistible urge to sleep, in other words the irresistible naps, this is a normal body coping mechanism to recover from chronic sleep deprivation or other homeostatic dysregulations. Hence, the first thing to do is to eliminate chronic sleep deprivation by sleeping adequately and in phase with the circadian rhythm to avoid circadian misalignment. Then, if naps continue to occur, it is possible to use blue light therapy at wake-up to reduce the number of naps. It can also be used after a nap to reduce the sleep inertia. Indeed, even low intensity light therapy (100 mLux) was shown to reduce objective daytime sleepiness as monitored with EEG, with fewer differences with higher illuminance. See also the next sections on ways to make the sleep pattern more monophasic, as having a monophasic sleep pattern means that daytime naps are eliminated.
If the naps frequently happen in the 2 hours after eating, treating reactive hypoglycemia may help, see the section about food above.
What if you have an appointment and can't sleep during your night, should you get a nap or not? Certainly, yes. Whether health-wise or for cognitive performance, keep in mind that some sleep is always better than no sleep (as demonstrated by this study), and that no drug can replace sleep.
If taking naps works well for you (and you have time in your work schedule to do that regularly), then you could even try to do a biphasic sleep (eg, sleeping just after work for some hours, then wake up and sleep a second time late into the night). Biphasic sleep is apriori a healthy sleep scheme, since both monophasic (one long sleep session during the night) and biphasic (two shorter sleep sessions interspesed with awake times) are natural for the human body.
If you use light therapy, you can use it after taking a nap (as long as you are waking up before your circadian night).
A lab-controlled study demonstrated that napping does not alter the circadian rhythm nor the core body temperature, but only the homeostatic sleep pressure process S (adenosine). Hence, whereas the circadian rhythm can be regulated by bright light exposure, homeostatic sleep pressure can be regulated by naps.
It is worth noting that napping is not rare, actually it is estimated that about two thirds of the worldwide population is chronically sleep deprived, and usually take midday naps or prolong sleep during weekends to try to compensate for the social jet lag cost. Note also that napping is commonly practiced in all countries and is not a specific habit in countries with high temperature.
Weird insomnia and temporary phase reversals may be biphasic sleep and circadian siesta
A phenomenon that is not described formally in the scientific literature but is part of the experience of a lot of individuals with non24 is the random occurrence of "weird insomnia". These insomnia manifest as a premature wake-up, followed by another sleep period a few hours later. Here is what it looks like, on night 8/6 (the one with a big gap in the middle and 2 sleep periods of about equal ~4h duration):
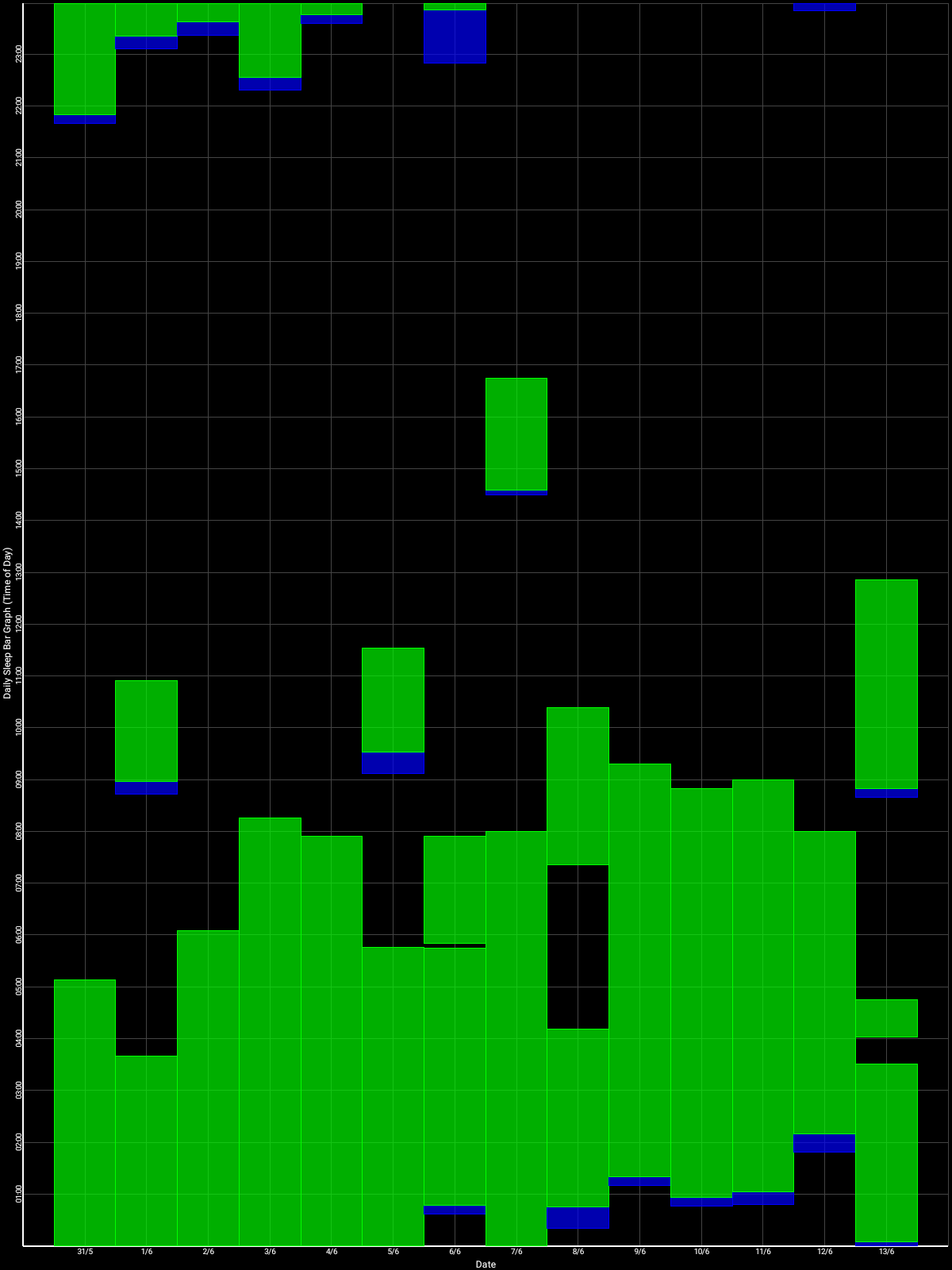
This particular instance happened during my own self-experiment of entrainment, using light therapy (not long), melatonin, dark therapy and food control. All parameters were unchanged with the previous days and all external factors were controlled as much as possible. There was no difference in subjective feelings of tiredness prior to this night. There was no prior sleep deprivation nor oversleeping for a week. And yet, unexplicably, I prematurely woke up too early, and had to sleep 3-4h later to catch up to get a full night of sleep. This seemingly lack of obvious reason for this insomnia is why it is deemed "weird". This description perfectly fits the definition of biphasic sleep.
Naturally, humans do have a biphasic (aka bimodal) sleep as evidenced by the bimodality of the circadian rhythm and core body temperature, "with one peak at the temperature trough and, contrary to previous reports, a second peak 9-10 h later" as studied on 15 individuals with non-24, which translates to the midafternoon nap and nighttime bedtime for typical sleepers. In other words, this means that humans naturally have two sleep gates: one at the biological night, and one at the biological midafternoon ("siesta"). Another study on humans monitoring (rectal) core body temperature determined that the siesta happens at the 12h harmonic of the circadian rhythm, in other words the siesta is about 12h apart from the start of the circadian night (ie, natural fall asleep time). This midafternoon trough in the circadian rhythm and core body temperature which underlies the siesta coincides with an elevation of peripheral melatonin produced by the digestive system after the lunch. Although the circadian siesta is often called the "post-lunch dip" or the postprandial sleepiness, it happens even without eating and it does not necessarily happen in the afternoon, as it depends on the individual's circadian rhythm, so that individuals with DSPD or non-24 can observe their dip happening (much) later depending on their current circadian rhythm phase. This secondary sleep gate can of course be detected by monitoring the core body temperature but it can also be detected using wrist skin temperature and is independent of feeding. The biphasic nature of the circadian rhythm was already known since at least 1981. In fact, the bimodality of the circadian rhythm was suspected as being a major factor of insomnia when combined with a phase delay since 1987, with Czeisler et al's work providing a strong link between the two seemingly disparate disorders. Food timing does not appear to affect the timing of the dip, although it varies greatly on its own from day to day.
Although the circadian rhythm and core body temperature profile are biphasic for all humans, regardless of their sleep pattern, this does not mean their sleep pattern necessarily is biphasic. So, what factors can make the sleep pattern into biphasic or uniphasic?
The strongest evidence we have of a major factor is that both biphasic and uniphasic sleep can be induced in the same individuals by varying the exposure duration to bright light: biphasic sleep can be naturally induced by a too short exposure (10h) to bright light during the awake period, and eliminated (ie, uniphasic sleep) by a longer light exposure (16h). Hence, both monophasic (one long sleep session during the night) and biphasic (two shorter sleep sessions interspersed with awake times) are natural sleep schemes for the human body. But this suggests that longer exposure to circadian-effective light during the circadian day should improve sleep parameters, such as during the summer, and this is indeed what studies such as by Figueiro et Rea found. Although all humans can likely achieve a biphasic sleep, biphasic sleep propensity may be a genetically inheritable trait similar to the siesta which was found to have a 65% heritability.
Biphasic sleep was historically considered healthy and even recommended by the medical community at the time (see also here and here). Nowadays however, the modern medical community rather advise a single continuous sleep period, called uniphasic sleep, not so much for health reasons but rather because this is more adequate to fit in current societal norms of office hours for work, hence, a ideological and political motivation for this shift in recommendations. Some scientists claim that the human sleep is naturally biphasic as evidenced by the biphasic nature of most of its rhythms, from circadian to physical and cognitive performance rhythms. Whether biphasic sleep is as healthy as a single continuous sleep period remains unknown since studies are lacking on that question, but since biphasic sleep can be naturally induced depending on the duration of light exposure, this suggests biphasic sleep is a natural bodily adaptation mechanism and hence likely serving some biological purpose. Furthermore, some scientists even hypothesized that monophasic sleep may be an unnatural state of sleep caused by the sleep restriction intrinsic to capitalism's ethos of maximizing labor's productivity:
> Not least, that sleep in humans and other animals is often not a single consolidated block of sleep but can be ‘biphasic’ or even ‘polyphasic’ with two or more periods of sleep separated by short periods of wake [45,46]. How such fragmentary sleep is generated is uncertain and will require additional inputs to the model depicted in figure 4.
> [...]
> Significantly, and as mentioned above, a single period of consolidated sleep (monophasic sleep) may not be the ‘universal state of sleep’, and could represent an artefact of a shortened night, and greatly compressed sleep. Biphasic sleep (sleeping during two periods interrupted by wake) or polyphasic sleep (multiple sleep/wake episodes) is the normal situation for most animals, and may have been for humans before the Industrial Revolution [184–186]. Although there is no universal agreement [187], the original concept that the natural state of human sleep is polyphasic was partly developed based upon human historical research [188,189], and therefore provides a good example of how historical studies can inform contemporary science. Indeed, laboratory-based studies subsequently supported the idea that human sleep is polyphasic [45,190]. This raises the important point, that if the natural state of human sleep is indeed polyphasic, then we need to re-think our interpretation of ‘disrupted sleep’ at night.
Source: https://doi.org/10.1098/rsfs.2019.0098
We have established above that the circadian rhythm is naturally biphasic, and that both biphasic and monophasic sleep patterns are natural for humans depending on bright light exposure duration and genetics, and that there is no established health advantage for one or the other pattern.
This is pertinent for the non-24 disorder, as it is possible that, due to the aberrant bright light exposure of individuals with non-24, a biphasic sleep pattern may spontaneously appear, superficially randomly, but if we account for bright light exposure (eg, with a necklace sensor), we would see that these "weird insomnia" nights may be highly correlated to a previous day of short exposure to bright light, and hence a higher likelihood of a biphasic sleep pattern emerging after.
Although this remains untested for non24, the "weird insomnia" or biphasic sleep can potentially be improved in a few ways:
- by using blue light therapy and by increasing the duration of blue light exposure, eg either by being exposed longer to natural sunlight, or by using very long light therapy using light therapy glasses or lamps for several hours. Given the result of the previously mentioned study, this is the most logical step to take to try to rtransition from a biphasic sleep pattern into a monophasic one, if this really is the cause of the "weird insomnia" phenomenon. In addition, photic history works by modulating melatonin profiles, with blue light increasing melatonin levels more than other colors, similarly to taking prolonged release melatonin pills, hence the increase of melatonin secretion may be one of the reasons why sleep becomes more monophasic with longer exposur edurations. Indeed, blue light therapy has a paradoxical effect: although it inhibits melatonin during light exposition, prior light exposure increases the amount of produced melatonin the following night, what is named photic history. The effect is more pronounced with blue light than amber light. An EEG study on humans demonstrated that the use of blue enriched light therapy increased performance and reduced the brain wave frequencies associated with sleepiness during the circadian siesta, and other studies found similar results, such as a study on undegrad students finding that blue light enriched light therapy relieved post-lunch dip impairments in mood, subjective sleepiness and performance when compared to normal indoor lighting. However, blue light exposure needs to be properly timed in phase with the circadian PRC curve, otherwise it can further increase sleep fragmentation if mistimed and hence the propensity of "weird insomnia". Blue light exposure at wake-up should be combined with dark therapy in the biological evening since it was shown that light therapy in the morning is less effective if an individual is exposed to light in the previous evening or night due to photic history. Another study shows that low intensity light therapy (100 mLux) is sufficient to reduce objective daytime sleepiness as monitored with EEG, with fewer differences with higher illuminance, which shows that focusing on high intensity is fruitless, timing and duration are more important.
- by using melatonin pills to consolidate the 2nd half of the night, with likely more efficiency for this purpose with prolonged-release melatonin such as Circadin. This increases the melatonin levels in the bloodstream throughout the night and hence the chances that the person stays asleep.
- Napping during the dip. Although it may be inconvenient, if possible it should not be avoided, as studies have shown that undegrad students who avoided post-lunch nap had a significantly decreased performance, increased subjective sleepiness and negative mood, hence, sleeping during the dip improved performance. Another study shown that sleeping before post-lunch dip also improved performance and suppressed the alpha brain waves that are associated with sleepiness.
- Avoid alcohol at lunch. In a clinical trial, alcohol given in the early afternoon (post lunch) greatly impaired (x2 to x4) performance and alertness during the following evening, demonstrating a carry-over effect.
- Reduce carbohydrates content, as this exarcebates the biphasic dip in the circadian rhythm.
More anecdotally, during the author's self-experiment, both seemed to help, but very long light exposure combined with melatonin was the most effective compared to 1h light therapy and melatonin or melatonin alone. He also tried to use blue light therapy to "pass over" the dip, which worked but not all the time. In particular, he noticed that when sleep deprived (ie, slept 1 ultradian cycle (2h) or less than what was optimally needed, eg, sleeping less than 6h when 8h were needed), then the dip was harder to pass over. And it was irresistible if slept only half of what was needed (eg, 4h instead of 8h optimally), then the only solution was to nap to avoid staying in a low performance "zombie-like" state for hours until the dip passed on its own.
Interestingly, biphasic sleep could be a potential lead to demonstrate a link between sighted non24 and light hyper or hyposensitivity.
This kind of insomnia should not be underestimated, as it happens seemingly randomly, is hard to control, and can make the sleep chaotic for several days after despite following properly the therapies. This can be easily blamed on the participant's behavior despite no evidence this is due to behavior, as it happened during the self-experiment despite thorough factors control and high motivation. Instead of targeting the behavior, it would be more advisable to try to increase the light exposure duration and provide prolonged release melatonin.
Biphasic sleep may also explain the regularly experienced phenomenon of a temporary phase reversal, where the individual's sleep schedule seems entrained for some days (usually through the use of alarm clocks), before "flipping up" by 8-12h in one day, then either reverting to the previous sleep schedule the next days or another sleep schedule in-between. This is likely not a phase reversal, but simply the individual sleeping at their biological afternoon trough, in other words taking a long nap, which is a sound strategy for the body to recover sleep if it is unable to sleep during its biological night. Especially for non-24 without an entrainment therapy since the circadian rhythm and hence biological night continues to delay further and further, which makes the biological night totally out of phase with the individual's wished schedule despite the use of alarm clocks, the body then can't have a recovery sleep during the biological night, so then the afternoon nap is promoted.
Hence, biphasic sleep can be hypothesized to be a redundant scheme for the body to get more opportunities for a long recovery sleep: with 2 opportunities spaced throughout the 24h cycle, if the first sleep gate during night is missed, the other sleep gate during the midafternoon will be used. Behaviorally, this can superficially appear as a phase reversal, when in fact this is simply a long afternoon nap, which can potentially be hinted by the observations that the sleep after the phase reversal that happens in the biological afternoon, although long, is not as long (1 ultradian cycle shorter at least) nor of a quality on par with a biological night sleep.
UPDATE 2021-02-25: Unfortunately, although very long bright light therapy seems to reduce the number of occurrences of these insomnia episodes (with a premature wake up in the middle of the circadian night), they still happen, at a rate of about once or twice per week. This happens to both the author of this document, and to his father. Core body temperature shows that indeed this is likely due to a dysregulation in the core body temperature (and hence the circadian rhythm), as the CBT increases at exactly the time of these premature wake ups. This insomnia component was never described before in the scientific or medical literature about non-24, this is a new finding. It was likely not found before due to the difficulty in getting individuals with non-24 to stay entrained for long enough to observe this phenomenon. Here is a sleep log of an entrained non-24 individual showing patterns of "weird insomnia" episodes:
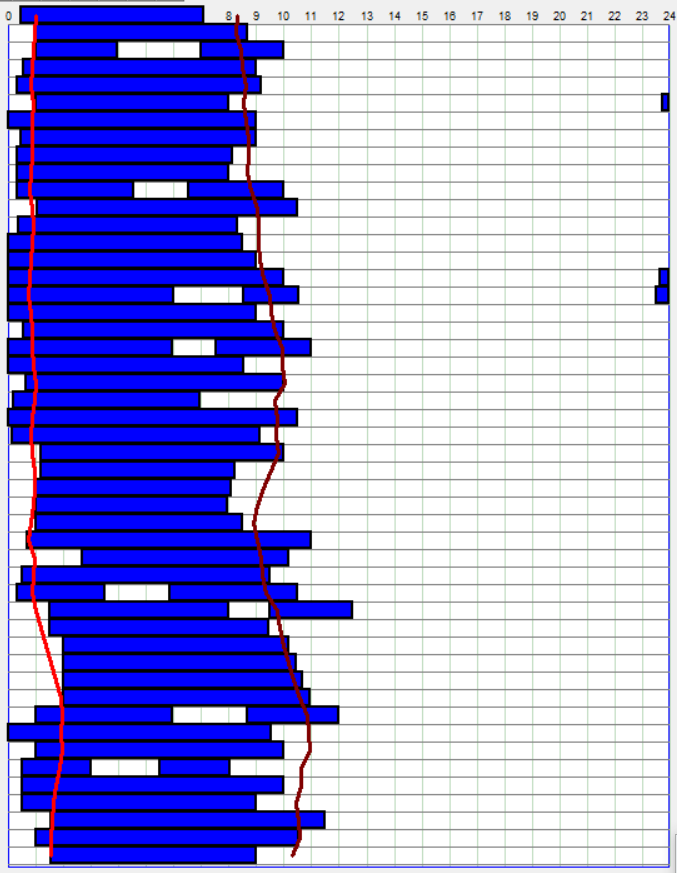
Update as of March 2022: YES, weird insomnia is likely just an expression of an incomplete circadian bifurcation, also called LDLD for light-dark-light-dark! It is incomplete as it requires 1 day with a consistent light exposure schedule, dim light at night, and then 3 days to allow a 12h phase shift. If this hypothesis is correct, then witnessing a "weird insomnia" pattern should cue the patient that they are able to bifurcate and do a 12h phase shift if that is more convenient for them. More information on this circadian waveform manipulation can be found elsewhere in this document.
TODO: a model for the circadian siesta: Modeling napping, post-lunch dip, and other variations in human sleep propensity, 2009 https://pubmed.ncbi.nlm.nih.gov/19294959/
A thread where other individuals with non-24 who reported the same phenomenon.
Note that this is different from polyphasic sleep proponents, although technically biphasic sleep is a polyphasic sleep, usually polyphasic sleep refers to sleep patterns schemes involving very short bouts of sleep (eg, 2h) interspeced throughout 24h with claims of increased productivity, intelligence, health or others. The current scientific consensus as of 2021 is that there is no evidence supporting these claims about the benefits of polyphasic sleep (excluding biphasic sleep, ie a long sleep and a shorter nap), and that adverse health effects are to be expected.
Sleep inertia aka brain fog, mind fog
Sleep inertia, colloquially named brain fog or mind fog, is the time spent with a numb mind at wake up from a sleep or nap session, or more formally, "the phenomenon of low vigilance on awakening even though sleepiness should be lowest at the end of a sleep episode". Sleep inertia is variable between individuals and whether there is a sleep disorder, with direct consequences on general health since "poor sleep efficiency and long wake after sleep onset" increase the risk of cardiovascular diseases.
A functional MRI study by Raphael Vallat, one of the world top researcher in neuroimaging, found that the neurological basis of sleep inertia likely involves a slower switching rate between the internal awareness (consciousness) network and external awareness (task) network (see a summary here). Indeed, when fully awake, our brain switches about every 20s between these two networks (0.5 Hz, although there are multiple timescales), in a balance of internal thoughts and attending to external stimuli. During sleep inertia, this switching rate is significantly slowed down. According to Raphael Vallat, there is not much that can be done to avoid sleep inertia. But there are factors that increase or reduce its duration, especially for those with an underlying sleep disorder that increase the duration of sleep inertia. Indeed, in line with Raphael Vallat's work, sleep deprivation appears to further impair the decoupling of the DMN with the external awareness network, which arguably would worsen sleep inertia. Another team reproduced similar results using Graph Theory. To summarize, at the neurological level, sleep inertia is characterized by a disconnection, or incorrect connection, of the default mode network (which regulates self/internal awareness) with the external awareness network (sensing the environment). In other words, under sleep inertia, the self is disconnected, or at least less connected, from the environment, which mirrors what the patients feel like (a sense of being numb, out of touch with reality).
However, sleep inertia does in fact not stem from neurological changes, but is, as often with sleep disorders, due to bodily and especially thermoregulatory changes. Indeed, an excellently designed lab-controlled study demonstrated that the disappearance of sleep inertia was significantly correlated with distal temperature (vasoconstriction) that happens at the end of the circadian night. In other words, this study found that sleep inertia is ultimately a consequence of the circadian rhythm and its associated core body temperature and distal temperature regulations, with no influence of the homeostatic sleep pressure process S (which means that caffeine cannot improve sleep inertia since it works on adenosine, the sleep homestat's hormone). Furthermore, sleep inertia clears up in direct proportion with the thermoregulatory changes, and since heat buildup is slower in the morning than the heat loss in the evening, sleep inertia takes as long to clear up. Hence, it is safe to assume that increases in sleep inertia frequency and duration are primarily the product of circadian misalignment, and that circadian realignment can reduce it. This hypothesis is strengthened by recent evidence from human case studies finding hyper photosensitizing drugs such as methylphenidate and buproprion to be effective in reducing severe morning sleep inertia in individuals with DSPD. Furthermore, this means that compounds primarily affecting the sleep homeostat but not the circadian rhythm such as caffeine cannot clear up sleep inertia, as observed in night shift workers where caffeine improves vigilance at the expense of an worsened sleep quality and duration. Interestingly, sleep-related thermoregulation shows an asymmetrical pace, with a faster decline and increase of core body temperature and distal skin temperature respectively versus a slower recovery and decline of CBT and DST respectively on wake up, similarly to ratings of subjective sleepiness and hence of sleep inertia. This means that sleepiness kicks in faster before falling asleep than it wears off when waking up, which has interesting implications for people with a circadian rhythm disorders as it means that planning when one will fall asleep beforehand can be tricky without a routine, which is impossible for individuals with a non-24 disorder.
Sleep inertia is at its highest at wake-up. The duration and intensity of brain fog correlates with past sleep deprivation (see also here). Sleep fragmentation can also cause sleep inertia, as night-time disturbances caused much more sleep inertia (aka brainfog) than to sleep deprivation, and particularly when the disturbances happens during the deep (slow wave) sleep stage.
Indeed, premature awakening from the deepest sleep stage N3 induced the most sleep inertia, which suggests that chronobiological "smart" alarms that monitor the user's sleep stage via actigraphy to wake them up when they are in a light sleep stage can be helpful to reduce sleep inertia. This is indeed the current document's author's experience, as he used chronobiological alarms for a decade to complete his university degree. The most effective likely are wrist-worn alarms, as they are not only more accurate in detecting sleep stages by actigraphy, but also because they can vibrate instead of ringing an auditory alarm, with somatosensory stimulation being arguably more effective than auditory stimulations to wake up an individual. However, sleep inertia is not dependent on the sleep structure prior to awakening.
Higher doses of melatonin may cause drowsiness just after and up to the next morning. This can be reduced by reducing the dose of melatonin.
The blood-brain barrier and the equilibrium of metabolites in the brain are regulated by the circadian rhythms and sleep. Indeed, sleep increases endocytosis and the transport of metabolites in and out of the brain, and when this is inhibited, there is an increase in sleep propensity, which may potentially be one of the biomechanistic causes of sleep inertia.
Some studies argued that long naps may cause sleep inertia (aka brainfog, the difficulty to wake up and with lower cognitive performance for 10 min to a hour after) and hence short naps should be preferred, but subsequent studies found that it's rather the prior sleep deprivation that exaggerates sleep inertia, not the nap duration. Indeed, other studies have shown that short naps can also produce sleep inertia, confirmed the findings of another study that night-time awakenings by disturbances caused much more sleep inertia than sleep deprivation. This study also suggests more precisely that to avoid sleep inertia, the strategy should be to avoid waking up in the middle of deep sleep, which happens usually 25 to 30min after falling asleep, hence either shorter naps (less than 25 minutes) or full naps (one ultradian cycle of 1h30) should be preferred. Furthermore, humans also have a natural tendency for biphasic sleep, ie, for a nap in the middle of the day, usually named the "siesta". The siesta is governed by the circadian rhythm, which itself is biphasic, with a trough at around the middle of the day (midafternoon), which coincides with the decrease of energy concomittant with an elevation of melatonin after lunch and with the siesta. The siesta can be eliminated by getting exposed for more than 10h to bright light.
As is well established with jet lag, sleeping in circadian misalignment is also another factor that can increase the duration and intensity of sleep inertia.
If you experience brain fog (aka performance reduction or sleep inertia), using light therapy can be helpful, particularly blue light which is more effective at reducing brain fog and increasing vigilance. Indeed, bright light therapy was shown to cause a secretion of cortisol, which has an effect similar to a "cup of coffee". Bright light in the morning is well known to not only inhibits melatonin but also have antidepressant properties. In practice, this should clear up the brain fog under 30min to 1h of blue light therapy, as brain fog is likely due to melatonin residues in the blood stream, which can be inhibited most effectively by blue light. If the brain fog sustains all day long, then it may be a sign of circadian misalignment (ie, sleeping all your needed hours but outside of your biological circadian night) or of severe sleep deprivation that will require several nights of good quality and long sleep to recover from. A systematic clinical review and practice guidelines found that bright light at circadian night is effective at increasing alertness but less effective during the circadian day, whereas bright light exposure is more effective at decreasing sleepiness during the circadian day than the circadian night, with the effect being dependent on environmental factors including prior light exposure (ie, photic history) and individual factors including the individual's circadian rhythm. They also note that the alerting effect of bright light exposure is likely to be sustained beyond exposure, and that it is mediated not only by the sleep processes C and S but also a proposed third sleep process A, which encompasses other factors such as posture.
Blue light also increases serotonin levels and hence vigilance, particularly at wake-up when sleep inertia is at its highest, and hence bright light is a well-known tool to clear brain fog due to melatonin left overs in the morning as well as having an antidepressant effect likely due to the increase in serotonin levels. Indeed, blue light is the most effective to reduce sleep inertia and increase vigilance compared to other colors such as green light which has no effect on vigilance and only temporary inhibition of melatonin for 90min contrary to blue light). Blue light inhibits melatonin faster than natural endogenous synthesis cessation, which means that blue light can be used at wake-up to more quickly eliminate sleep inertia due to melatonin left-overs, whereas amber light does not. Blue light alone is sufficient to constantly suppress melatonin as long as the subject is exposed. Although bright light indeed immediately alters bodily thermoregulation to transition to a more alert state, melatonin is not necessarily suppressed, hence if for a subject the sleep inertia is due to melatonin carryover from the previous evening's intake, then bright light therapy may not be sufficient to completely eliminate this kind of sleep inertia, although it will still boost the alertness level. It is hence more accurate to see melatonin-induced drowsiness as a separate system independent from alertness from circadian rhythm and bodily thermoregulation.
Anecdotally, from the current document's author's experience, the duration of brain fog is indicative of a bad quality or too short quality sleep or sleeping out of phase with the circadian rhythm during the previous night(s). For example, waking up being drowsy for hours isn't predictive of whether the individual will be able to sleep during my next circadian night nor whether the next sleep session will be bad or good (it can even be optimal). However, it indicates that the previous night was certainly not optimal, so it's then necessary to review sleep parameters such as sleep duration, potential hindrances such as new food consumed or noise or circadian rhythm monitoring devices if they work properly and take steps to mitigate these issues if they are deemed possible culprits explaining the lower quality of the previous sleep session. In summary, brain fog may be indicative in retrospect, but has no predictive value for the next sleep sessions (although it can be predictive of the wakefulness and cognitive impairments for the rest of the day).
Although sleep inertia can happen with oversleeping, it's likely a consequence of previous sleep deprivation that led to oversleeping, rather than the oversleeping itself. Sleep inertia is far more common with undersleeping.
Sleep inertia in and of itself is already a debilitating condition, several testimonials are available here due to its high prevalence among covid-19 survivors.
More experimentally, vitamin D seems to inhibit melatonin for a yet unknown reason, so this may potentially be a future treatment to help clear up sleep inertia.
Nootropics include new psychoactive compounds that are not (yet) considered drugs. Usually, they are commonly found dietary supplements but delivered in a long-release form, to make their properties longer lasting and with a smoother delivery (instead of having a sudden strong but short-lived effect), and hence more akin to a medicine. Nootropics can target memory, wakefulness promotion, etc. One wakefulness-promoting nootropic is long-release caffeine, such as Lucovitaal Caffeine 400mg tablets (200mg of caffeinated per tablet). Although not directly targeting melatonin and cortisol, the two culprits of sleep inertia, caffeine targets adenosine, which regulates sleep pressure. By ingesting long-release caffeine tablets, instead of caffeinated drinks (eg, coffee, tea, energy drinks) or food, there is no diuretic effect as is caused by the ingestion of hot fluids like coffee, and the inhibition of sleep pressure thanks to caffeine is stretched over hours, often the whole circadian day, instead of just a hour or two with coffee, and the dosage is much smaller than taking several coffee, since smaller doses of the tablet is released over time, and in the end only 200mg is released after a whole circadian day of 10h. Caffeine tablets induce some of the same increases of the brain executive network as seen with hot liquid coffee, but not all (see also here a french vulgarization article): there is a reduction of connectivity with the Default Mode Network that is only activated when resting, but not an increase with the visual network as is observed when drinking hot liquid coffee. In practice, ingest one tablet (200mg) of caffeine tablet at the circadian/natural wake up, not later, as to avoid unwanted phase delays in the circadian phase by caffeine. For those who are highly sensitive to caffeine (eg, drinking one coffee in the circadian afternoon prevents them from sleeping until the next day!), then one tablet of 200mg is more than sufficient, but for those that are less sensitive, more tablets may be necessary, but no more than 400mg, the maximum recommended daily dose for adults (although there is no known lethal dose for caffeine). Anecdotally, long-release caffeine tablets at wake up have been highly effective for the current document's author, whereas liquid coffee has never been helpful in the past, despite very high sensitivity to caffeine. This is because the long-release form allows for much more finer grained control over the delivery of effects, as explained previously.
If your main issue is that you experience difficulties waking up at a precise time and not necessarily the fatigue associated, then you can look into smart alarm / chronobiological alarm clocks, such as Sleeptracker Pro watches (discontinued) or FitBit, or smartphone apps such as Sleep As Android, which can vibrate when it detects that the user is in a light sleep stage via actigraphy. Indeed, waking up someone during their light sleep stage, which is very close to wakefulness, is a very efficient to forcefully wake up despite sleep deprivation. Furthermore, doing so by vibration is more effective, in addition to being silent for co-sleepers, as humans are highly sensitive and alert to touch and vibration, much more than to sound. Waking up during a light sleep stage is highly effective as to the user it feels like they are already awake when the alarm triggers, so that they are much less likely to fall back asleep or snooze. The window of detection of the light sleep stage is configurable, usually the default is 30min, which means that at the earliest, the smart alarm triggers 30min before the programmed alarm time if a light sleep stage is detected, and at worst at the programmed alarm time if no light sleep stage is detected. The combination of both features (smart alarm + vibration) is hence highly effective to wake up, and has the distinct advantage over sound that it doesn't bother partners living close by or in the same bed (which would induce sleep deprivation for them, a common but avoidable side effect that partners of individuals with circadian rhythm disorders often experience). However, they do not fix sleep deprivation so the tiredness and cognitive impairments will still appear during the day, but at least you will have woken up on time. Alternatives include Axbo and Sleep As Android app on Android smartphones (but apps are less accurate than wearables). Avoid faradic stimulation (electrically induced pain) devices such as Pavlok which are not supported modern science and will only increase sleep deprivation and pain. Combine with napping whenever possible to reduce sleep deprivation.
Ultradian cycles define the discrete gateways to sleep (sleep gates)
Summary: On top of the circadian rhythm, sleep is also regulated by another process called the ultradian rhythm, with cycles lasting about 1h30-2h with a ~15min gap between them, often colloquially termed the "sleep cycles", but they do happen all the time, even when we are awake. The ideal time to sleep being when both the circadian rhythm and ultradian rhythm are low, as reflected by the core body temperature. Because of them, sleep patterns move in a discrete manner, with the circadian phase shifts building up under the hood until the cumulative shift is enough to reach the next (or previous) ultradian cycle, which then is reflected with a delay (or advance) in the sleep pattern. This explains why fatigue feelings only last a dozen minute, when both the circadian rhythm phase and the ultradian cycle are low, and if this "sleep gate" is missed, then it's often necessary to wait 1h30-2h later for the next low phase of an ultradian cycle which coincides with renewed fatigue feelings. This also explains why individuals with non-24 who freerun (usually with a <25h circadian period) may superficially appear to be able to maintain a stable sleep schedule for a few days, with then a sudden big shift in their sleep pattern, which in fact simply reflects that their circadian phase was still shifting all along, but the shift needs to accumulate for a few days until it's enough to reach the next ultradian cycle and hence to be reflected in a delayed sleep pattern. This means that a sleep schedule stabilization of a few days is always meaningless, longer periods (at least 2 weeks) are always necessary to assess if an intervention or therapy is effective.
Ultradian cycles are any cycle smaller than 24h, hence any cycle shorter than circadian. In sleep science, an ultradian cycle refers to the 1h30-2h blocks of vigilance/sleep cycles. There are smaller ultradian cycles in other biological processes. Ultradian cycles were initially discovered by Nathaniel Kleitman - who is considered the father of sleep science - and were initially named Basic Rest-Activity Cycle or BRAC. These ultradian cycles are exactly the duration of one full sleep cycle (including going through the various deep sleep stages and REM sleep, until it starts again with the next cycle). Even more interestingly, medical doctors observed during the Tripp experiment, where a radio presenter did not sleep for 200h to raise funds for a children charity, that hallucinations due to sleep deprivation also are following a 90 minutes pattern, in other words an ultradian cycle, which strongly suggests that ultradian cycles happen all the time, including when awake, but are simply suppressed when the homeostatic sleep pressure is minimal. Indeed, recent research support the initial hypothesis of Kleitman that ultradian cycles (or BRAC) are in fact happening all the time:
> A large number of these studies have shown ultradian cycles with periodicities centred around this privileged periodicity, in support of Kleitman’s hypothesis. Such cycles were shown in various electroencephalographic (EEG) frequency bands such as α (8–9 Hz; Kripke and Sonnenschein 1978; Gertz and Lavie 1983) and δ (0.5–3 Hz; Kripke 1972), or the total power of the EEG (Manseau and Broughton 1984), in the tendency to fall asleep during the day (Lavie and Scherson 1981), and in autonomic indices of arousal such as pupillary diameter and reactivity to light (Lavie 1979), respiratory rate (Home and Whitehead 1976) and heart rate (Orr et al. 1976).
Notably, it is thanks to Kleitman's research that all fields of medicine are now making a distinction between rest (resting state) and sleep, which is crucial for example in neurobiological studies.
To illustrate the ultradian cycles, let's see how the circadian rhythm is usually represented (in green a typical sleeper's melatonin profile, in blue the same for an individual with DSPD, source of the figure):
But in reality, the circadian rhythm is not smooth, here is what it more realistically looks like when integrating the ultradian cycles on top of the circadian cycle (source of the image unknown):
These ultradian cycles are of importance to fall asleep, because we feel most sleepy (and hence have more ease to fall asleep) at the lows between these blocks. Indeed, there is a gap (or low point if we imagine a continuous curve instead of discrete blocks) of about 20-30 min between each ultradian cycle, which is sometimes called the "gateways to sleep". That's why if you feel sleepy but fight the feeling and stay awake, after 20-30min you will feel more awake again for about 1h30. Hence, ultradian cycles can be a great tool to know when you are going to sleep, as if you feel a bit sleepy but it's too early for you to sleep (or you miss the window opportunity), you can calculate that in about 1h30-2h you will get another opportunity to sleep, and prepare on time to be ready for it. Meanwhile you can do something else, as it is better to get up if you can't sleep than staying in bed.
Also, ultradian cycles are like a rebounding ball, with a peak in the middle: there is always one ultradian cycle where the gap will be the peak of tiredness. The other ultradian cycles will be associated with less tiredness, whether before because the process S (sleep pressure) is still building, or after because of dopamine accumulation which counteracts the sleep pressure and creates a "forbidden zone of sleep" or more formally the "wake maintenance zones".
All these processes lead to an "all or nothing" access to sleep, a discrete event: either you can sleep now or you can't and need to wait later. Hence why the moments when sleep onset (ie, falling asleep) is possible is dubbed as "sleep gates":
"The onset of the nocturnal sleep period (the sleep gate) was found to be a discrete event occurring as an 'all or none' phenomenon."
This interaction between the processes C, S, dopamine and ultradian cycles explains why if you stay awake past your ideal sleeping time, you will find it more and more difficult to fall asleep, despite the accumulating sleep deprivation and sleep pressure, because of dopamine hiding the feeling of tiredness as more time passes on. That's also why after an all-nighter, you will have a harder time sleeping, despite the obvious sleep deprivation. This dopamine buildup may also be one of the factors causing chaoticity in the sleep patterns after extensive sleep deprivation. Hence why freerunning allows not only to avoid sleep deprivation and be more healthy, but it's also a virtuous cycle where your sleep will stabilize and you'll feel more the natural bedtime tiredness, and hence why it's necessary to freerun before starting any therapy.
Melatonin regulates access to these sleep gates, by allowing them during the biological night.
These ultradian cycles can also explain why freerunning appears as sudden phase delays or phase advances after a few days of stabilization. Indeed, contrary to common assumptions, freerunning sleep patterns do not progress in a continuous manner, rather, they move suddenly with a cumulative offset every few days, when the circadian rhythm phase has shifted enough to reach the next/previous ultradian cycle. For example, for a circadian period of 24h and 30min, the non-24 individual will often be able to maintain a stable sleep period for 3 days, and then the cumulative shift of 30 min x 3 days = 1h30 will be sufficient for the circadian night to start at the next ultradian cycle, which is when the individual will notice that they unfortunately can't sleep at the same time as the last 3 days. To summarize, sleep does not appear to move continuously, it can only "jump" from one start of an ultradian cycle to the next/previous one, but under the hood, the circadian phase is continuously shifting. It's the superposition of both the circadian rhythm and the ultradian rhythm that creates sleep (and wake) opportunities, the ideal timing for sleep being when both overlap in their lowest phases.
The ultradian rhythm, as well as infradian (seasonal) rhythm, are unfortunately much less studied. The scientific journal Chronobiology International estimated that only 5% of the submissions their received related to ultradian rhythms, although the editor pledged to prioritize these topics of study in the future.
It's known since 1979 that the pupillary light reflex (PLR), and more precisely pupillary diameter and reaction time to bright light exposure, can reflect ultradian cycles.
Wake maintenance zone and interactions with dopamine
The Borbély's model of sleep includes two processes: the process S which is the homeostatic sleep pressure that builds up like a timer over time for as long as the individual stays awake, and another process C for the circadian rhythm which varies during the day in cyclic fashion, and resets everyday whether the individual sleeps or not.
But there is another process that also builds up AND is periodic, mixing both properties of the processes S and C. This third process is the dopaminergic buildup, which likely interacts with the suprachiasmatic nucleus since it possesses dopaminergic receptors (TODO: find ref). Since this dopaminergic process possesses two properties, we will discuss them separately, although they of course interact.
About periodicity: multiple studies observed that a dopamine build up happens in a periodic circadian fashion, with "a zone of minimal sleep tendency approximately 1-3 h before habitual bedtime", and multiple others of lower sleep tendency (but not minimal) at various other times in the day as shown here and here. These time spans are scientifically called the wake maintenance zone(s) or the forbidden sleep zone(s). It is a paradoxical counterbalance to the sleep pressure produced by adenosine, and inversely, adenosine should produce sleepiness and hence counterbalance the alertness promoting effect of dopamine. This increase of dopamine leads to increased subjective and objective alertness and focus during these hours before sleeping and further along after a long period of sleep deprivation, and its periodicity doesn't stop even if the individual does not sleep.
Figure 2 of de Zeeuw et al, 2018, we can see how there are bouts of increased efficiency/scores for nearly all tasks, just before the usual sleeping time (at 2 on the axis):
About buildup increasing with time: this process continues to buildup dopamine the more time spent awake, and hence the more sleep deprived (see a summary here). A study of 40 hours of sleep deprivation observed a similar increase. This zone is one of the reasons it's extremely difficult to phase advance (ie, sleep earlier) than phase delay (sleep later) for individuals who are sleep deprived. In other words: when being sleep deprived, there will be a tendency to sleep later and later. This has detrimental consequences for individuals with a circadian rhythm disorder as this can needlessly increase their daily phase delay.
However, dopamine buildup is counterbalanced by adenosine buildup, since adenosine inhibits dopamine. Indeed, adenosine, through the activation of the A1R receptors, suppresses glutamate, dopamine, serotonin and acetylcholine secretions. Some scientists even argue that adenosine could be considered a "master regulator" of various neurotransmitters. Hence, the forbidden sleep zone is only transient, until adenosine builds up to a point that dopamine is too suppressed to have any meaningful effect on sleep. Furthermore, adenosine inhibition of dopamine may explain some effects of prolonged sleep deprivation, such as more difficulties to learn or do any task that requires reinforcement, and motor tasks, since dopamine gets more and more inhibited over time.
Individuals with non-24 often tend to skip sleeping (ie, pull an "all-nighter") in order to meet appointments and obligations. Because of the dopaminergic build-up creating an increasing but periodically varying wake maintenance zone, the less the individual sleeps, the less sleepy they will feel, and the more difficult it will be to sleep in the end. This creates a particularly vicious cycle for people with circadian rhythm disorders, since non-24 and DSPD causes people to be sleep deprived when they try to conform to a schedule. This compounds on the reduced effect of zeitgebers on the circadian rhythm when sleep deprived, which increases circadian misalignment and jetlag, interestingly due to adenosine buildup. Hence, it is a prerequisites for them to first try to follow their natural rhythm, to reduce this and other confounding effects on their sleep if they want to either log it in a sleep diary or try to control their circadian rhythm a bit.
After an all-nighter, the individuals may complain that their body doesn't know when to be asleep or awake anymore, likely because of this dopamine buildup. This buildup may also explain why after such a prolonged period of sleep deprivation, the individuals will tend to sleep chaotically at a very different time than their circadian rhythm should allow for, because paradoxically it may be easier to sleep out of phase than in phase because the wake maintenance zone preceding the biological sleeping period will only be reinforced by the previous prolonged period of sleep deprivation. This may produce the illusion that the individual could reset their circadian phase to an entrained phase synchronized with the day-night cycle, which is completely incorrect and short-lived since their circadian rhythm has not changed. This illusion may also affect clinicians capacity to diagnose the circadian rhythm disorder, as they may get the impression that the individual can manipulate their circadian rhythm at will, which is not the case, and a simple look at the sleep fragmentation and duration should clear any doubt.
Hence, all-nighters should be avoided for individuals with a circadian rhythm disorder to avoid the dopamine build-up from unnecessarily worsening the condition.
The author of this document used to be a slow sleepers all his life, taking at least 30min to fall asleep but usually more than 1h. After a few months of sleeping in phase with the circadian rhythm and the reduction of sleep deprivation, the sleep onset delay was naturally reduced to 15min or less.
In practice, the ultradian gates to sleep can be used as follows: don't just go to sleep whenever you want or planned, rather plan ahead and define a period when you know you will be more likely to fall asleep according to your circadian rhythm. During this time period, prepare in advance (brush your teeth, prepare bedroom, save your work and reminder notes for later, etc) to sleep at anytime and then do passive activities (with dark therapy) during the rest of that time period to wind down until sleepiness feeling kicks in (even if subtly). Take this feeling as a cue to go to bed, falling asleep should then happen fast.
The forbidden zones of sleep or wake maintenance zones is also colloquially named "second wind" (see Wikipedia, although the author does not recommend this article as it is confusingly sourced and written, and hence unreliable).
TODO: rewrite the paragraph below:
Also, the impact of the dopaminergic system on the circadian rhythm is even worse for people with ADHD and a circadian rhythm disorder ([paper1](https://www.maynoothuniversity.ie/research/human-health/neurobehavioural-cognitive-science/projects/link-between-adhd-and-circadian-body-clock), [paper2](https://www.ncbi.nlm.nih.gov/pubmed/30927228), [paper3](https://europepmc.org/article/PMC/4323534), [paper4](https://www.nature.com/articles/s41386-019-0592-4), [paper5](https://journals.sagepub.com/doi/full/10.1177/1087054716669589), [Washington Post article](https://www.washingtonpost.com/news/to-your-health/wp/2017/09/22/could-adhd-be-a-type-of-sleep-disorder-that-would-fundamentally-change-how-we-treat-it/)). See also [this post](https://www.reddit.com/r/DSPD/comments/fcdfqo/is_adhd_and_dspd_often_comorbid/).
Note that the effect of the dopaminergic system should not be confused with the bimodality of the circadian rhythm which produces "two consistent zones in the circadian temperature cycle during which normal subjects rarely fall asleep" and which certainly contributes to insomnia.
There are alternative theories which do not involve the dopaminergic system but emphasize the role of the circadian rhythm and the sleep homeostat (adenosine):
> The phase relationship between the circadian rhythm of distal skin temperature and CBT may also provide a thermophysiological explanation of the so-called “wake maintenance zone” in the evening just before endogenous melatonin secretion and distal vasodilatation begins (39). At this circadian phase, the circadian system counterregulates with high effort the homeostatically increased sleepiness and sleep pressure to maintain wakefulness (17). In thermophysiological terms, the “wake maintenance zone” can be characterized as the most vasoconstricted state of distal skin regions in relation to CBT over the entire circadian cycle (i.e., low inner heat conductance with high CBT and low distal skin temperature; Ref. 4).
Another review notes:
> it should be noted that daytime circadian alerting signals interact with sleep deprivation, resulting in exponentially scaling attentional impairment with extended wakefulness (for reviews, see REFS 7,8).
Which means that the more time spent awake, the more difficult it is to sleep with paradoxically the less alertness (ie, increased wakefulness but decreased alertness).
Laziness, willpower, motivation and introspection illusion
People with a circadian rhythm disorder aren't lazy, to the contrary, they put more efforts into waking up than others. Indeed, laziness has nothing to do with the circadian rhythm.
How much efforts? An excellent example is the NASA crews monitoring robotic Mars missions, where the trained crew was tasked with following a non-24h martian sleep-wake schedule to better monitor the robotic missions, ended up with the crew rebelling and dropping the schedule as they felt it was unbearable, after a single month! This crew was composed of highly trained staff, and the authors praised them for sustaining this "broken" non-24 schedule for a full month. But as the authors state:
> The authors attributed this result to the high motivation of the crew, although motivation has limited ability to override circadian and homeostatic regulation of alertness and performance and is, in fact, subject to these influences itself.
Why did the authors praise the crew's high motivation in following a non-24h schedule, despite rebelling and prematurely stopping the experiment 30 days in only? Because chronic sleep deprivation is quite a feat, and is used as a form of torture by the USA (see also here) and before by the nazis through night-time musical disturbances. The effect of sleep deprivation can also be observed on more recent and well documented cases such as Peter Tripp, Randy Gartner (see also here) and Jason Russell of Kony 2012 fame. Experiments and therapies disrupting someone's natural sleep schedule are nowadays considered unethical. Unethical medical experiments on humans are unfortunately common.
Hence, thinking that sleep issues are caused by a lack of motivation is an inversion of logic. Actually, motivation is actually deeply influenced by sleep as noted above, and it was demonstrated on a cohort that sleep deprivation drastically impairs motivation for social and physical activities, and the authors further suggest this may have deep implications in the pathogenesis and treatment of a wide array of diseases. Since there is not one circadian clock but lots of them throughout the body and down to every cells, willpower can obviously not change the molecular biology of the circadian processes, just like willpower cannot cure diabetes (but treating the circadian rhythm misalignments may cure or at least certainly improve these other afflictions).
The accusations of laziness are unfortunately just instances of sleep-shaming based on a just-world, suffering-reward and ableism ideologies. It is also a clear example of the classic fundamental attribution error, underestimating situational factors while overestimating personality-based explanations, by assuming that sleep and wakefulness issues are due to the person's fault rather than explainable by an uncontrollable condition. Victim blaming allows to rationalize the unfairness of this possibility and maintain a just-world belief.
Laziness is often confused with tiredness, but their signs and causes are entirely different, and people with a circadian rhythm disorder foremost suffer from tiredness, not laziness.
It's rather easy and common for the afflicted to rationalize their disease as not only their fault but even their preference. This stems not only in the fundamental error attribution, but also in introspection illusion. Indeed, if you became convinced that you were lazy due to your sleep issues, then it's likely that you have a sleep disorder since a long time, as you had time to rationalize (or be convinced by others) that your issues were your fault, which is a classic fundamental attribution error, underestimating situational factors while overestimating personality-based explanations. Furthermore, if you think that you dislike routines, or like living at night, or dislike working, or further atypical preferences, they may be a rationalization of the sleep disorder, as humans have a tendency to piece together what they are presented with, with what they think they prefer, even though this is not necessarily the case. For example, if you were given to choose one between 2 different jams, and then later on you get presented with the jam you rejected as being your preferred jam, you are most likely to accept it as if it was your choice and actually appreciate the flavor you apriori rejected, just like 80% of the participants of such an experiment. This is an instance of choice blindness, a kind of introspection illusion and change blindness. Humans are often unaware of the real reasons behind their actions, but will nevertheless try to rationalize them, such as preferences or mental states (eg, stress, anxiety). Furthermore, this is not a temporary thing: hijacking a preference causes a durable change in preference, as you are then more likely to unknowingly choose the rejected item in the future.
Revenge bedtime procrastination vs circadian rhythm disorders
Revenge bedtime procrastination is when you sleep later than when sleepiness kicks in because you want to do some enjoyable activity. In other words, if you wanted, you could have easily slept earlier. Afterwards, you pay the price by being more tired than if you slept earlier when you could have.
DSPD is when you do not feel sleepiness until late in the night. You can't easily sleep earlier, as you feel very much energized until late. The price you pay afterwards is not dependent on what time you sleep, because you cannot sleep earlier than your body dictates, but rather on the time of wake up. If you wake up too early compared to what your body needs, you will feel fatigued and have chronic jet lag symptoms. Left to your own device, you feel best when waking up later.
Hence, DSPD is something you suffer all the time, whereas revenge bedtime procrastination happens only the nights you procrastinate. With DSPD you feel best when sleeping and waking up later, whereas with bedtime revenge procrastination sleeping and waking up late (even without an alarm clock) makes you miserable, but you do it just to get some spare time.
It's possible to both have DSPD and to do bedtime revenge procrastination, they are not exclusive. DSPD is not something you can control by behavior, whereas bedtime revenge procrastination can be.
In summary: If you can't sleep because you don't feel sleepy then that's not procrastinating, but a circadian rhythm disorder.
Note that revenge bedtime procrastination is not a medical concept but has emerged in 2021 on social networks. However, bedtime procrastination is a psychological concept that has weak evidence to support it (ie, most or all studies are confounded by poorly designed experiments and uncontrolled factors).
Should you use an alarm clock?
Alarm clocks are not meant to help with adjusting the circadian rhythm, they only are a way to get up to be on time for appointments. Indeed, they don't prevent your circadian rhythm from freerunning under the hood, as if it was the case, circadian rhythm disorders would not exist since anyone using alarm clocks would be entrained.
That's why actually not using an alarm clock is more helpful if you want to get entrained, because then you reduce external factors and also reduce sleep deprivation, which messes up with the circadian rhythm in major ways. But if alarms are necessary because of appointments, then there's nothing to do about it in the short term, and maybe wait for holidays to switch off the alarms and fully dedicate to an entrainment therapy.
Furthermore, alarm clock increase sleep deprivation, which reduces the effectiveness of bright light therapy, one of the few effective therapies for circadian rhythm disorders.
Also, the wake up time is much more predictive of your circadian rhythm than bedtime (see also here). Hence, with an alarm clock, you are deprived from a very strong indicator to track your sleep and the progress of the entrainment therapy. Indeed, it's possible to wake up at the same time or even earlier regardless of sleep time (whether you slept later or before usual), and in my own experiments, the stability of the wake up time seems to be a strong sign of successful entrainment.
Do not wake up earlier with an alarm clock to do the therapies. Although one study on typical sleepers studied a phase advance protocol combining a chronotherapy (using an alarm clock to wake up 1h earlier every 3 days) and light therapy at wake up, in practice I did not find any advantage in using an alarm clock or combining light therapy with a chronotherapy. I would rather advise to just wake up and sleep when you naturally feel inclined to do so, and do the therapies when you are awake. This was the most efficient strategy in my experience, as this gets as much benefits from light therapy, while reducing the potential and unnecessary sleep deprivation induced by alarm clocks and which can hinder the effectiveness of the phase advance produced by the light therapy. In my experience, it is useless to try to force yourself to wake up earlier: either the therapy is working and you will naturally sleep and wake up earlier, or you don't and the therapy is not effective and behavioral interventions won't work. See also here.
So remember: alarm clocks are for appointments, not for therapy. If your entrainment works you should wake up at the time you want without an alarm clock.
Hormones and sex specific interactions with sleep and circadian disorders
Sleep disturbances and women
Non-24 is arguably more difficult to treat in (biological) women than in men, as they also have a menstrual rhythm in addition to the circadian rhythm. Indeed, the natural hormonal variations and their impact on both the core body temperature and the circadian rhythm on a menstrual periodic timescale. The core body temperature is raised during the luteal phase or under contraceptive pills, and lower during the follicular phase with a similar temperature profile to men. Women taking oral contraceptive experience reduced slow-wave deep sleep. However, another study found on the contrary that core body temperature and melatonin profiles were unaffected by menstrual phase, but menstrual phase interacts with REM sleep, which shows there still remains some debate on this topic. Hence, "gender, menstrual cycle phase and hormonal contraceptives significantly influence body temperature".
Furthermore, the "use of oral contraceptives has been found to increase night-time melatonin levels and the menstrual phase may affect the melatonin level (Webley and Leidenberger 1986; Wright and Badia 1999)", so there is an interaction between melatonin and contraceptives, including progestin which increases melatonin levels.
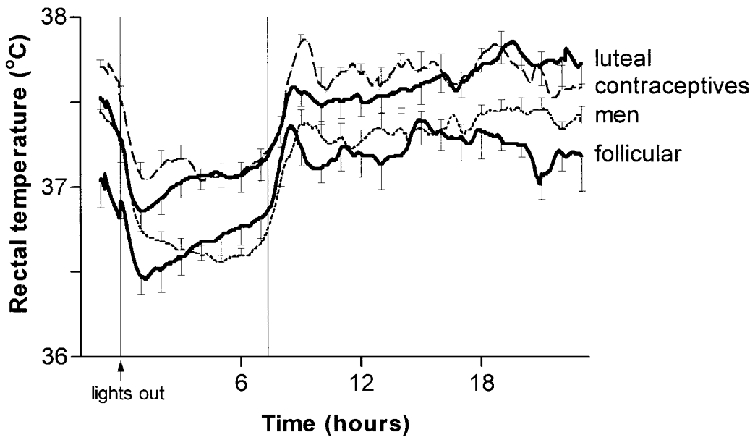
Average core body temperature (rectal temperature) for 1 h before lights-out and 23 h afterwards in eight men, eight women taking hormonal contraceptives, and eight naturally cycling women in the mid-follicular and mid-luteal phases of their menstrual cycles. This shows that the menstrual cycle's hormones have an effect on the core body temperature, and hence on the circadian rhythm. Figure reproduced from this study.
"Continuous monitoring of body temperature during the menstrual cycle is commonly performed in daily life. The basal body temperature (BBT) decreases at the end of the luteal phase."
"Sleep-deprived women lose heat rapidly in response to a mild cooling stimulus. Sleep-deprived humans may be more vulnerable to heat loss with reduced ability to warm even at temperatures thought to be associated with thermal comfort."
According to the 2005 study on the biggest cohort (57 participants) on sighted non-24 subjects yet, there were 41 (72%) men and 16 (28%) women. This high disparity is in contrast with observations that can be made on community support groups such as the N24 discord, where participants introduce themselves, and which allows to observe that biological sex is much more balanced than in this study's results.¹ Also apriori there is no known genetic predisposition for non24 that would be specific for male sex. One possible explanation is that the non-24 disorder is under-diagnosed in women, even more so than the under-diagnosis rate of men, as women are twice as likely to be diagnosed as anxiety or other non-specific psychiatric disorders instead of the proper diagnosis such as ADHD, so the higher rate of misdiagnosis can be assumed to be similar for non-24. Another potentially compounding explanation is of a side-effect of cultural and societal expectations, as in western patriarcal societies, women's societal role is still foremost expected to be of home and children care, which if done as full-time can make them and their disorder more invisible to others but also to themselves, since individuals with non-24 often discover they have a sleep disorder while trying to maintain a set sleep schedule for a work position.
Women higher in progesterone such as during the luteal phase of the menstrual cycle and men lower in testosterone, both of which can be caused by sleep deprivation, were more vulnerable to emotional swings, showing that sleep deprivation can impact sex hormones which in turns can impair emotional regulation. Sperm physiology fluctuates according to ultradian (2h) and infradian (6 to 12 months) cycles.
In a (rare) 2007 review on this topic, it was found that there are strong links between menstrual cycle and circadian rhythm disturbances, with associations between circadian misalignment and menstrual irregularities, longer menstrual cycles, breast cancer, decreased sleep quality around menses and menstrual-related mood changes. The review also found differences in sleep quality and body temperature between luteal and follicular phases of the menstrual cycle.
Low melatonin levels are associated with endometrial cancer and breast cancer and is suggested to be used as a screening indicator of these cancers, and inversely timezones with lower light exposure and hence lower melatonin inhibition such as the Arctic see lower rates of breast cancers. These cancers are also much more prevalent in women working in night shifts. Breast cancer survivors are not spared, as the ones sleeping in misalignment with their circadian rhythm are more likely to have more metastases.
Women with unmanaged non-24 have further practical limitations as they cannot do activities outdoors at night without significantly increased risks of aggressions.
Since Restless Sleep Disorder is likely to stem from an iron deficiency, women with restless legs syndrome or periodic limb movement disorder may be more prone to acute onsets disturbing their sleep during their menstrual periods. Supplementing with iron around these periods may help, although this is experimental (no evidence, just a hypothesis).
Several key processes regulating the reproductive system in women display a circadian rhythm, and are hence at least partially regulated by the circadian rhythm (read more in this PhD thesis).
Anecdotally, a pregnant woman with non-24 reported that the usual sleep disturbances associated with hormonal changes during pregnancy are worsened with non-24.
The timing of intake of time-sensitive medication, such as hormonal medication such as birth control pills, is a legitimate concern for people with a circadian rhythm disorder. Recent evidence supports that cardiovascular medication cause less side effects and is more effective when taking relative to the patient's circadian rhythm type (chronotype). This has not been studied for birth control, but this is likely applicable too.
While this answers the phase (people with a late chronotype should use medication later than those with an earlier chronotype), what about those with a longer circadian period, such as people with non-24? There is currently a dearth of information, but a woman with freerunning non-24 reported after years of experimenting that the adequate timing was relative to their circadian rhythm, hence, taking birth control later and later in absolute time every day, but at the same relative time relatively to their biological clock. Interestingly, birth control appeared to lose efficacy for them when they tried to take it at the same absolute time each day, suggesting that their biology really considered such absolutely timed intake as taking the medication earlier and earlier each day relative to their biological clock.
Note the author of this present document is male, and hence this section could be further expanded and there may be specific tips and additional therapies or adjustments necessary for the VLiDACMel protocol to optimally work for women. It would also be necessary to find if some studies were done on hormonal replacement therapy and other hormonal treatments, as the induced change in hormones is likely relevant to circadian rhythm disorders.
¹ TODO: do a formal calculation, the sample size is much more than in the study (but only a few are formally diagnosed - this may change with wearables - or use sleep diaries to count as likely non24?)
- TODO: read: Sex differences in circadian timing systems: implications for disease, 2013 https://pubmed.ncbi.nlm.nih.gov/24287074/
- The Pineal Gland and its Function in Pregnancy and Lactation, 2020 https://doi.org/10.1016/B978-0-12-814823-5.00002-7
> [...] During labor, melatonin may synergize with oxytocin, promoting uterine contractility. Melatonin has a protective effect during pregnancy through its free-radical scavenging and antioxidant capacities, intervening to balance the oxidative challenge that accompanies the high metabolic demands during pregnancy, labor, and lactation.
This is in line with testimonials from pregnant women with non24 reporting increased sleep disturbances during pregnancy.
- Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome, 2019 https://doi.org/10.1210/jc.2018-02385
- Chapter 40 - Sex and Gender Differences in Sleep Disorders: An Overview https://doi.org/10.1016/B978-0-12-803506-1.00046-2
- Pregnancy increases the risk of restless legs syndrome. Historically, sleep apnea has been underdiagnosed in women, there is a gender-bias in diagnosis.
Sleep disturbances and men
> We find that erectile dysfunction, lower urinary tract symptoms, and hypogonadal symptoms all have a linear relationship with sleep, as worse symptoms occur with poorer sleep. Male infertility, interestingly, has an inverse U-shaped relation to sleep in which men with too little and too much sleep seem to be more at risk for infertility than those with 7–8 hours of sleep. Finally, the literature has not demonstrated a significant clinical relationship between hypogonadal symptoms or testosterone levels and sleep. Overall, a large number of men experience poor quality sleep. Given the impact that poor sleep can have on general health and men’s health, in particular, screening for poor sleep quality and recommending interventions to improve sleep are becoming imperative during clinical evaluation and treatment.
https://www.ncbi.nlm.nih.gov/pubmed/32257858
> Studies have associated non-standard shift work schedules and poor health outcomes, including increased risks of diabetes mellitus, dyslipidemia, hypertension, heart disease, peptic ulcer disease, and depression, in shift workers. However, few studies have focused on the role that shift work plays in men's urologic health. Current evidence supports associations between non-standard shift work and increased hypogonadal symptoms, poor semen parameters, decreased fertility, lower urinary tract symptoms, and prostate cancer. These associations are strengthened by the presence of SWSD, which affects up to 20% of shift workers. Unfortunately, interventions, such as planned naps, timed light exposure, melatonin, and sedative hypnotics, aimed at alleviating excessive nighttime sleepiness and daytime insomnia in non-standard shift workers experiencing SWSD, are limited and lack strong evidence to support their efficacy.
https://pubmed.ncbi.nlm.nih.gov/29371140/
Nightmares and sleep quality
Nightmares are a common worry among sufferers of sleep disorders.
In the current document's author's experience, dreams and nightmares are not related to the sleep quality nor the circadian rhythm disorders. Sleep quality is solely determined in the author's case by sleep duration and circadian alignment (ie, sleeping under the low phase of the circadian rhythm). Nightmares were an almost daily occurrence during the first year of experiment on a daily basis, and they did not impair the entrainment (nor cause desynchronization). They also did not impact daytime mood, contrary to what psychologists hypothesized, whereas sleep duration and circadian misalignment did. This decorrelation between nightmares and sleep quality was observed in a study: "nightmares affect the experience of sleep quality but not sleep architecture".
This hypothesis is in line with the findings of a review on the effect of the psychotropic drugs on sleep and dreams, which found that antidepressants reduce dream recall, and also that dream recall is increased by micro-awakenings during the sleep session, so that drugs that improve sleep consolidation (reduce sleep fragmentation) seem to decrease dream recall, but this is only a correlation. With this hypothesis, a more fragmented sleep (such as sleeping outside of one's own circadian rhythm, or sleeping during the day with ambient noise and disturbances) will increase the likelihood of experiencing nightmares, which would be in line with the "arousal-retrieval model stating that nighttime awakenings enable dreams to be encoded into long-term memory and therefore facilitate dream recall".
Interestingly, it's established that effective antidepressants shift the circadian rhythm by increasing entrainment to bright light, hence further supporting the hypothesis that the frequency of nightmares increases with circadian misalignment. Anecdotally, this is in line with the current document's author results during the 2nd year of experiments, with far more rare occurrences of nightmares when the circadian rhythm is entrained and sleeping in circadian alignment, as nightmares frequencies drastically increased whenever sleeping in circadian misalignment with the circadian night (even if the circadian rhythm was still entrained and stable in its phase). In other words, while there potentially a strong case for circadian misalignment causing increased frequency of nightmares, this does not mean that increased frequency of nightmares can impair sleep or the circadian rhythm.
Nevertheless, nightmares can be more troublesome for some people. Nightmares are more likely to happen during vivid dreams, and vivid dreams happen during REM sleep, towards the end of the night, and the end of the night is when micro-awakenings are more likely too. Hence, vivid dreams including nightmares are more likely to happen towards the end of the night. Anecdotally, in the present document's author's experience, this is the case, and it also is more frequent during sleep sessions in circadian misalignment, as despite the reduced short duration due to the lack of circadian rhythm support, deep sleep stages are shorter and less frequent, and REM sleep stages are more frequent then.
There are compounds which are known to increase the vividness of dreams, and hence the likelihood of nightmares, such as melatonin, magnesium, vitamin B6 (which is often used by lucid dream experimenters as it also increases dream recall) especially when combined with zinc such as in ZMA (zinc magnesium B6) compounds. It seems these compounds may be more likely to increase dreams vividness and nightmares occurrences when the dosage is too high or when taken too close to bedtime, so decreasing the dosage and taking them earlier in the day (ie, the morning rather than the evening) may help.
Eating, particularly a heavy meal, just before going to bed may increase the likelihood of nightmares (studies here and here - this last one is not peer-reviewed). Spicy foods may also disturb sleep. Hence, it may be preferable to eat earlier, which is advised to avoid the carbs and melatonin interaction anyway. If a meal just before bed is really necessary for you, eating a smaller meal may help in reducing sleep disturbances and nightmares.
Nightmares and vivid dreams are a frequent occurrence for people with an advanced disease such as advanced cancer, which is not surprising since the dreams of psychiatric and insomniac patients also reflect their daytime worries. Nightmares are prevalent in 19-81% of people with PTSD depending on the severity of PTSD and their exposure to physical aggression.
Furthermore, the vast majority (98%) of dreams just randomly recombine elements of the waking life (ie, they are NOT symbolic representations of the unconscious contrary to Freud's beliefs). Dreams content is influenced by the most recent events experienced in the day (see previous ref, mirror here). Also, personally significant and novel experiences are more frequently integrated in dreams than common, repetitive daily activities. Interestingly, non-REM sleep also serves the role of a 10x accelerated and random access offline replay of memories (see also here for humans and here and here). Hence, it is important to have some time to wind down and do pleasurable hobbies before sleeping, and thus avoid stressing or mood depressing activities at the same time. This technique is called positive presleep suggestion. Interestingly, expanding on Stephen Laberge's landmark works on lucid dreams, a study where scientists could bidirectionally communicate with lucid dreamers by asking them questions during their sleep shown that their recollection of the questions after they wake up differed substantially from the actual questions they had answer during their dream, which shows that our dream recall is often fragmented and distorted, what we recall from a dream or a nightmare is not exactly what happened. Interestingly, this bidirectional communication is possible because although the body is paralyzed during sleep, the eyes are not, and can be controlled by the sleeper during their dream.
Nightmares can be a good indicator of increased REM sleep, and hence of circadian wake up time and potentially of circadian misalignment. Indeed, deep sleep stage is reduced and REM sleep stage is increased when sleeping out of phase (ie, circadian misalignment). REM sleep stage is also increased towards the end of one's sleep. Vivid dreams are more frequent during REM sleep. Hence, having nightmares are more likely to occur towards the end of the night, and when being circadian misaligned. Thus, although waking up after a nightmare is often assumed to be because of the nightmare, it is likely the other way around: that the nightmare occurred because the body was already getting ready to wake up.
Hence, experiencing nightmares often signals that the body was ready to wake up. This can be used to confirm that the current wake up time is in phase with the circadian rhythm if the sleep duration is long enough and the wake up time is stable over several days, or if these 2 parameters aren't fulfilled, the time at which the nightmares occur (with or without a wake-up) can indicate when your circadian wake up time is expected by your body, which may be very different from what the individual think their sleep schedule should be. For example, if an individual think their sleep schedule is from 1am to 9am (8h of sleep), but they wake up from nightmares at 5am, this indicates increased REM sleep around 5am, hence the individual may rather have a circadian sleep schedule of 9pm-5am. If the individual can sleep during this window of time for 8h (their natural sleep need), then they found their circadian sleep window. Otherwise, if the sleep duration is much less (eg, <5.5h), then it's likely that the individual is still sleeping out of phase and the increased REM sleep is simply a consequence of circadian misalignment that is not indicative of where the circadian wake up time is, but simply that there is a circadian misalignment.
Side-note: the relative perceived time spent in dreams is in fact equivalent to reality's time, except when doing motor tasks which take more time to do during dreams. The dream-lag effect is also very interesting and could be linked to spaced repetitions learning or intermittent reinforcement. The existence of both the day-residue effect and the dream-lag effect hints at the existence of a circaseptan (ie, 7-days) rhythm in humans (in addition to circadian and ultradian rhythms, and menstrual for women).
Side-note 2: for those interested in lucid dreams, here is a great tutorial by a lucid dream researcher to get started (this may be used as a way to reduce nightmares by gaining more control over dreams). There is also an app designed by researchers which uses sounds at specific times to induce lucid dreams, which has some scientific support since previous studies on rats by the well established MA Wilson found that rats dreams could be engineered by sound cues during non-REM sleep. An updated version of the app has been announced.
Side-note 3: although sleep necessity was previously thought to be due to brain cleanup processes including dreams, which was shown to be incorrect, it now appears that dreams major purpose beside memory consolidation may be to allow for creative thoughts by recombining memories in innovative ways allowing for innovative insights compared to individuals who do not sleep. Although this hypothesis of a key role of sleep for memory consolidation remains unconfirmed and controversial, there is now an experimental framework that will allow the testing of this hypothesis in the upcoming years. A related but slightly different hypothesis is that dreams primary functional purpose is to generalize from personal experiences, which technically involves reducing overfitting in neural networks as for artificial intelligence systems, and this may explain the strangeness of some dreams and nightmares.
ADDENDUM, TODO: REFORMAT and add references:
Lucid dreams and dreams content has little to do with the circadian rhythm and sleep deprivation, except that of course 1) if you are heavily sleep deprived you are more likely to stay longer in deep sleep stages and less in dreaming stages (mostly REM but also happen a bit in stage 1 and 2), or 2) if you sleep outside of your circadian rhythm, you are more likely to experience lighter sleep (REM, stages 1-2).
Note also that if you try to sleep past your circadian wake up time, it's normal that you are more likely to wake up with a racing heartbeat, it's because your circadian rhythm causes your body to generate cortisol, the hormone of wakefulness/stress, so that even if you try to "sleep in", you are much more likely to wake up (and often from a nightmare - caused by cortisol).
Sleep paralysis can on the other hand be a sign of sleep apnea. It's also much more common for younger people.
That said, I also had a lot more frequent sleep paralysis occasions when I did not manage my sleep disorder, and so when I was both chronically sleep deprived + sleeping in circadian misalignment. But to my knowledge, this is not studied in the research literature.
Can this therapy cure non24 and other circadian rhythm disorders?
Curing an illness means that after some period, treatment can be discontinued without the loss of the benefits. Management means that the detrimental effects of an illness can be reduced or even eliminated as long as the treatment is continued.
Currently, there is no known therapy that can cure non24. Indeed, it was shown that the circadian rhythm and melatonin profiles shifts back to its natural state only a couple of days after stopping light therapy that was administered for a week and sometimes as fast as under 15 min of stopping light therapy. Similar return to baseline levels over a couple of days were observed with melatonin treatments. Light therapy and melatonin being the most effective tools currently known to shift the circadian rhythm, this strongly suggests that no currently available treatment can permanently modify the circadian rhythm.
Hence, the only currently known treatments, when they work for the individual, which is not guaranteed for everyone, are only managing the disorder, not curing it. The treatments must hence be continued life-long to maintain the benefits.
Any improvement is better than none as it reduces sleep deprivation, and any reduction of sleep deprivation is well worth it. Take what you can out of treatments, don't aim for the ideal time that you may not reach, just aim for a better and more stable sleep schedule than you experience now.
The adherence can also be problematic, as a previous study found that combination therapy with melatonin and light/dark therapy had a high drop out rate, due to the difficulty to maintain a "behavioral and environmental structure that is required to maintain stable entrainment". However, it must be noted that this study had several major limitations: it used conventional blue light therapy lamps which cumbersomeness was reported as one of the reasons at least one patient dropped out; the duration of light therapy was small, only 1h, which did not account for the differences in light hypersensitivity; it required waking up earlier (ie, a "strict sleep schedule") hence prior sleep deprivation; finally, the melatonin and light therapy timing were calculated based on sleep onset (ie, falling asleep time), although this is an unreliable estimator of the circadian rhythm. All these points are counter productive and can in fact be easily fixed by using light therapy glasses emitting blue light, and using the light therapy at wake up instead of forcing a strict sleep schedule and instead of using the sleep onset time.
If this therapy does not work for you, and this is a real possibility, then there are a few other things that can be checked:
- a MRI (magnetic resonance imagery, more precisely a T1-MPRAGE) of your brain to check if there is any lesion in the pineal gland or the suprachiasmatic nucleus.
- a blood test checking B12 and vitamin D levels.
Do we have other biological rhythms?
Beyond the circadian rhythm, which is by definition "circa"/about a day (24h), we also have evidence of the existence of more rhythms in humans:
- ultradian rhythm, with cycles of 90-120min and which regulates the sleep gates
- semi-circadian rhythms, with several organs showing a 12h rhythm.
- seasonal rhythm, with a varying wake up time and melatonin secretion duration according to sunrise time (because of sunlight exposure).
- circannual rhythm, with weight gains more frequent during the summer, as is also observed in the animal kingdom.
These biological rhythms should not be confused with the pseudoscientific biorhythms theory, which assumes the existence of 3 immutable rhythms that are set at birth and remain the same throughout life, which would theoretically allow to predict their influence at any point in life based just on the birthdate of an individual. To the contrary, all the observed biological rhythms above are mutable, so that they are influenced at all times by a variety of stimuli/zeitgebers such as light, and hence cannot be reliably predicted (as any individual with non-24 knows too well).
Is pulling all-nighters a sustainable strategy?
A bit of theoretical infos that i think will help understand the pros and cons of pulling an all nighter.
There are 2 major processes underlying sleep : the circadian rhythm and the sleep homeostat (also called the sleep pressure). The circadian rhythm is like a clock, it has a day time and a night time, and it isn't affected by when you sleep or how long you stayed awake, it just periodically marks periods of wakefulness and sleepiness, it alternates between both at regular intervals. On the other hand, the sleep homeostat is a timer that starts counting from the moment you awaken from sleep, and gets reset when you sleep. The sleep homeostat serves as a failsafe mechanism to allow yol to sleep anytime if you were awake for too long, even if your circadian rhythm clock currently indicates "daytime" (eg because you pulled an all nighter).
Now that you know these 2 processes, you can understand what happens when you pull an all nighter. You will basically skip sleeping when it's night time for your circadian rhythm, letting your sleep homeostat build up through the roof. When you finally can go to sleep according to the restricted schedule you planned (ie, at night, which you usually can't because of DSPD), your circadian rhythm will still indicate "daytime" as it usually does (which is what prevents you from sleeping at night), but your sleep homeostat will be at a so high level that you will still be able to sleep.
That said, there are issues with this approach. The biggest issue is that you build up a huge sleep deprivation debt by disregarding your circadirn rhythm, and to recover from it you won't need just one night of sleep. Think about it: you need to sleep everyday. If one day you don't, of course you are going to need multiple days to recover, as you very likely won't feel refreshed as you should be after the first night of sleep after the all nighter (more likely you will feel worse than usual, especially the brain fog is usually worse). Although the body will try to somewhat compensate by sleeping a bit more than usual, it cannot sleep long enough for 2 nights of sleep, at some point it needs to be awake to ensure you eat food, drink and fulfill other essential needs, so that partial sleep deprivation will remain after the first "recovery" night as it is called in scientific works.
Secondly, since you will be sleeping only thanks to your sleep homeostat, hence using only one of the 2 sleep processes, your sleep won't be as long nor as restorative as when you sleep in circadian misalignment. In my experience, you can expect to be unable to sleep more than 1 or 2 ultradian cycles less than your ideal sleep duration. Eg if you ideally need 8 hours of sleep, you won't be able to sleep more than 5h30 in circadian misalignment, and if you're not used to do that usually it's less (more like 4h30).
Thirdly, this won't change your circadian rhythm. So if for example your current circadian night is from 7am to 3pm, even if you sleep at night after an all-nighter and wake up at 7am, you will still feel asleep up until 3pm after the end of circadian night, even are awake since 7am. So avoid driving during this period of time as accidents are much more likely (accidents due to sleepiness are the leading cause, alcohol only comes second).
Comorbidities with other disorders (mood, neuroatypism, motor, addictions)
This section covers comorbidities of circadian rhythm disorders with other disorders/diseases including mood, neuroatypism and movement (including some disorders that would commonly qualified as "mental", but this is a too vague word that will not be further used). For other comorbidities (such as metabolic) that are much more frequent and often caused directly by sleep deprivation or circadian misalignment, such as cardiovascular diseases, see the "Health issues of a circadian rhythm disorder" section.
Sleep disorders and circadian rhythm disorders are highly comorbid with psychological disorders
Sleep disorders are associated with wide range of psychiatric and physical disorders and diseases. Both these disorders and the circadian rhythm disorder develop often concurrently at various stages of life. It's difficult to know which is causing what, whether it's the sleep disorder that causes the other disease, or if the other disease is worsening the sleep disorder, or if they are both independently caused by another third factor but they interact with each others, although circadian dysregulation often precedes the appearance of other symptoms and there is some evidence of a causal role. Nevertheless, most evidence is currently only correlational as of 2019, and hence there is no evidence in humans yet that early childhood circadian disruption may be more prone to other comorbid disorders (contrary to some claims, although some animal evidence exists but needs further confirmation). Yet, the association is so strong that some researchers suggest to create a new diagnostic group of "circiatric disorders", for disorders where both a circadian rhythm disorder and a psychiatric disorder are associated. Other researchers go even further, stating that sleep disorders are present with every major brain disorders and always warrant adequate investigations and treatment:
> The basic scientific findings regarding sleep loss have not yet been routinely applied in the clinic. [...] Sleep abnormalities are robustly observed in every major disorder of the brain, both neurological and psychiatric. Sleep disruption merits recognition as a key relevant factor in these disorders at all levels, from diagnosis and underlying aetiology, to therapy and prevention.
Regardless, it's known that sleep disorders can worsen the severity of a wide array of other diseases (ie, comorbidities), such as autism, depression, anxiety and ADHD, but also physiological diseases, with scientists recommending clinicians to be more cognizant about the effect of sleep deprivation for their patients with comorbidities. There are also anecdotal reports from some individuals that getting treatment for their other disease (such as ADHD) also helped or sometimes cured their sleep disorder, including non24, although their reliability remain uncertain.
A recent systematic review, supporting the arguments already produced by a 2001 review by Harvey, found that psychological disorders are mostly independent from sleep disorders, and complete management of the psychological disorders does not improve the sleep disorders, as such they conclude that sleep and circadian rhythm disorders "require independent attention irrespective of co-morbid conditions".
Another interesting hypothetical explanation for this high correlation but lack of apparent causation is that chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if appearing and untreated from a young age, hence it is not inconceivable that chronic sleep deprivation from childhood may be the pathway, the pathogenesis, that develops neurological disorders that are often comorbid with sleep disorders.
ADHD
ADHD is a strong case for an apriori psychiatric disorder having a strong or even causative circadian rhythm disorder at its root. Indeed ADHD is strongly associated with insomnia as shown by this figure. Furthermore, some individuals were misdiagnosed with ADHD when they really had DSPD. More recently, a systematic review found consistent evidence of circadian rhythm disruption in ADHD, with ADHD being associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels, and it's estimated that circadian rhythm disorders such as DSPD (likely including a fraction of misdiagnosed non-24) is present in 73-78% of children and adults with ADHD (see also here and here)! This assessment of a DSPD disorder being common in ADHD was confirmed using objective measures such as melatonin sampling, core body temperature and distal-proximal temperature. See also this nice introductory video by SciShow Psych explaining the links between DSPD and ADHD and why DSPD worsen hyperactivity. Symptoms of ADHD are worsened proportionally to the circadian misalignment according to this, this and this studies, with scientists suggesting that targeting treatments to the circadian rhythm disorder may yield more benefits than targeting ADHD behavioral symptoms:
> In contrast to these results, which were based on small samples, other studies found that symptoms of inattention were most evidently associated with disturbed sleep, delayed circadian rhythm, and greater sleep need (Bae et al., 2010; Caci, Bouchez, & Bayle, 2009; Gau et al., 2007; Rybak, McNeely, Mackenzie, Jain, & Levitan, 2007; Voinescu, Szentagotai, & David, 2012). [...] Adults with comorbid ADHD and insomnia were found to have significant circadian rhythm delay, the severity of ADHD symptoms and neuropsychological deficits correlating with the delay (Gamble et al., 2013; Rybak et al., 2007). In contrast to the controls, the patients with adult ADHD had the same prevalence of delayed sleep phase syndrome independent of age, the authors suggesting that delayed sleep phase syndrome in ADHD is not age related (Bijlenga, Van Someren, et al., 2013). [...] If ADHD medication affects the circadian rhythm, the effect on sleep may be less obvious and appear later (Stein et al., 2012).
https://doi.org/10.1177%2F1087054716669589
> Dr Andrew Coogan, a neuroscientist based at the Department of Psychology, has published the results of a study linking Attention Deficit Hyperactivity Disorder (ADHD) in adults to the circadian rhythm, commonly referred to as the body clock. The study shows a direct link between the 24 hour body clock and the symptoms of ADHD. It opens the door for a possible therapy for the condition, currently usually managed by an expensive medication regime. The study indicates that people with ADHD have ‘out of sync’ body clocks. The more disrupted their body clock is, the more severe the symptoms of their ADHD become.
https://www.maynoothuniversity.ie/research/human-health/neurobehavioural-cognitive-science/projects/link-between-adhd-and-circadian-body-clock
This leads some scientists, based on the findings of a previous systematic review finding nearly systematic associations of ADHD with circadian rhythm disorders and that mutations in circadian rhythm genes were associated with core ADHD symptoms, to argue that at least a part of ADHD symptoms are in fact caused by the circadian rhythm sleep disorder:
> Ultimately, the main question is addressed: whether ADHD needs to be redefined. We propose a novel view on ADHD, where a part of the ADHD symptoms are the result of chronic sleep disorders, with most evidence for the delayed circadian rhythm as the underlying mechanism. This substantial subgroup should receive treatment of the sleep disorder in addition to ADHD symptom treatment.
https://www.ncbi.nlm.nih.gov/pubmed/30927228
Interestingly, an analysis of the geographical prevalence of ADHD shows that solar intensity explains 34%–57% of the variance in ADHD prevalence, showing an association between bright light exposure and the prevalence of ADHD, which may be explained by a better regulation of the circadian rhythm disorder by increased bright light exposure and hence reduced ADHD symptoms, leading a proportion of people in these geographical areas to never feel the need to seek a diagnosis of ADHD in the first place. Circadian rhythm disorders of people with ADHD can apriori be treated with the same usual treatments, as melatonin does not interact with methylphenidate, and light therapy can be used but with care due to the sensory hypersensitivity inherent to ADHD or induced by drugs such as methylphenidate (see also this review for a list of hyper photosensitizing drugs and this other list) — as indeed photosensitivity seems to be present in 70% of people with ADHD —, hence extra care should be given to using only the minimum light intensity setting (500 lux) on light therapy glasses and keep eyes closed the first 30s of starting a light therapy session to let the pupils contract and reduce the chance of uncomfortable side-effects associated with sudden bright light exposure. Just like for other psychiatric disorders, recent evidence points towards a common genetic basis and neural correlates underlying both the ADHD disorder and the associated sleep disturbances. Females are better at masking their symptoms and hence often remain undetected when they have ADHD or autism, being instead diagnosed with anxiety. A dopaminergic dysregulation model is hypothesized to be a potential cause of ADHD.
This hypothesis of a genetical interaction between ADHD and circadian rhythm genes, with potentially a hypo or hyper photosensitivity, is further strengthened by a trial showing that individuals with ADHD saw improved symptoms and advanced circadian rhythm phase with bright light therapy, strongly suggesting that bright light therapy may be an effective treatment for ADHD management:
> Single nucleotide polymorphisms in circadian genes were recently associated with core ADHD symptoms, increased evening-orientation and frequent sleep problems. Additionally, alterations in exposure and response to photic input may underlie circadian problems in ADHD. BL [bright light] therapy was shown to be effective for re-alignment of circadian physiology toward morningness, reducing sleep disturbances and bringing overall improvement in ADHD symptoms. The susceptibility of the circadian system to phase shift by timed BL exposure may have broad cost-effective potential implications for the treatment of ADHD. Conclusions: We conclude that further research of circadian function in ADHD should focus on detection of genetic markers (e.g., using human skin fibroblasts) and development of BL-based therapeutic interventions.
https://pubmed.ncbi.nlm.nih.gov/30234417/
There are other diseases associated with the circadian rhythm such as sensorimotor disorders including Periodic Limb Movement Disorder (PLMD) and Restless Legs Syndrome (RLS, also known as Willis-Ekbom disease (WED)), often associated with ADHD. PLMD is an intrinsic sleep disorder affecting over 17% of insomniacs and "11% of hypersomniac patients complaining of excessive daytime sleepiness" and 4% to 11% in the general population although they are underdiagnosed. RLS is not necessarily associated with circadian rhythm disorders, but the acute triggering of their symptoms show a circadian pattern. This was also demonstrated for Periodic Limb Movements in Sleep (PLMS/PLMD). This means that melatonin can trigger the symptoms of RLS and PLMD through an interaction with the dopaminergic system. Although RLS is not necessarily associated with circadian rhythm disorders, it is estimated that 35-44% of the people with ADHD also have RLS, and 78% of people with ADHD have DSPD (or misdiagnosed non24), hence having ADHD increases the likelihood of having both RLS and a circadian rhythm disorder. In the unlucky case the individual both has RLS and a circadian rhythm disorder, melatonin supplementation is then counter-indicated, which reduces the possibilities for the treatment of the circadian rhythm disorder. However, despite the RLS symptoms triggering by melatonin, some users reported a positive side-effect on mood and motivation and on the circadian rhythm. This effect of melatonin on the dopaminergic system would merit further studies. It's very interesting to notice that both ADHD and RLS share the similarity of having dysregulations in the dopaminergic system, and given the strong link of the dopaminergic system with the circadian rhythm (see the "forbidden zone of sleep"), this suggests that dysregulations of the dopaminergic system may be a cause of circadian rhythm disorders for some individuals. Iron supplementation were found to be a very promising treatment over the long term for children with PLMD or RLS with significant improvement in sleep parameters. Interestingly, iron deficiency impairs adenosine metabolism, and hence the sleep homeostat, which is one of the two processes underlying sleep with the circadian rhythm disorder. Studies on rodents even suggest that adenosine dysregulation due to brain iron deficiency plays a pivotal role in RLS, with iron deficient mice reproducing a circadian rhythm disorder, which furthermore provides a strong evidence of a link between RLS , iron deficiency and circadian rhythm disorders. Clonazepam is another treatment that seems to improve the stability and duration of sleep episodes for people with PLMD with or without RLS according to a placebo controlled trial, with a usual dosage of 1mg at bedtime. Anti-depressants should not be prescribed to people with PLMD or RLS as they can worsen the sensorimotor symptoms, except for Bupropion which is also indicated for treatment of depression in people with ADHD.
Until 2020, there was no consensually recognized link by scientists between neuromotor disorders such as PLMD and RLS with sleep disorders, with iron supplementation being considered an alternative therapy. This changed in 2020 when a panel of experts reached a consensus to define the Restless Sleep Disorder diagnosis and its treatments. The Restless Sleep Disorder is theorized to stem from an iron deficiency in the brain (hence acknowledging that this is a neuromotor dysfunction) that may also underlie the often comorbid ADHD and autism, and that the best available treatment is daily oral iron supplementation combined with a yearly infusion of intraveinous iron sucrose (not stimulants nor benzos) (see also here and here). Indeed, several systematic reviews, including a 2019 Cochrane Systematic Review, found iron supplementation, both oral and intraveinously, to be effective treatments to improve the symptoms of RLS with no more side effects than placebo and are hence considered a safe treatment, and with similar effectiveness to dopaminergic agonists such as pramipexole but arguably fewer side effects. Before this emerging body of evidence for iron supplementation, RLS used to be treated with either a nonergot dopamine agonist or a calcium channel α-2-δ ligand. When this treatment works, side effects of iron supplementation such as constipation are rare, so that experiencing side effects can be indicative of being non responsive to the treatment. Since Restless Sleep Disorder is likely caused by brain iron deficiency, it isn't always associated with a blood anemia, which is more formally called a "non-anemia iron deficiency". Those who have RLS and PLMD will not be surprised, but they will find relief in the proper acknowledgement and understanding of this kind of neuromotor disorders' etiology (ie, cause) and treatment, which opens the door to a better healthcare for these patients. Although as some patients note (see also here), RSD is more appropriately seen as a rebranding of PLMD than RLS, since it lacks several key symptomatologic items of RLS.
There is an interesting hypothesis that has yet to be investigated to the present document author's knowledge: the possibility that several key items of psychiatric and psychological disorders may in fact not be due to the disorder but to chronic sleep deprivation. For example, a meta-analysis on children with ADHD found that sleep deprivation worsened their attention, but not their activity. We may extrapolate this finding by suggesting that the attentional deficit in ADHD is not due to the ADHD disorder but to the chronic sleep deprivation. Another example is the emotional dysregulations in autism spectrum disorders. Interestingly, studies found that REM sleep plays a key part in the consolidation of emotional memories, and that its disruption impairs this kind of memory. This again may be extrapolated to suggest that the emotional dysregulations observed in autism are not in fact intrinsic to the disorder but mostly due to sleep deprivation. If this hypothesis is correct, then treating the co-morbid sleep disorder may yield much more improvements than currently assumed for a wide variety of psychological disorders, and may also yield crucial insights that can help devise more targeted clinical symptomatology and diagnostic scales for these disorders, by removing the items that are in fact extrinsic, caused by the sleep disturbances rather than the psychological disorder.
Preliminary evidence suggests that children with dyslexia may have a dysregulated circadian profiles of melatonin and cortisol.
Given the cursory data from spontaneous testimonials on peer communities (ie, reddit, discord), the author of the present document suspects that ADHD is highly common for people with DSPD, and autism (ASD) is more common for people with non-24. This seems to be supported by an informal survey of the r/DSPD subreddit.
Although not a solution for sleep issues, Jornay PM is a new delayed-release and prolonged-release formulation of methylphenidate, which was reported to be effective in helping to manage ADHD symptoms directly from wake-up including in kids from 6 years old and older.
Mood disorders such as major and seasonal depression, psychoses and hallucinations
Mood disorders and especially depression, whether major or seasonal (which are now thought to be the same, the differenciation was made on a psychological hypothesis, not empirical evidence), with depression being the most common comorbid disorder for DSPD, and although data is lacking, likely similarly for non-24. Beside sleep deprivation and sleep disorders, there is an accumulating wealth of evidence from animal studies that strongly suggests that circadian rhythm disruption is a major trigger or causative factor of several psychological mood disorders, such as major depression, anxiety and schizophrenia. In fact, and especially given the cyclical nature of depression which mirrors seasons like the circadian rhythm does, some scientists go as far as stating they think that previous investigations into the pathogenesis of depression, and even its definition, were flawed, with circadian misalignment and manipulation being the most promising pathogenesis and therapeutic hypothesis yet for at least a large subpopulation, if not all, of patients affected by major mood disorders such as major depression.
Historically, antidepressants were initially discovered by repurposing a drug used to treat tuberculosis, with little understanding of their effects on the brain (see also here). The most prevalent theory for the pathogenesis of mood disorders is the serotonin deficiency hypothesis. However, it never was empirically demonstrated robustly, previous studies being largely biased. Even worse, a 2022 umbrella systematic review shown that the serotonin theory of depression is not supported by evidence, and if anything, SSRI antidepressants may actually lower serotonin concentrations (see also this vulgarization article on The Conversation by the same authors). Another review of major theories of factors underlying major depression found that they are lacking empirical evidence, except maybe for cortisol, which may constitute another clue that major depression may be a circadian rhythm disorder. As the authors in The Conversation write:
> Although a famous early study found a relationship between the serotonin transporter gene and stressful life events, larger, more comprehensive studies suggest no such relationship exists. Stressful life events in themselves, however, exerted a strong effect on people’s subsequent risk of developing depression.
This result is not new: although their updated 2022 review allows a more global overview of this issue, a previous 2009 meta-analysis from another team "yielded no evidence that the serotonin transporter genotype alone or in interaction with stressful life events is associated with an elevated risk of depression in men alone, women alone, or in both sexes combined." In consequence, the authors state that there is no evidence that SSRI antidepressants have any proven efficacy. This is in line with a previously published medical evidence review in 2019 which found no clinical efficacy of antidepressants for the treatment of major depression disorder:
> The benefits of antidepressants seem to be minimal and possibly without any importance to the average patient with major depressive disorder. Antidepressants should not be used for adults with major depressive disorder before valid evidence has shown that the potential beneficial effects outweigh the harmful effects.
And these results are accounting only for published results, which are known to be heavily biased, with a study finding that 57% of all registered trials on SSRI antidepressants ended up finding negative results and hence the majority remaining unpublished. When accounting for these unpublished results, it appears that "most (if not all) of the benefits are due to the placebo effect".
There are however well established harmful effects of SSRI antidepressants use, especially over the long run, such as SSRI-induced indifference, which affects 20-40% of users and may be a precursor to increased suicidality, and may be reduced or eliminated by dosage reduction or discontinuation, although this is often missed by clinicians due to its effects onset being insiduous and delayed from the start of the therapy. Nevertheless, the increase in suicide risk from SSRI antidepressant use is underestimated due to the systematic under-reporting by the pharmaceutical industry (see also here). Some may argue that at least the serotoninergic theory of depression, as the first popular biogenetic explanation of depression, allowed to reduce stigma on patients, but a study found "mixed blessings" (see also this vulgarization article):
> People who attribute mental health problems to brain disease or heredity tend to blame affected people less. However, they are also more pessimistic about recovery, more willing to socially exclude affected people and more likely to see them as dangerous. [...] This also affects patients views of their own diseases. Participants in the chemical imbalance group blamed themselves as much as their control group peers. They were also more pessimistic about their chances of recovery and less confident of their ability to manage their depression. Further, only participants in the chemical imbalance group believed pharmacological treatment to be more appropriate and effective than psychotherapy. In sum, the chemical imbalance view led people to feel less hopeful and capable in the face of their problems and more disposed to use medication.
Faced with the very strong evidence of a lack of empirical foundation of the "chemical imbalance" serotoninergic theory of depression, leading psychiatrists claim this theory is an "urban legend" that was never academically supported by the psychiatric field, which is unambiguously false as even textbooks supported the dissemination of the theory and usage of antidepressants to treat major depression, and this attempt at misdirection only divert from criticisms of the theory itself. For an opposite viewpoint, see the replies by Robert J. Pies, who is very vocal in both public comments and academic papers. There is also a systematic review by Cipriani et al in 2018 which found a positive, although small, beneficial effect of antidepressants, although the methodology is disputed (see here and here).
Another issue lies in the definition of major depression and insomnia in the DSM-III to DSM-IV, with overlapping symptoms: insomnia includes mood dysregulations symptoms, and major depression includes insomnia as a symptom. Hence, insomnia being a comorbidity of major depression is by definition, and is hence biased. Nevertheless, recent years have seen the publication of modern, well designed studies isolating confounds and which determined the contributions of sleep disturbances to mood dysregulations and inversely, and especially the role of circadian disturbances. Indeed, in the DSM-V, seasonal affective disorder is not a standalone diagnosis anymore, but a specifier (ie, subtype) of major depression disorder.
Since the 2010s, a few scientists investigated other much less explored but more promising hypotheses for the pathogenesis of major depression, including dopamine dysregulation and sleep-wake or circadian rhythm dysregulations, and some authors even trying to reconcile the serotoninergic hypothesis with the circadian dysregulation hypothesis. Indeed, it is now strongly suspected that major depression is tightly associated, or even caused, by an underlying circadian or sleep disorder, and molecular and biological markers of circadian dysregulations are often observed in depressed patients.
On one hand, treating the sleep disorder can help with the other comorbid disorders, such as major depression, but also potentially a wide range of other diseases including schizophrenia by improving functioning and reducing suffering, or in the case of mood disorder, sleep disturbances and circadian disturbances may be one of the causes. Indeed, a large-scale 2018 cohort study using the UK BioBank dataset over 91 105 participants aged 37-73 years found that "circadian disruption is reliably associated with various adverse mental health and wellbeing outcomes, including major depressive disorder and bipolar disorder". It appears that a delayed melatonin profile is associated with unipolar depression, and depression symptoms caused by night-time light exposure (and hence circadian misalignment) can be cured by eliminating the light exposure. Individuals with DSPD who have a circadian misalignment (ie, it's not behavioral) are more prone to depression, showing a link between circadian misalignment and depression. Previous studies were already finding similar results, with a higher severity of depression with more delayed sleep phases, which prompted scientists to suggests that "circadian disturbances are at the core of depression". The increased difficulties in social interactions, the lack of routine (and hence increased cognitive load) and the depressed mood are not surprising for someone with non24 or extreme DSPD who is living at night and sleeping during the day. A more mechanistic link can be found in sleep deprivation, as sleep deprivation increases pain perception (hyperalgesia) and also decreases the effect of pain medication such as opioid and serotoninergic pathways, hence reducing the effectiveness of serotoninergic drugs which likely includes those used to pharmacologically treat depression. This suggests that treating depression requires to treat the patient's sleep disturbances as this is key for optimal depression therapies efficacy. And indeed, a study in 2006 by Lewy et al demonstrated that melatonin (administered relatively to the current circadian phase) can improve depression symptoms in proportion with the degree of circadian realignment, which shows a clear link between circadian misalignment and depression severity. The wide spectrum of melatonin activities makes it a potential candidate to treat diverse neuropsychiatric disorders including epilepsy, schizophrenia, depression and anxiety disorders, hence treating the circadian rhythm disorder with a therapy including melatonin may also directly affects the co-morbid neurological or mental diseases. Entrainment, or simply light exposure, can objectively improve depression, and in addition treating sleep issues can reduce the severity of depression along the way. Bright light therapy was shown to be as effective as antidepressants to treat depression by a systematic review, and the combination of both was even more effective (see also here), which is logical if antidepressants improve mood through hyper photosensitization, with nevertheless a major difference being that antidepressants take 2 to 8 weeks for effects whereas bright light therapy may be effective in as little as one week. Not only is light therapy as effective as drug antidepressants and effective much faster, it also has a much safer profile with no known serious side effects for the general population, such as no increased risk of suicide contrary to drugs. Furthermore, blue light improved the activity of brain areas associated with emotion processing more than other colors such as green, which demonstrates that blue light is likely more effective to treat depression (eg, SAD) than other colors, and strongly suggest that the same pathway (ie, ipRGC cells) underlies both use of light therapy (circadian rhythm shifting and mood regulation). Interestingly, melatonin only improves the mood symptoms and the sleep of depressed individuals with DSPD, but no improvement was observed for depressed individuals without DSPD, which is another hint that circadian rhythm disorders are not causally related to mental disorders, they are only associated because they worsen each other, and may co-occur simply because of the burden of sleep disorders. In general, it was observed that all effective antidepressants actually shift the circadian phase, with some scientists arguing that circadian phase shifting may be the primary pathway for how antidepressants can improve mood disorders. Given that 30% of all major depression disorder cases are resistant to treatment, one has to wonder why the rate would be so high if antidepressants were indeed effective circadian phase shifters. Although there is no study yet on this aspect, it is arguable that if indeed their effect on mood is a consequence of their circadian phase shifting effect, then, just like all other zeitgebers-based therapies such as melatonin and light therapy, intake timing of antidepressants should also be assumed to be crucial. In other words, the limited efficacy of antidepressants may not be because of the molecules per se, but due to an inadequate timing of intake, which is relatively easy to improve once PRC curves for each antidepressant is established.
Not only seasonal affective disorder is now thought to be the same as major depression, both being at least partially if not fully caused by circadian disruptions, other conditions are now progressively being merged too: postpartum depression was found to vary according to seasons, with a higher prevalence when delivery happens during winter months, and postpartum sleep deprivation was found to be the strongest predictor of postpartum depression.
Another therapeutic approach for mood disorders including major depression involves another chronobiotic: agomelatine (Valdoxan), a melatoninergic agent, which can be used as a drop-in replacement for melatonine to treat both mood and anxiety disorders on top of having the circadian shifting and sleep inducing effects of melatonine, thanks to the unique combined effect agomelatine has as a MT2 agonist and 5-HT2C antagonist. However, the circadian shifting effect should be considered a bonus, not the primary effect which is to be an antidepressive and antianxiolitic. Indeed, agomelatine is efficacious to treat anhedonia in major depression. Cipriani's famous 2018 review also found agomelatine to be an effective antidepressant. Just like other antidepressants, agomelatine can cause gastrointestinal side effects according to a systematic review.
Agomelatine was the first antidepressant extending beyond monoaminergic transmission and the first melatoninergic antidepressant, since then, other melatoninergic agents are being investigated for the treatment of depression (see also here). Agomelatine was later found to have an antianxiolitic effect in addition to its antidepressant and circadian manipulation effects. Both the antidepressant and antianxiolitic effects caused by the 5-HT2C antagonism that is unique to agomelatine among melatoninergic molecules (mirror, see also here):
> The antidepressant agomelatine had a similar effect than melatonin when applied alone but blocked the melatonin-promoted Gq activation due to its 5-HT2C antagonistic component.
Several feedbacks from users with non24 report that agomelatine is effective to treat mood and anxiety disorders such as major depression, but has limited effect on the circadian rhythm, similar to melatonine. However, it's worth noting that there appears to be no withdrawal symptoms, similarly to melatonine.
Also, a major advantage of agomelatine compared to previous classes of antidepressants is that melatoninergic agents are not known to increase the risk of suicide, and also they are generally considered very safe, with very few and mostly benign side effects.
On the other hand, depression can impair the individual's motivation and ability to stay committed to a treatment (therapeutic compliance). This can create a vicious cycle, where a comorbidity increases sleep deprivation and fragmentation, and these in turns worsen the comorbidity expression, as it happens with epilepsy. However, there is no evidence that treating depression can treat a comorbid circadian rhythm disorder, and no evidence that antidepressants can improve insomnia. Indeed, "even in maintenance treatment with activating antidepressants as many as 30-40% of patients may still suffer from insomnia". Even worse, many antidepressants actually cause or worsen insomnia, but there are other classes of antidepressants that impact sleep less (see here and here). Despite these issues with the pharmacological treatment of depression, the mood and motivation improvement obtained through the management of depression may indirectly improve sleep issues by improving motivation and compliance. But treating sleep disturbances for their own rights with targeted treatments on sleep is crucial to treat depression, since "midnocturnal insomnia is the most frequent residual symptom of depression".
Mood, including higher cognitive functions such as the ability to read other people's subtle emotional or social cues but also leaders abusive behaviors and decision making particularly moral judgments, are severely impaired by sleep deprivation, regardless of education and sleep deprivation quantity. Indeed, judges dole out more severe sentences on the "sleepy mondays", the mondays after DST time change, which is less than 1 hour of sleep deprivation once a year. This can have a societally wide impact as minorities are more prone to sleep deprivation due to discrimination. Sleep deprivation also increases depression and impairs autobiographical memories, causing remembrance of more negative memories. Even modest changes in sleep quality (increases or decreases) causes changes in pain perception the next day (decreases and increases respectively) — literally, sleep deprivation is painful, and pain can be treated by increasing sleep duration and quality. Circadian misalignment, in particular with aberrant light exposure, can also cause major cognitive, learning and mood impairment. Sleep deprivation is detected visually by other people, making you appear less socializable with (ie, "ugly") and "sadder" (see also here). Leaders supervision's quality can vary dramatically from day to day based on their sleep quality.
Severe sleep deprivation will even lead to hallucinations and psychosis and paranoia, with more hallucinations happening with proportionally more time awake. Interestingly, in the famous Tripp's case, the medical doctors observed that the hallucinations happened on an approximately 90 minutes period, which is exactly the duration of ultradian cycles during sleep, suggesting that ultradian cycles (and potential dreaming) happen all day long, but are somehow inhibited during wakefulness unless there is prolonged sleep deprivation. Interestingly, delirium shows circadian fluctuations, and melatonin supplementation may reduce it. TODO: add ref (from thoughty2 video)
A study demonstrated that sleep deprivation and sleep disorders impair interoception, which is the ability to feel one's own internal body signals including the ability to feel sleep pressure, with impaired introception being associated with major depression according to a 2019 systematic review.
Sleep deprivation can impact sex hormones which in turns can impair emotional regulation.
Bright light exposure is known to have an antidepressant effect, likely due to the inhibition of melatonin, since melatonin inhibition by propranolol also has an antidepressant effect. Hence, a reduction or lack of bright light exposure can worsen the mood. Light therapy improves mood as much as antidepressants according to a systematic review and meta-analysis, and is even recommended by the authors as a first-line treatement for seasonal and non-seasonal major depression alone or in combination with antidepressants for greater effect, which makes sense given the very low risk of side effects with light therapy compared to antidepressants. Actually, it is now strongly argued by some scientists that the effectiveness of antidepressants stem from their circadian rhythm shifting capacity. Indeed, all effective therapies and drugs for major depression disorder also affect the circadian rhythm A clinicial trial using agomelatin found that the degree of mood improvement in depression in young individuals was associated with more circadian shifting as evidenced by melatonin sampling, providing further support to the hypothesis that the efficacy of antidepressants is tied to their circadian shifting effect, and can explain why the combination of light therapy and antidepressants supercede the effect of any alone.
Although it was hypothesized more than 50 years ago by Franz Halberg that non-24 may be associated with the bipolar disorder, a study found little to no evidence. However, another systematic review found that bipolar disorder is often associated with circadian disruptions, but not an association with non-24 specifically. Another systematic and clinical review found that light therapy is also promising to indirectly improve a wide range of other psychiatric disorders such as schizophrenia or bipolar disorder by improving sleep and reduce suffering.
Patients with an advanced disease such as advanced cancer are more prone to sleep disorders including circadian rhythm disorders, and hence some authors recommend that all patients with an advanced disease should be systematically screened and treated for sleep problems.
Although anxiety is often suggested as a comorbidity of circadian rhythm disorders, there is little evidence. Interestingly, women are much more likely to be misdiagnosed with an anxiety disorder instead of ADHD, as females are more prone to show inattentiveness symptoms whereas males are more prone to hyperactivity. ADHD being much more strongly associated with circadian rhythm disorders such as DSPD, this may explain the spurious association between anxiety disorders and DSPD.
Autism and sleep disorders
The links between autism spectrum disorders (ASD) and sleep disorders, including circadian rhythm disorders, are so strong and prevalent that a dedicated section is necessary to treat the topic.
Indeed, it is now widely recognized that "poor sleep exacerbates problematic daytime behavior", especially for children and adolescents with severe symptoms associated with ASD, as sleep patterns predict worsened behavior in individuals with low-functioning autism (for 81% of the participants, hence a high accuracy!). Autistic individuals with an unstable sleep also have worsened symptoms. Sleep deficits also lead to difficulties in communication, as well as increased restrictive and repetitive behaviors. In light of the crucial importance of sleep for autistic children and teenagers, the American Academy of Neurology published guidelines in 2020 to recommend to systematically screen autistic individuals for sleep issues, and for sleep to be a primary target of treatment as a major way of improving the quality of life and the symptoms of autism. A 2018 review even suggested that autistic children should be profiled based to design better targeted interventions.
Interestingly, some of the typical behavioral symptoms associated with ASD can also be induced in non-ASD adult individuals by sleep deprivation, such as difficulties in recognizing others emotions (see also here and here), mood depression, irritability, impaired ability to suppress unwanted thoughts especially when interrupted with reminders and other daytime behavioral disturbances. Similar findings were observed in neurotypical kids ("general pediatric population"). Another example is interoception: while theoricians previously suggested that individuals with ASD may have a deficiency in interoception (ie, feelings from the internals of one's own body, such as the heartbeat, or temperature, or sleep pressure), a study found the opposite held true, with children with ASD displaying similar or increased interoceptive sensitivity, another found that alexithymia, not ASD, was associated with impaired interoception, and ultimately, another study found that sleep deprivation and sleep disorders profoundly impair interoception. This demonstrates an important overlap between ASD symptoms and sleep deprivation symptoms in terms of both mood and cognitive dysregulations, and one may wonder if some cases of ASD may simply be sleep-deprivation phenotypes.
Note that interestingly, sleep deprivation didn't impair the recognition of primitive survival oriented facial expressions according to one study.
Sleep issues are indeed highly prevalent with individuals with ASD: 44% to 83% of children and adolescents with ASD have sleep issues (see also here), and sleep issues only gets worse with time, as adults with ASD have twice more sleep issues than adults without ASD (summarized here). Indeed, contrary to the common belief that one can "grow out of sleep issues", they in fact do not remit with time. Not only sleep issues are increased for adults with ASD, but also other metabolic (diabetes, obesity, hypertension, dyslipidemia, gastrointestinal), immune disorders as well as psychiatric disorders such as bipolar, depression and anxiety.
Although ASD is strongly associated with sleep issues, "they are not bidirectionally associated: sleep problems do not precede and worsen autistic behavior but rather co-occur with autistic traits in early childhood. Over time, children with ASD have an increase in sleep problems, whereas typically developing children have a decrease in sleep problems. Our findings suggest that sleep problems are part of the construct ASD." In other words, sleep issues are not the cause of ASD, they just worsen the symptoms, and sleep issues may be one of the primary facets of ASD.
What is the source of these sleep issues for individuals with ASD? About 28.3% of them have hyperserotonemia, which means they produce too much serotonin, and is one of the most reproduced endophenotypes of ASD. Serotonin can be converted into melatonin. This can lead to a (paradoxical?) decrease of endogenous melatonin levels. And indeed, low melatonin levels in "in urine, plasma and pineal gland" due to "dysregulated melatonin synthesis or clock gene anomalies" was observed in several individuals with ASD (see also here). Furthermore, mother's level of melatonin can impact their child, as low maternal melatonin level increases the likelihood of autism spectrum disorder for their children. Hence, the culprit of these sleep issues can be for some individuals with ASD due to circadian misalignment. Melatonin pills supplementation showed great success to treat individuals with ASD and sleep issues, including a study showing sustained efficacy for children with ASD of 2mg-5mg of prolonged-release melatonin over more than 1 year of treatment, and is hence recommended since 2020 as a first-line therapy to try for these individuals.
Finally, both ASD, sleep issues and digestive issues seem to be linked, with "ASD children with GI symptoms reported more severe ASD core symptoms than others".
Side-notes: ASD individuals are often misdiagnosed with borderline personality disorder (and inversely) (see also here). It's interesting to note that the social interactions difficulties that autistic people experience may not be entirely due to their behavior but also to the negative apriori prejudice toward style (and not content) of behavior by neurotypical individuals.
Genetic studies have also linked autism and sleep and circadian rhythm disorders (such as FOXP1 gene), with some researchers even going as far as to suggest that circadian rhythm disorders may underlie autism (original studies here and here).
Misdiagnosis between autism (ASD) and ADHD is common, because there are overlaps in behaviors. A 2016 study determined that there are 6 questions from the SRS scale that discriminates between ADHD and ASD with high reliability (almost always true for individuals with autism and only rarely for ADHD):
- Trouble with the flow of normal conversation (SRS 35)
- Difficulty with changes in routine (SRS 24)
- Appropriate play with peers (SRS 22)
- Difficulty relating to peers (SRS 37)
- Atypical or inconsistent eye contact (SRS 16)
- Regarded by other children as 'odd' (SRS 29)
It's worth noting that these items pertain to males, as females are more competent at masking their behaviors and hence remain often undetected when they have autism or ADHD.
Some of these items interestingly overlap with the Modified Check-list for Autism in Toddlers.
Alcohol and alcohol dependency (alcohol use disorder)
Contrary to common beliefs, despite being a sedative that eases falling asleep, alcohol impairs sleep and makes it non restorative. It may often be used by patients as a counterbalance to stimulants during the day such as caffeine and nicotine, although this is unsustainable and damaging over the long term.
Alcohol (ethanol) disrupts both the biological and molecular (peripheral) circadian clocks (see also here), in addition to increasing sleep fragmentation. Hence, alcohol elimination or at least reduction should be a primary target to improve circadian rhythm disruptions.
On the other hand, according to a 2020 systematic review, alcohol use, even when occasional with a single dose, is strongly associated with circadian rhythm and melatonin disruptions, with more alcohol causing more disruptions, with a DSPD-like pattern of melatonin secretion (secretion during the day instead of the night) being a strong marker of alcohol use disorder. Note that a single dose of alcohol under various condition did not alter entrainment to bright light according to a 2016 study. However, the hepatic circadian disruption is different whether alcohol intake is acute (momentary) or chronic (habitual). During alcohol withdrawal, the more severe it is, the more circadian rhythm disruptions are observed, so for individuals with alcohol use disorder, the extent of circadian disruption is a marker of the severity of alcohol withdrawal symptoms. Although assumedly treating alcohol use disorder can improve the circadian rhythm disorder, treating the circadian rhythm can also be a therapeutic approach to improve alcohol use disorder, as a rat study shown that melatoninergic agents, including melatonin and agomelatin, both restored the circadian rhythm and improved or even remitted the alcohol use disorder, but only when administered in phase with the circadian rhythm to cause a phase advance. Otherwise, if the melatoninergic agents administration was mistimed, there was no phase advance nor improvement in alcohol use disorder. For most individuals who withdrawn from alcohol use disorder, circadian rhythms resynchronized within about 2 to 3 weeks of abstinence, except for melatonin levels.
This demonstrates a bidirectional influence of alcohol use disorder on the circadian rhythm disturbances, and the circadian rhythm disturbances on alcohol use disorder, and hence that they require to be both treated in parallel for optimal therapeutic efficacy on both fronts, as "circadian-based interventions could play a critical role in preventing and treating AUD.". More hypothetically, this may also suggest that people with a circadian rhythm disorder may be more prone to an alcohol use disorder.
Cocaine use disorder
Similarly to alcohol, addiction to cocaine was shown to be improved by treating the circadian rhythm of rats with melatonin.
This emerging body of research suggests that circadian rhythm disturbances may be a major factor in at least the maintenance or even the onset of addictions.
Nicotine addiction
Nicotine is a stimulant and a paradoxical relaxant. It is hence commonly used off-label by patients with various disorders when they lack proper treatment. One well known example is schizophrenia, with rates of smoking far exceeding those in the general population. More than 40% of all tobacco is smoked by people with mental illness, but they are less likely to be given support to quit. It is also likely that a significant proportion of people with sleep disorders also use nicotine as an off-label neurostimulant and wakefulness promoter. Hence, it is not surprising that nicotine use is associated with sleep complaints, but whether nicotine is contributing to these complaints is highly debatable, as it may very well be that nicotine is used as a stimulant to compensate for a pre-existing sleep disorder. There is some evidence from a 2018 systematic review that tobacco smokers have a modified sleep architecture, with less deep sleep and more time spent in light sleep N1, and a more fragmented sleep, but this is not a direct evidence of the effect of nicotine on sleep, but of smoking, which can include pre-COPD pulmonary obstructions (not far from being a form of sleep apnea). Although nicotine is a known neurostimulant, its effects on sleep are poorly studied, as they are often confounded with smoking effects, which obviously cause obstructive respiratory illnesses, but it remains unclear whether nicotine itself, via its neurocognitive effects, can affect sleep. There is however no evidence it can affect the circadian rhythm or core body temperature.
Anecdotally, this is the case of the present document's author, who experienced non-24 since early childhood (or birth) with no difference since starting nicotine use via smoking then vaping in his 20s, and the 2nd level ancestor also did not use nicotine while displaying non-24 like symptomatology. Although there is still some debate as to whether the effects of nicotine is confounded by other components of cigarettes, the present document's author experienced all the same neurostimulant and paradoxical relaxant and even weight regulation effects using e-cigarettes with only three components in the e-liquid: nicotine, 20% vegetal glycerin and 80% propylene glycol. No aroma nor any other component used for a whole year. Only when nicotine was removed from the e-liquid, replaced by a strong menthol flavor, were the neurostimulant, paradoxical relaxant and weight regulation effects lost, as the author noticeable experienced withdrawal symptoms, including temporary suicidal thoughts. (Side-note: exact e-cigarette setup: Innokin GoZee+ with a 1.6 Ohm resistance and a 5 Watts configured Forz box battery, vapeenfer.com classic menthol contentrate flavor in dose of 5ml + 15ml of 50/50 VG/PG + 10ml 100% PG = 30ml of e-liquid with no nicotine but a throat hit-like sensation, if not sufficient, spice pepper flavor can be added, this concentration is roughly equivalent in terms of throat hit to 10-12mg/ml of nicotine).
Hence, for people ith a sleep disorder complaints who are smoker, it can be a very viable risk reduction strategy to transition to e-cigarettes with nicotine in the e-liquid (see also here), as cardiovascular complications are the major cause of death among smokers and e-vapor avoids all or nearly all of the cardiovascular risks associated with smoking cigarettes and drastically reduced risks of carcinogenics exposure (because there are very few components in e-liquids and there is no smoke hence no tar), which makes e-cigarettes a recommended risk reduction tool for smokers with COPD (see also here and here), and no secondary smoke with e-cigarettes, although nicotine can still accumulate in an unventilated room (see also here, here and here) and may increase respiratory impairments in young adults exposed to secondhand nicotine vaping and hence should be avoided in the presence of children. Secondary smoke of cigarettes can trigger 13 months earlier menopause in women. Beside nicotine, other components of e-liquids, such as propylene glycol, vegetal glycerin (glycerol), and menthol, are all considered GRAS (generally recognized as safe for human consumption). Studies on modern smokeless tobacco, a proxy for pure nicotine until more studies are done on e-cigarettes, show that risks are much reduced from smoking tobacco (see also here, here, here and here). E-cigarettes thirdhand risk exposure is also much more reduced than thirdhand smoke from cigarettes (eg, re-aerosolization of particles from cloths) which is a serious source of contamination and health hazard for non-smokers (see also here). The above applies only to DIY e-liquids, as for branded e-liquids there can be up to 70 unlisted constituents. It's worth noting that aerolizing nicotine creates acroleine and glycidol derivatives that may be the causes of the adverse health effects of e-cigarettes. There is some evidence that the cardiorespiratory effects of vaping can be reversed instantaneously by cessation, for both nicotine and nicotine-free vaping, and interestingly, it appears that nicotine-free vaping also induces some reversible alterations of pulmonary epithelial cells.
For those contemplating nicotine cessation (ie, removing nicotine even from e-cigarettes), it is worth mentioning that weight loss is a frequent side effect of successful cessation, with a mean weight gain of 4 kg after 1 year and 5 kg over 5 years, with most of the weight gain appearing in the first 6 months after nicotine cessation (see also here). The meta-analysis also found that smoking cessation helps, such as varenicicline, bupropion and nicotinic substitutes do not reduce the weight gain after one year, they only make it more progressive over time. The anorexigenic effect of nicotine has been found to rely on the stimulation of POMC neurons. Weight gain after smoking cessation is the self-reported major cause for failure for 50% of women and 30% of men, and various factors that affect the quantity of weight gain that can be expected, such as starting nicotine younger, and also stopping nicotine younger, with about a third gaining more than 10 kg after 5 years.
There is also a less known beneficial effect of nicotine: protection against Parkinson neurodegenerative disease, as indeed smokers are 40% less likely than non-smokers according to epidemiological data, but this effect may only work for women.
Bupropion can be an especially interesting therapeutic option for individuals with DSPD who are smoking or vaping nicotine, as it can treat nicotine addiction and is approved for smoking cessation, and has shown some efficacy for treating ADHD. Furthermore, a case study found bupropion may be effective at shifting the circadian rhythm to treat DSPD and sleep inertia, arguably through hyperphotosensitization, and hence it likely needs to be combined with bright light therapy for this purpose. (TODO add ref: Just make sure to use a dose of 300mg or lower, as doses above can cause major side effects such as epileptic seizures, as was observed in the earliest studies in the 80s that used 800mg). However, the effects of bupropion on the circadian rhythm are only temporary, as it appears from users reports that the effect fades away after at most 6 months.
Modafinil is another commonly prescribed wakefulness agent / stimulant to people with sleep disorders. Unfortunately, clinical trials demonstrated a reinforcing interaction between modafinil and nicotine. Indeed, modafinil increases nicotine's effect, in both ways: withdrawal is worsened, but nicotine intake's effect (positive mood, energy) are increased too (see here and here).
Other comorbidities
Neuroscientific progress is not linear, this is a "delusion": it has an erratic course and sometimes veers backward and in many cul-de-sacs with strong influences from external factors including subjective such as fashion, social, socioeconomic and "the full gamut of human failings" and with psychoanalytic thought found in almost all social discourses, as eloquently illustrated by this historical retrospective on epilepsy research. Indeed, the history of epilepsy research provides a very interesting model of how the scientific knowledge progresses over time about a mysterious medical condition of unknown cause ("idiopathic"), with nowadays dozens if not hundreds of potential causing and triggering factors or epilepsy have been identified, as reviewed in a subsequent book by the same authors on the causes of epilepsy.
If you suffer from an addiction and wish to pursue a rehabilitation, beware of scams, as a lot of rehab centers and programs have shady practices and do not take the sleep of their patients into account. Indeed, insomnia and sleep issues is often an issue during rehab due to the withdrawal syndrome, so that any rehab center without any procedure nor staff to help their patients at night should be avoided. Choose a science-based rehab center and with staff and procedures at night.
Circadian rhythm disorders and especially non-24 are often misdiagnosed as schizotipical/schizoid/schizophrenic disorders by psychiatrists, psychologists and psychotherapists, because of the dissociative symptoms such as depersonalization/derealization (see also here and here), and social isolation caused by the chronic sleep deprivation.
Dissociative Identity Disorder (DID) and PTSD often co-morbid with "complex sleep disorders" and so one can assume that circadian rhythm disorders likely are more common in this population. Indeed, some scientists, including the leading works from Dr. Dalena van der Kloet but this comes back to at least 1970 with Gove's theory or maybe even centuries earlier, even argue that the commonly accepted psychological assumptions underlying current treatments for dissociative disorders and trauma-induced disorders are incorrect and not supported by empirical evidence, and they rather argue that these symptoms and the social difficulties rather stem from sleep disorders (see also here and my discussion on reddit). If the latter scientists are correct, then Post-Traumatic Stress Disorder (PTSD) should in fact be more properly labelled Post-Traumatic Sleep Disorder, with the bonus side effect that the acronym would remain unchanged. Mechanistically, another study found that "sleep deprivation in the immediate aftermath of trauma could be a potential contributor to posttraumatic stress disorder development and maintenance via interference with natural extinction processes". Note that it's the combination of trauma and sleep disturbances that leads to trauma-induced disorders such as DID and PTSD, sleep disturbances alone are not sufficient. Another study on autobiographical memory consolidation following sleep deprivation found that sleep deprivation decreased recall of positive memories but increased recall of negative memories. Yet another study rather found that sleep selectively stabilizes negative aspects of negative memories, which supports the hypothesis that sleep attenuates the emotional weight of negative memories. A systematic review on the effect of sleep deprivation found that 52% subjects experienced dissociative symptoms (depersonalization and derealization). This hypothesis of early life trauma inducing lifelong sleep dysregulations which in turns cause the observed behavioral disorders is further supported by a 2019 rat study. This association, or even causative factor, may have been missed for a long time since historically, sleep disturbances in children have largely been ignored by the psychomedical field.
Here is an excerpt of van der Kloet's work:
> Conventional wisdom holds that dissociation is a coping mechanism triggered by exposure to intense stressors. Drawing on recent research from multiple laboratories, we challenge this prevailing posttraumatic model of dissociation and dissociative disorders. Proponents of this model hold that dissociation and dissociative disorders are associated with (a) intense objective stressors (e.g., childhood trauma), (b) serious cognitive deficits that impede processing of emotionally laden information, and (c) an avoidant information-processing style characterized by a tendency to forget painful memories. We review findings that contradict these widely accepted assumptions and argue that a sociocognitive model better accounts for the extant data. We further propose a perspective on dissociation based on a recently established link between a labile sleep–wake cycle and memory errors, cognitive failures, problems in attentional control, and difficulties in distinguishing fantasy from reality. We conclude that this perspective may help to reconcile the posttraumatic and sociocognitive models of dissociation and dissociative disorders.
Interestingly, another link between circadian rhythm disorders and PTSD and depression may lie in the glucocorticoid secretion and immunity system, which were found to be regulated by the circadian rhythm and ultradian cycles. Indeed, "disrupted circadian glucocorticoid cycling is a relatively consistent feature in clinical studies of patients with depression or PTSD" and "blunted circadian cortisol oscillations are a feature common to both PTSD and depression." Hence, what may be thought as chronic stress may in fact be rooted in a dysregulation of the circadian rhythm, itself dysregulating the stress system presenting itself as if it was chronic stress. Sleep disturbances are common in PTSD (70-91% report at least one), although this includes nightmares as a disturbance and with inconsistent evidence of objective sleep disturbance (ie, lack of evidence that PTSD is associated with sleep disorders). A phase-3 clinical trial found that 67% of PTSD patients were cured by a MDMA-assisted therapy.
Dissociative disorders can also be of organic origin, such as epilepsy (see also here).
Sleep paralysis has been consistently associated with sleep disturbances and insomnia.
Someone with one psychological disorder is likely to have others as well: "Clearly, high comorbidity with another disorder is a feature of many psychological disorders." Why is this the case requires further investigation, but may be an evidence of overdiagnosis by psychologists and psychiatrists (see also here).
Bright light therapy is also investigated to treat several other mood related or circadian modulated diseases and disorders, such as premenstrual syndrome (PMS) premenstrual depression or dysphoria (PMDD), bulimia, postnatal and postpartum depression, perimenopausal depression, and monopausal sleep disorder.
Narcolepsy is an interesting potential comorbidity, or potentially even a misdiagnosis. Indeed, there are studies observing circadian dysregulations as measured with distal-to-proximal temperature samples, a proxy for core body temperature relative changes, in narcolepsy, similar to DSPD or potentially non-24, with a high circadian phase in the night and decreases in the day, explaining the high vigilance at night and the daytime sleepiness during the day.
Adaptations of this protocol for other circadian rhythm disorders (DSPD, nightshift workers)
The tools (zeitgebers) to modify the circadian rhythm are the same for all other circadian rhythm disorders. These therapies are even used by Canadian Air Forces and NASA's Mars missions crews. What changes between various conditions are mostly two points:
- the individual's intrinsic circadian rhythm, since zeitgebers efficacy is dependent on the timing of use/intake/exposure relatively to circadian rhythm.
- the goal: phase advance (waking up earlier) or phase delay (sleep later) or entrainment (stay stable)?
The following subsections will describe some adaptations that the author would suggest to optimize the therapeutic efficacy of the treatments presented in the present document, although please bear in mind the author could not thoroughly test these suggestions since he is not afflicted by these conditions.
Adaptations for DSPD
Delayed Sleep Phase Disorder (DSPD) is a highly prevalent disorder that potentially affects 5.1% of the global population, with at least 0.6% of the global population carrying a known gene that is associated with Famillial DSPD and is especially prevalent in adolescents (7-16%). DSPD accounts for 10% of all sleep disorders and is often misdiagnosed as sleep-onset insomnia, despite being as easy to diagnose as measuring the pupillary light reflex speed. Indeed, as show in a case study, diagnosed insomniacs who can sleep soundly with sleeping pills such as zolpidem but who still struggle with waking up on time are likely to have a circadian rhythm disorder such as DSPD. About half (47%) of individuals with DSPD are light hypersensitive, suggesting they can be responsive to light therapy. DSPD was first defined in 1981 by Weitzman et al as "a chronobiological disorder with sleep-onset insomnia". Contrary to sleep shaming prejudices, individuals with DSPD actually spend less time in bed than typical sleepers, with their "cardinal symptoms" being insomnia and fatigue, and not excessive daytime sleepiness. In addition, they do not require more extra sleep hours during the weekend than weekday compared to the general population, meaning that they suffer as much social jet lag as anyone else, but not more, which means that their insomnia difficulties during the workweek are not caused by a hypothetically too different sleep pattern during the weekends.
This review offers a concise but accurate definition and description of DSPD and its etiology:
> The developmental changes in the circadian and sleep systems we have described may be exaggerated in adolescents who receive a diagnosis of delayed sleep phase syndrome (DSPS). DSPS is a disorder with a typical onset in the second decade of life or earlier [75]. Weitzman, Czeisler, and colleagues [76,77] first described delayed sleep phase insomnia as a distinct syndrome characterized by a cluster of features including a chronic inability to fall asleep and wake at a desired clock time, consistency in reported sleep times at later hours than other individuals, and otherwise normal sleep when measured by all-night polysomnography if the delayed schedule is allowed. An important characteristic of the syndrome is that patients are able to initiate and maintain sleep on their normal delayed schedule; difficulties manifest only when they attempt to synchronize their sleep schedule with requirements of normal everyday schedules of society. As a result, patients with DSPS are locked into a sleep schedule that is out of phase with usual work and school requirements. Other consequences of DSPS include sleep loss, disturbed sleep, excessive daytime sleepiness, and impaired waking function.
Chronotype repartition among the general population is known to follow a bell shaped curve, in other words a gaussian distribution, which strongly suggests a random and natural variability. In other words, the repartition is normal (in the mathematical sense), no two individuals have the exact same chronotype/circadian rhythm, and it's ingrained in our biological, genetic code. Indeed, it is estimated that ~40% of sleep disorders are inherited, and 46% to 70% of the circadian rhythm is genetically inherited, with minor influence from environmental factors, with similar heritability for the propensity to regularly do a siesta. In fact, heritability of the circadian rhythm is so strong that it was shown that rats kept in the dark for generations maintained the same circadian rhythm, which strongly suggests that behavioral exposure to light patterns cannot affect the circadian rhythm between generations (neither negatively - by bad habits - nor positively - by following an entrainment therapy, children likely won't benefit from genetic improvements). The repartition of chronotypes in the general population is about 30% of morning larks, 40% of night owls and the rest in-between (see also this informal review). Circadian rhythm disorders such as DSPD or non-24 are not accounted in these statistics, and are likely on the tails of the bell-shaped curve ("extreme" chronotypes). A genetical study by Dr. Alina Patke estimated that potentially 0.6% of the population is carrying a gene mutation CRY1 that may cause Famillial DSPD (ie, inheritable DSPD), while another large-scale study found a heritability of 13.7% of diurnal preferences. This biological diversity is further supported by some evidence that prehistoric mammals were likely all nocturnal to avoid the oversized reptilian predators that were the dinosaurs, and only later some mammals, including humans, switched into bright light vision (see also here for a layman summary). In summary, it is likely that most cases of DSPD are genetically inherited.
Contrary to common misconceptions, individuals with DSPD are not lazy, as DSPDs put (much) more efforts to wake up on a daily basis than their morning lark peers:
> [...] Individuals with evening chronotype (preference for later timing of sleep and activity) have been shown in some studies to have a shorter phase angle between circadian markers and sleep, indicating that they sleep and wake earlier in their circadian phase (Duffy, Rimmer, & Czeisler, 2001; Mongrain, Carrier, & Dumont, 2006).
Indeed, evening chronotypes suffer from more frequent circadian misalignment than other chronotypes due to the societal norm of the diurnal 9-5 work schedule, which causes decreased well-being and increased anxiety and depression along with other physiological health issues such as cardiometabolic:
> Circadian misalignment is a potential explanation for the link between diurnal preference and mental health and wellbeing, with evening people tending to be more misaligned [13, 49].
> [...] Observationally, we demonstrated that more misaligned individuals (i.e., higher CPD) were more likely to report depression, anxiety and have lower wellbeing.
> [...] Societal determination of work time and free time can interfere with an individual’s diurnal preference [13,14,15]. For example, evening people (late preference) experience this mismatch when they are forced to wake early for work, while early types might be forced to stay up longer on weekends to adhere with social norms [16]. This phenomenon has been coined “social jetlag”, and can be quantified by calculating the shift in sleep patterns (in hours) between work and free days [17].
> [...] Our findings fit with previous evidence that evening people may experience more circadian misalignment, as their chronotype is often mismatched with diurnal (9–5) schedules, which are the societal norm [16]. Individuals with a physiological tendency towards delayed sleep and circadian timing are especially prone to further delay by modern schedules and lighting, resulting in greater social jet-lag [50]. Our findings also build on existing evidence of circadian misalignment in shift workers, who often work against their diurnal preference, with some studies suggesting that these individuals have a higher prevalence of depression and lower wellbeing [51, 52].
Despite, or rather due to, the efforts of individuals with DSPD to nevertheless conform to typical 9-5 work schedule, at the expense of chronic sleep deprivation and their health, they are at least 5 times more likely than morning larks and intermediate chronotypes to retire early on a disability pension. Unfortunately, this result leads some scientists, who clearly lack an expertise in circadian rhythm science, to suggest to avoid prolonging the work career of evening chronotypes, instead of tackling the root issue of circadian misalignment and inadequate work schedule. Indeed, modern society require more and more night shift jobs, we live in a 24h society especially in urban areas, and this kind of work is perfect for individuals with DSPD. Alas, the current widespread misconceptions about sleep and the circadian rhythm makes leaders preferentially choose morning larks or intermediate chronotype to do night shift jobs, at the expense of their health and causing increased work accidents and underperformance during night shifts. If jobs were assigned to corresponding chronotypes, this would be a win-win for everyone, including for the customers. And there is some hope for change, thanks to the worldwide movements to maintain the flexible work schedules that were widely adopted during the COVID-19 pandemic.
A study suggests that half of the people diagnosed with DSPD may not have circadian misalignment. Indeed, In a study on a cohort of individuals with DSPD which monitored melatonin levels through salivary samples, the researchers found that half of the individuals did not in fact have a delayed melatonin profile and had less delay in their sleep-wake schedule: they were behavioral DSPD, but not circadian DSPD (see also here). Hence, it's important to know which kind of DSPD an individual has, as the treatment can be different. For a non-circadian DSPD, a behavioral intervention (ie, sleep hygiene) may be sufficient, and may explain why there are sporadic reports of "cure" on peer group forums such as reddit, that never work for a big part of the other patients, as they have a circadian DSPD that requires a therapeutic intervention such as light therapy and melatonin. A major difference between behavioral and circadian DSPD is the energy levels during late hours: we can hypothesize that individuals with behavioral DSPD do not sleep optimally when their night is delayed later as it would fall late in their circadian night and hence would experience a shorter night since their wake up time is still defined by their non-DSPD circadian phase, and hence would likely sleep better if they were going to bed earlier, whereas circadian DSPD consistently report that their sleep deprivation subsides as well as various other health and cognitive improvements when they sleep under their biological delayed night, and accumulate sleep deprivation when they try to sleep earlier.
DSPD is highly comorbid with ADHD (73-78% of children and adults with ADHD also have DSPD) and autism, and these comorbidities and social features may mask the charateristics of DSPD, delaying diagnosis and treatment. In some cases, DSPD can even be misdiagnosed as ADHD.
The major difference between DSPD and non-24 lies in the intrinsic parameters of the circadian rhythm: whereas non-24 have an always shifting circadian rhythm, DSPD have a fixed circadian rhythm and hence ideal sleeping time, just like typical sleepers, but delayed. More technically, we could say that N24 don't have a preferential intrinsic sleep schedule due to the ever changing circadian rhythm, but DSPD do have one.
In theory, individuals with non-24 can use their natural freerunning ability to wait and be in phase with any sleep schedule they want, and then "freeze" (entrain) their circadian rhythm in place to maintain the target sleep schedule. To do that, individuals with non-24 only need therapies that produces a relatively small phase advance, just the same amount as their natural daily phase delay (eg, for an individual with a 25h day, hence a 1h daily phase delay, a therapy producing a 1h phase advance is theoretically sufficient for entrainment).
On the other hand, individuals with DSPD already have a fixed circadian rhythm, hence the phase advance therapies will only "stretch" their circadian rhythm. In theory, this means they can only wake up earlier as much as the phase advance they get from the therapies.
Let's take an example: with a therapy producing a phase advance of 1h, an individual with DSPD with a circadian rhythm fixed at 4am-12am will see their sleeping schedule shifted to 3am-11am after therapy. With the same therapy, an individual with non-24 such as a 25h day who wants to wake up at 8am can freerun and wait until their sleep is from 12pm-8am before starting the therapy. With this 1h phase advance therapy, the DSPD individual cannot consistently wake up before 11am, whereas the non-24 individual can choose virtually any time by leveraging their freerunning ability.
This changes the goal of the therapies: whereas non-24 just need a small but reliable phase advance, DSPD need to combine multiple phase advance therapies to get more phase advance.
In practice, the current protocol should be adapted as follows:
- No need to wait before starting the therapy, since there is no need to freerun before being in phase.
- A longer light therapy can be used to get more phase advance.
- Other weaker zeitgebers such as physical exercise can be used in complement to light therapy, melatonin and food to get additional phase advance.
- The rest is left unchanged. For example, zeitgebers can be expected to work the same because all humans have apriori the same fundamental physiology, so for example light therapy should still be most effective at natural wake-up time, and the longer the exposition the more phase advance. The health issues of sleep deprivation and circadian misalignment for DSPD individuals who try to constraint their sleep should also be the same, since the same basic biological processes are at play (oxydants accumulation due to reduced endogenous melatonin secretion, cognitive deficiencies increasing the risk of accidents, etc).
Although it was hypothesized that forced freerunning (phase delay chronotherapy) could be used too by individuals with DSPD, in some cases this may have turned into a non-24 disorder, hence it is now disadvised to try this approach until there is further clinical trials.
Reddit user DiminishedGravitas wrote an extensive review of his own experiment and adaptations of the protocol for DSPD: https://www.reddit.com/r/DSPD/comments/mnzwtq/waking_up_6_hours_earlier_after_6_days_of/
See also the Q&A on DSPD and the similar set of recommendations from the Circadian Sleep Disorders Network.
Another avenue is to adapt one's sleep schedule, especially by adopting a biphasic sleep schedule, which has anecdotally been reported by DSPD reddit members as a good strategy. An experimental but very promising extension of this idea is to use bright light therapy with a precise artificial schedule to cause a stable biphasic sleep pattern called LDLD, which ensures robust circadian alignment, kind of an improved biphasic sleep where the nap is really a second night with melatonin production and everything, not just a nap. Scientists are strongly suggesting this may be a promising avenue to reduce the detrimental health issues of long term shift work, although this require further validation or even proof that LDLD is achievable consistently in humans, as this is for the moment only demonstrated in animals.
Adaptations for Advanced Sleep Phase Disorder (ASPD)
Advanced Sleep Phase Disorder is a circadian rhythm disorder that is characterized by individuals sleeping and waking up too early, often before or at the first rays of sunrise. Typically, these individuals sleep at about 5-9pm and wake up at about 2-5am. Although this disorder may at first appear as a sort of extreme morning lark, and hence may seem more socially acceptable, it can still be very disabling, especially for social duties. Although this disorder most often affect older individuals, since the circadian rhythm tends to get shorter and/or more sensitive to zeitgebers with older age, individuals of all ages can be affected. A demographics study in Cyprus estimated the prevalence of ASPD in the general population at 0.5%.
For the standard therapy, see the section elsewhere in this document: "Phase-delay bright light therapy (true chronotherapy)".
Adaptations for night shift work disorder
Whereas non-24 and DSPD are intrinsic circadian rhythm disorders, shift work disorder along with jet lag are extrinsic circadian rhythm disorders, meaning they are caused by external factors, such as work constraints.
Night shift disorder is kind of the inverse of the non-24 disorder: whereas individuals with non-24 naturally freerun and accumulate sleep deprivation and health issues by trying to constraint to a fixed sleep schedule, night shift workers are typical sleepers with a naturally fixed sleep schedule but who suffer from sleep deprivation and health issues by being constrained to a rotating (ie, freerunning) work schedule. In both cases, this result in progressive misalignment between the internal circadian rhythm and the day-night cycle and the work schedule.
In any urban economy, about 20% of the workforce is required to work night shifts. Sleep disorders, affecting 50% of the worldwide population, associated with night shift workdisorder are becoming a public health issue due to the sheer prevalence of night shift work: "In industrialized countries, 75% of the total workforce is estimated to have been involved in shift work and night work.", with circadian disruptions due to mistimed and uncontrolled exposure to bright light during the biological night and sleep deprivation being a major factors contributing to the health risks associated with night shift work, leading to life-threatening complications such a "50-100% increased prevalence in breast cancer" and depression (see also this account):
> Shift and/or night work generally decreases the time spent sleeping, and it disrupts the circadian time structure. In the long run, this desynchronization is detrimental to health, as underscored by a large number of epidemiological studies that have uncovered elevated rates of several diseases, including cancer, diabetes, cardiovascular risks, obesity, mood disorders and age-related macular degeneration. It amounts to a public health issue in the light of the very substantial number of individuals involved. The IARC has classified shift work in group 2A of “probable carcinogens to humans” since “they involve a circadian disorganization”.
> The potential and multifactorial mechanisms of the effects include the suppression of melatonin secretion by ALAN, sleep deprivation, and circadian disruption.
> A further complication of phase manipulations is that effective phase-shifting may require complex schedules of light (Revell and Eastman, 2005; Dumont et al., 2009). In humans and other animals, the phase-resetting actions of light are greatest during subjective night: light at the beginning and end of subjective night delays and advances the pacemaker, respectively (Aschoff, 1999). Inappropriately timed light can therefore not only undo an achieved phase-shift, but actually move an individual’s clock further from the desired schedule. In particular, bright morning light at the end of a night shift has strong phase-advancing effects that counteract the desired shift (Crowley et al., 2003). Empirically, even in individuals with permanent schedules, for whom a stably altered phase may be reasonably expected, the clock generally remains entrained to a phase typical of day workers. Only one in four shift-workers with a permanent schedule outside of the regular nine to five workday are able to even partially shift circadian phase as measured by melatonin, while only 3% fully shift (Folkard, 2008). Thus, despite a solid understanding of how the human circadian rhythm responds to light, the lack of practically implemented strategies for shift-workers underscores the need for an alternative target of circadian manipulation that may be more responsive and more tractable than phase.
It was estimated in 2021 that shift work disorder affects 6.7% of the general population, and is hence the most common type of circadian rhythm disorder.
The circadian misalignment induced by nightshift work also increases the likelihood of drowsiness and driving accidents: "In naturalistic conditions, subjective and objective sleepiness and driving events are increased following night shifts, even during short (~30 minutes) commutes and exacerbated by an interaction between circadian phase and duration of wakefulness."
To illustrate the burden of circadian misalignment, the following anecdote about a NASA's monitoring crew for Mars missions is especially enlightening:
> Prior missions have demonstrated that working Mars day schedules without appropriate countermeasures can cause severe problems with sleep, performance, and compliance. Reports from the earlier Mars Pathfinder missions that did not employ dedicated circadian and sleep countermeasures indicated less success in adaptation to the Mars day schedule than our current study. Based on NASA surveys of 24 Mars Pathfinder veterans, those supporting the Sojourner Rover indicated that fatigue significantly affected their performance at work to the extent that they discontinued work on the Mars day schedule after only one month and described the schedule as “broken.” JPL managers described the scientists' and engineers' discontinuation of the Mars day schedule as a “rebellion.”
>
> Performance data were not reported in the MER technical report, although Bass and colleagues reported one MER team member was injured after a series of Mars time shifts when he mistakenly walked into a wall and another reported falling asleep at the onramp to the freeway. A previous two-week “Mars analog” study (but conducted on Earth time) in four subjects did not show decrements associated with time awake in PVT performance or subjective sleepiness (KSS). The authors attributed this result to the high motivation of the crew, although motivation has limited ability to override circadian and homeostatic regulation of alertness and performance and is, in fact, subject to these influences itself.
Occupational studies estimated 6.5 years of cognitive decline in long-time shift workers compared to age-related controls, which persists for 5 years after conclusion of shift work.
Although the first sleep deprivation is felt as the worst, habituation to sleep deprivation does NOT enhance cognitive performance, as it stays constantly reduced compared to no sleep deprived performance: "Although healthcare workers perceive themselves to be less alert on the first night shift compared to subsequent night shifts, objective performance is equally impaired on subsequent nights." The wide array of cognitive impairments consecutive to sleep deprivation are well known since decades, especially in medical schools, which prompted the following question to be raised in a 1973 paper: "It is suggested that rested house officers make better physicians than sleep deprived ones, and this question is raised: why are schedules that make repetitive sleep deprivation mandatory part of the training programs at many medical centers?"
Beyond cognitive impairments, there is moderate grade quality evidence that shift work and long work hours significantly increase the risk of (breast) cancer and cardiometabolic diseases such as strokes according to a 2020 systematic review, with night shift work leading to "a 4% increased risk of ischemic stroke for each 5 years of shift work". A recent conference report found that "for each hour that an employee’s work schedule deviated from their natural body clock, their odds of having high cardiovascular risk increased by 31%". In line with studies on the melatonin-insulin interaction, a study found that eating meals during night shifts led to an insulinoresistant-like state, with "postprandial insulin, glucose, and triacyglycerol (TAG) levels were significantly elevated during night shifts compared to the day time shift", and with triacyglycerol levels remaining elevated even after returing to a day shift schedule. There are lots of studies documenting the wide increase in health risks for night shift workers.
Since circadian misalignment also reduces the immunological system, it also increases the risk of infections such as COVID-19 and its severity. In practice, it was found by a large and well controlled study that shift workers were 2 to 3 times more likely to get infected by COVID-19, with the highest odd being for those who performed irregular/rotating night shift work. Sleep deprivation also independently increases the odds by 12% for each 1-hour decrease in sleep.
Night shift workers hence suffer from similar health issues due to chronic sleep deprivation and circadian misalignment as other intrinsic or extrinsic circadian rhythm disorders. The treatment is hence also similar, and is focused on reducing the misalignment of light exposure by using a combination of light therapy and dark therapy, and avoid sleep deprivation. However, note that all these treatments can only speed up the circadian phase shift, but it will still be progressive, as there is no currently available treatment to proceed to a complete phase reversal. Indeed, no living organism can adapt to such a rapidly rotating schedule (eg, a day shift of 9am-5pm followed the day after by a night shift of 9pm-5am — yes this really exists), and hence why rapidly alternating schedules are disadvised by shift work societies.
> Most observations on subjects with complete, or near-complete, phase shiftschange in longitude during travel (41, 82, 198) or strict light-darkness routine in the Arctic (225)-have found that about 3 days elapse before the temperature rhythm is adjusted to the new phase, and the same has been found with workers on a night shift (24). An even more rapid adaptation can occur (18g), but a week may be insufficient (I 76>, and night workers in hospital have been found to take as long as 3 weeks to adapt their temperature rhythm (238). Most significantly, on returning to day work their rhythms took much the same time to return to the normal pattern, clear evidence of an endogenous rhythm that was here exactly out of phase with the environmental periodicity.
Source.
> Under conditions of circadian perturbation in which environmental conditions abruptly change, e.g., flying across multiple time zones, the temporal synchronization of circadian oscillators with the environment is suspended. Within the SCN, circadian neurons become desynchronized from each other with resynchronization taking 5–7 d after long-phase delays and 9–13 d after long-phase advances (Nagano et al. 2003). Similarly, peripheral oscillators across tissues and organs also need to resynchronize with each other and the SCN, and generally take longer to resynchronize than the SCN (Yamazaki et al. 2000). However, the length of time necessary to achieve stable phases attuned to the new photoperiod varies between tissues and organs (for review, see Harrington 2010), with ∼8 d necessary for full peripheral resynchronization following a 6 h phase advance (Davidson et al. 2009; Kiessling et al. 2010).
Source.
Depression is not uncommon with nightshift workers. This can likely be explained by two interrelated factors: uncontrolled/lack of bright light exposure, and circadian misalignment.
The lack of bright light exposure or aberrant exposure in the biological night (see also here) was shown to cause depressive symptoms in non depressive individuals. And as shown abovve, this also contributes to circadian misalignment.
Interestingly, light exposure was shown in a recent systematic review to be as effective as antidepressants to treat both seasonal and non-seasonal depression (see also here and here).
So that's one thing you can try, is to control more tightly your exposure to bright light, by being exposed to bright light during your shift (your relative day), and avoid bright light exposure when you go home to sleep after your shift by wearing blue blocker glasses and a black silk eye mask (this is the protocol of this study on night shift workers).
Another potential area of improvement is to maintain the shifts obtained in the circadian rhythm by maintaining the same sleep schedule on week-ends, according to a study of five strategies used by nurses. Another strategy, napping regularly, is also viable. In one rare study on mitigation strategies for rapidly rotating shift work schedules, long napping before shifts was found to significantly reduce the sleep debt incurred by the necessarily shorter sleep after the shift due to circadian misalignment preventing full sleep. Given the significant reduction or elimination of health issues, cognitive impairements and work errors due to sleep deprivation, a 2020 review concluded that napping while on duty should be allowed for public safety shift workers, in line with a previous systematic review. A 2020 systematic review found that most shift workers practiced daytime napping and caffeine consumption, "in line with best-practice fatigue-management strategies, but contrary to existing sleep hygiene recommendations". Another review found that intra-shift naps improved sleep-related performance and reduced sleepiness, contrary to caffeine which improves vigilance at the expense of an worsened sleep quality and duration, which suggests that napping is likely preferable to caffeine both to reduce the error rates and the worker's health.
Although there is a whole body of studies showing the importance of bright light exposure management and napping for night shift workers to reduce the impact on their health, to the extent this is part of the guidelines of the Working Time Society, do not expect too much out of it: zeitgeber based tools such as light therapy/dark therapy can work wonders yes, but they can only nudge your circadian rhythm. If you have a "normal" circadian rhythm, it's unlikely you will ever feel fully comfortable doing a nightshift job, although some people have a more malleable circadian rhythm than others, but not everyone.
An experimental but very promising adaptation is to combine both of the previous strategies: using bright light therapy with a precise artificial schedule to cause a stable biphasic sleep pattern called LDLD. Scientists are strongly suggesting this may be a promising avenue to reduce the detrimental health issues of long term shift work, although this require further validation or even proof that LDLD is achievable consistently in humans, as this is for the moment only demonstrated in animals.
More experimental interventions, mostly tested on animals only at the moment, include time-restricted feeding, that may reduce cognitive impairments such as anxiety and depression due to shift work in mice.
Given that the individual's chronotype determine their capacity to adapt to specific sleep-wake schedules and the deleterious effects on health when these are not matched, including predisposition to cardiovascular and metabolic diseases such as diabetes, several scientists call for employers to match shift work schedules with employees' chronotypes. Indeed, a study demonstrated that aligning work schedules with chronotypes allowed for clinically significantly improved workers sleep and mood, including 0.5h more sleep on work days and 1h less social jet lag, and that evening chronotypes better tolerated frequent night shifts. In 2021, a large-scale study demonstrated that mismatching the work schedule with the individual's genetical chronotype led to decreased well-being and increased anxiety and depression, in line with a previous study showing that mismatching chronotype and work schedule leads to a much more variable sleep schedule and increased circadian misalignment. This is line with previous studies using phasor analysis finding that shift working nurses had a similar circadian rhythm mismatch with bright light exposure as experimentally jet lagged rats.
At the employer's level, scientific and industrial societies recommend to proceed to targeted interventions on regulating electric bright light exposure on their workers only where necessary, as to reduce circadian dysruption as much as possible.
TODO: rewrite below
BEST: strategy to delay circadian rhythm: mix light-dark exposure + eye mask to sleep correctly in the dark + blue blocker glasses: A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. https://www.ncbi.nlm.nih.gov/pubmed/16887753 and formally advised by: Working Time Society consensus statements: Evidence based interventions using light to improve circadian adaptation to working hours. https://www.ncbi.nlm.nih.gov/pubmed/30700675
However, even with a full and stable change of shift to be completely at night for an extended period of time without progressive rotation (ie, the "night float paradigm"), it still reduces sleep duration and quality and affect performance and vigilance of the healthcare workers because of circadian misalignment. This goes counter to the circadian plasticity theory.
[25]: Light-Dark PRC accounts for 71% of the circadian rhythm variability: https://www.ncbi.nlm.nih.gov/pubmed/29589871
[26]: BEST: evidence of interaction between circadian and homeostatic sleep processes, confirming Borbély's theory: «In naturalistic conditions, subjective and objective sleepiness and driving events are increased following night shifts, even during short (~30 minutes) commutes and exacerbated by an interaction between circadian phase and duration of wakefulness.», Sleepiness and driving events in shift workers: the impact of circadian and homeostatic factors., https://www.ncbi.nlm.nih.gov/pubmed/30882154
> Of the 416 hospital workers who participated, two in five (40.9%) screened positive for a sleep disorder and 21.6% screened positive for depression or anxiety. After multivariable adjustment, screening positive for a sleep disorder was associated with 83% increased incidence of adverse safety outcomes. Screening positive for depression or anxiety increased the risk by 63%. Sleep disorders and mood disorders were independently associated with adverse outcomes and contributed additively to risk.
Sleep disorders, depression and anxiety are associated with adverse safety outcomes in healthcare workers: A prospective cohort study., https://www.ncbi.nlm.nih.gov/pubmed/30069960
BEST: Shiftwork Disorder Screening Questionnaire https://www.ncbi.nlm.nih.gov/pubmed/23204612
"Finally, the higher incidence of cancer could be caused, in part, by light during the night shift. Light suppresses the secretion of the neurohormone melatonin which is an antioxidant." -> melatonin supplementation and blue blocker glasses may reduce the cancer (and immunodepressive) incidence + 20 years of studies and experiments on circadian realignment + protocol for partial alignment: https://www.cdc.gov/niosh/nioshtic-2/20045415.html
Practical circadian interventions for night shift work, Eastman CI - "primary proposed mechanism for a possible shift work effect on breast cancer risk is also by hormonal perturbations" http://www.hazards.org/cancer/graveyardshift.htm - "The results provide further evidence that night shiftwork may increase the risk for breast cancer and suggest that the largest impact on risk is associated with the most disruptive shifts." - Echoing a concern raised separately by both Schernhammer and Stevens, Hansen said in the years after cessation of exposure, the passage of time “attenuates or fully eliminates” previously observed risks. (ME: so if we start to freerun and sleep according to our circadian rhythm, over time the risk decreases as the hormonal perturbations gradually level out).
Modafinil RCT on night shift disorder: Cognitive Performance Following Modafinil Versus Placebo in Sleep-Deprived Emergency Physicians: A Double-Blind Randomized Crossover Study, 2006 https://pubmed.ncbi.nlm.nih.gov/16436796/
- "Modafinil increased certain aspects of cognitive function and subjectively improved participants' ability to attend post-night-shift didactic sessions but made it more difficult for participants to fall asleep when opportunities for sleep arose."
[38]: Sleep strategies of night-shift nurses on days off: which ones are most adaptive?, 2014, https://www.frontiersin.org/articles/10.3389/fneur.2014.00277/full - my summary on reddit: https://www.reddit.com/r/Nightshift/comments/gezng0/should_i_change_my_sleep_schedule_whenever_i_get/fq1aa64
[39]: https://www.researchgate.net/publication/8001986_The_Nighttime_Nap_Strategies_for_Improving_Night_Shift_Work_in_Workplace
Other tips: https://doi.org/10.1097/cnq.0000000000000152
Adaptations for chronic jet lag
NOTE: This section is a work-in-progress.
Bright light therapy can also be used to shift the circadian rhythm in the desired direction, before and after the travel across timezones.
https://doi.org/10.1101/lm.038877.115
- "Under conditions of circadian perturbation in which environmental conditions abruptly change, e.g., flying across multiple time zones, the temporal synchronization of circadian oscillators with the environment is suspended. Within the SCN, circadian neurons become desynchronized from each other with resynchronization taking 5–7 d after long-phase delays and 9–13 d after long-phase advances (Nagano et al. 2003). Similarly, peripheral oscillators across tissues and organs also need to resynchronize with each other and the SCN, and generally take longer to resynchronize than the SCN (Yamazaki et al. 2000). However, the length of time necessary to achieve stable phases attuned to the new photoperiod varies between tissues and organs (for review, see Harrington 2010), with ∼8 d necessary for full peripheral resynchronization following a 6 h phase advance (Davidson et al. 2009; Kiessling et al. 2010)."
- "Chronic jet lag lasting several years decreases cognitive performance in flight crews compared with flight crews routinely crossing less than three time zones (Cho et al. 2000). Moreover, these cognitive decrements were accompanied by higher cortisol levels and temporal lobe atrophy (Cho 2001)." — see also for dose-dependent effect of circadian disruption with cortisol levels: http://dx.doi.org/10.1038/88384
- A study on mice found that continually phase advancing (chronic jet lag) mice by 6h/daily led to a 4.2x increased death rate (89% versus 21% in unshifted mices), and another study demonstrating premature aging and prediabetic profiles in circadian misaligned mices.
- Chronic Jet Lag Produces Cognitive Deficits, 2000 https://doi.org/10.1523/JNEUROSCI.20-06-j0005.2000
- Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits, 2001 https://doi.org/10.1038/88384
- Despite the field of artificial light design progressing, "it is likely that people like airline pilots, flight attendants and shift workers will never have an ideal lighted environment". Similarly to endogenous circadian rhythm disorders such as non-24. https://doi.org/10.1177/1477153517721598
Other therapies or circadian rhythm factors
Similar therapies
Anecdotally, there has been multiple reports of success stories using similar therapies to what is described in the present document:
- 1 year entrainment of a non24 by using light therapy glasses 30min-1h at wake up, dark therapy and melatonin (the poster was previously resistant to melatonin treatment alone - interestingly, he also at some point tried to stop melatonin and use only light therapy, but eventually had to go back to use melatonin for stable entrainment): https://www.reddit.com/r/N24/comments/8bw67q/how_my_non24_treatment_has_allowed_me_to_sleep/
- 3.5 years entrainment at least of a non24, using blue light therapy lamps (lots of them), dark therapy and strict sleep hygiene: https://non24.blogspot.com/2009/09/synopsis-from-my-teenage-years-until-my.html
- 10 years+ entrainment of a non24 using a custom bright neon light lamps setup and dark therapy: https://www.reddit.com/r/IAmA/comments/cjx2i/iama_non24_i_sleep_every_26_hours_no_matter_what/ (the 10+ years of entrainment was confirmed to me in private correspondances)
- Eliezer Yudkowsky achieved much better entrainment results for his non24 with properly timed melatonin than with the usually prescribed 1h before bedtime timing, in 2013, combined with light-dark therapy: http://www.hpmor.com/notes/98/
- 2+ years of entrainment for a DSPD using light/dark therapy and melatonin: https://www.reddit.com/r/DSPD/comments/h11xok/followup_from_cured_sufferer/
- DSPD phase advance with morning exposure to sunlight, but with lots of variability due to change in weather (as noted by the author themselves, suggesting that sunlight does work, but it's highly variable because of weather, so their wake up time varies from day to day due to the varying amount of lux): https://www.reddit.com/r/DSPD/comments/hilzak/spending_time_outside_around_sunset_and_twilight/
- A purchase comment for the UVEX Skype blue blockers and Luminette v2: https://www.amazon.com/gp/customer-reviews/R3SIEA9MI7JNYB
- Several testimonies for phase advance for individuals with DSPD using the Luminette: https://www.reddit.com/search/?q=Luminette or https://www.reddit.com/r/DSPD/search?q=Luminette&restrict_sr=1
- Some people diagnosed with insomnia also reported positive results with a very similar therapy to VLiDACMel (including bright light control and food scheduling - and interestingly sleep positioning to significantly improve obstructive sleep apnea): https://www.reddit.com/r/insomnia/comments/l603gy/i_think_ive_fixed_my_sleep_issues/
- Entrainment of sighted non-24 for 2/3 months using a ketogenic diet: https://www.reddit.com/r/N24/comments/l2tf29/im_not_experiencing_n24_anymore/
- An individual with DSPD saw a great improvement to their sleep by using a Luminette (switching from a DIY light therapy lamp), so much so that they could stop other sleep medications: https://www.reddit.com/r/DSPD/comments/kmcxtc/cant_afford_a_luminette_is_luminette_not/glx648l?utm_source=share&utm_medium=web2x&context=3
- An individual with DSPD tried the split-dosing method of melatonin, with great success, more than anything else this person tried before: https://www.reddit.com/r/DSPD/comments/kxejt7/lowdose_melatonin_to_advance_sleep_cycle_and/
- An individuals with DSPD could achieve a 4h phase advance under 4 months by using a combination of bright artificial and sunlight and dark therapy, meal timing scheduling (stopping to eat 3h before bedtime) and a few customized procedures such as a food item combining no carbs caffeine and multivitamins and minerals consumed very early in the morning: https://www.reddit.com/r/DSPD/comments/nz24c3/shifting_my_wakeup_time_from_10_to_6_am_earlybird/
- An individual with non24 reported that melatonin intake 12h before their last wake up time allowed them to robustly entrain.
- An individual with non24 reported on the contrary that (over-the-counter) melatonin destroyed their sleep quality and fragmented it despite trying various brands and formulas for 6+ years.
- A redditor with DSPD reported in private chat communications that they could phase advance several hours just by doing dark therapy using red tinted laser safety glasses. Upon investigation, it appeared they also unknowingly did indoors sunlight therapy, as they found with a lux meter app that their indoor sunlight exposure was beyond 2k lux, which is usually sufficient to saturate the ipRGC cells. This is in line with what a scientific author wrote: "just as light exposure can shift circadian timing, so too can the strategic avoidance or reduction of light".
- An individual with DSPD successfully advanced their circadian phase using 0.5mg of melatonin 3.5-4h before sleep and a light therapy lamp at wake up, in addition to dark therapy and physical exercise: https://www.reddit.com/r/DSPD/comments/porobw/good_results_with_lowdose_melatonin_and/
- An individual with DSPD successfully advanced by 4-5h (from 1pm to 8-9am) their circadian phase only by using Luminette light therapy, but not with melatonin exercise, sleep hygiene etc: https://web.archive.org/web/20210915201037/https://old.reddit.com/r/DSPD/comments/poung8/life_changing_light_therapy_glasses_luminette_3/
- An individual with DSPD successfully advanced by 4h (from noon to 8am) their circadian phase by using Luminette light therapy for 2h/day over 2 months and a low intensity setting (500 lux) on most days, and sometimes medium setting (1k lux) when an additional boost is required: https://archive.is/owqf5
- Successful entrainment of an individual with non24 but impaired sleep quality and reduced sleep duration: https://web.archive.org/web/20230105003604/https://old.reddit.com/r/N24/comments/pjxw3o/longer_i_entrain_my_sleep_more_tired_i_get/
- Successful entrainment of an individual with sighted non24 using 1h or so of Luminette combined with dark therapy and a few other zeitgebers optimizations: https://web.archive.org/web/20221228212909/https://www.reddit.com/r/DSPD/comments/zks4mh/wearables_for_tracking_circadian_rhythm/
- Successful entrainment of an individual with sighted non24 over a whole year, with a sleep diary showing the previous years: https://web.archive.org/web/20230103203722/https://www.reddit.com/r/N24/comments/102emd3/a_full_year_of_entrainment/
- Successful entrainment of an individual with sighted non24 using a modified VLiDACMel protocol, more particularly using as long light therapy as possible (up to 10h/day) on the highest (third) Luminette intensity setting (which lasts only about 3h to consume the whole battery, and then the user switched to a Beurer light therapy lamp placed very close to their eyes): https://web.archive.org/web/20230108225907/https://old.reddit.com/r/N24/comments/vy8acd/sleep_graph_before_vs_after_following_the/
- https://www.circadiansleepdisorders.org/treatments.php
- Several successful entrainment therapies testimonials for sighted non24, several including VLiDACMel or sunlight therapy: https://web.archive.org/web/20230422110418/https://old.reddit.com/r/N24/comments/12qj855/is_there_anyone_that_have_either_fixed_completely/ (includes someone entrained for over 1 year with VLiDACMel, and someone who was successful with only 300mcg melatonin alone) and https://web.archive.org/web/20230422111810/https://old.reddit.com/r/N24/comments/yy1bz2/has_anyone_here_successfully_treated_or_cured/ (includes someone reporting 200+ days of entrainment with VLiDACMel and another almost 1 year entrainment with VLiDACMel)
- Successful 3 months entrainment for sighted non24 using VLiDACMel https://web.archive.org/web/20230422124914/https://old.reddit.com/r/N24/comments/12uwq0h/victory_post_three_months_of_entrainment_control/
- Sudden stable entrainment for over 3 years of sighted non24, maybe by indoors sunlight therapy (but even so, it is a "miracle" case): https://web.archive.org/web/20230422110418/https://old.reddit.com/r/N24/comments/12qj855/is_there_anyone_that_have_either_fixed_completely/jgxjl8e/ and https://web.archive.org/web/20230422111810/https://old.reddit.com/r/N24/comments/yy1bz2/has_anyone_here_successfully_treated_or_cured/ and https://web.archive.org/web/20230422111810/https://i.imgur.com/Jg5BfAy.png
- Sighted non-24 entrained 10 years with only melatonin and sometimes orexin antagonists for palliative: https://old.reddit.com/r/N24/comments/12hhu2x/comment/jg35zor/
In private communications, the current document's author received a lot more feedbacks than are displayed above. For instance, some users with DSPD reported being able to phase advance by 3-4h in a matter of days upon starting bright light therapy with light therapy glasses, whereas previous attempts using light therapy lamps failed. Users with non-24 similarly reported great results, although almost always temporary (worked wonderfully for a few months and then entrainment was lost). There are also reports of side effects, especially biphasic sleep caused by either melatonin or light therapy.
Failure stories:
- This reddit thread with an informal survey suggests that light therapy, even with the Luminette, appears to work for maybe half of individuals with non24, but a significant portion of individuals with sighted non24 may not be responsive: https://archive.is/sA10c
- https://www.reddit.com/r/DSPD/comments/q0kv44/anyone_else_who_feels_awful_when_sleeping_earlier/ (mirror: https://archive.is/Uy0nP )
- Failure to respond to any treatment, including VLiDACMel: https://web.archive.org/web/20230422111810/https://old.reddit.com/r/N24/comments/yy1bz2/has_anyone_here_successfully_treated_or_cured/iwsm0cj/
And here is the list of previous therapies the author of the present document self-experimented but that failed to entrain on at least 1 month:
- sleeping pills (donormyl = doxylamine) a long time ago.
- melatonin only, 1h before bedtime or 2-4h before DLMO, this was advised by sleep specialists.
- light therapy lamp (medical grade, brand Beurer TL 30, for 30 minutes at the appropriate distance) + dark therapy (blue light filters, blue blocker glasses) + melatonin 1h before target bedtime, this was advised by sleep specialists. Note that recently a user could apparently get entrained with a very long light therapy (at least 3h) with a Beurer TL 30. So these lamps may work as well as the light therapy glasses, but they are just much more cumbersome.
- light therapy lamp combined with a smart plug to programmatically light it up like a sunrise lamp. Tried various patterns such as 5min earlier each day or just the same time each day. No result after months of tries.
- strict ketogenic diet only (+ dark therapy).
- full carbs diet only (+ dark therapy).
- intermittent fasting (or even complete fasting for a few days), with and without dark therapy.
- time-restricted feeding with big meal timing (with carbs), with and without melatonin 2-4h before DLMO.
- serotonin via 5-HTP, either in instant release in the evening to help to sleep, or in long release form in the morning to level up the fluctuation in energy levels.
- Chronotherapy, whether for phase advance (15min per day or 1h each 3 days) or faster phase delay (except when combined with ketogenic diet and late meal timing).
- Short light therapy (1h) combined with chronotherapy (1h earlier light therapy every 3 days, with an alarm clock).
Temperature-based therapies
- The "Wechsel Treatment": Fixing Your Circadian Rhythm Disorder With Targeted Hot And Cold Exposure is an experimental protocol proposed by Circacadoo which aims to regulate the circadian rhythm directly through its main signalling system: the body temperature modulation. To achieve this, the protocol includes both the use of a sauna-blanket for 1.5h in the evening to induce sleep, and a 2-min cold shower in the morning to force moving the core body temperature minimum. An update was posted later here. To improve the protocol, the use of heat traning suits may allow for the same effect as the sauna blanket in the evening but while allowing the user to move freely.
Other pharmacological (drug-based) therapies
According to Thorpy & Roth's classification, there are four classes of medications for sleep and circadian rhythm disorders: “sedative hypnotics", “stimulants", “chronobiotics", and “other”.
For non-24 and DSPD, the only therapies that currently exist are of the chronobiotics class. Beyond those presented in the current document (light therapy, melatonin), other therapies and drugs exist to treat non-24, which are as of 2023 all melatoninergic (ie, affecting the melatonin receptors): ramelteon, tasimelteon (costs $17K a month!) and agomelatine. Only tasimelteon/hetlioz is currently cleared by the FDA, both for blind and sighted non-24. But the data on effectiveness remains sparse, even more than for melatonin. All these drugs work on the melatoninergic receptors type 1 and 2 and can hence shift the circadian rhythm. The difference between melatonin type 1 (MT1) and type 2 (MT2) receptors is that type 1 causes drowsiness whereas type 2 causes circadian shifting/consolidation. A third melatonin receptor (MT3) was identified but is poorly understood.
There are however experimental drugs currently in development that use novel pathways, such as cordycepin which works on adenosine receptors (ie, adenosinergic drugs).
Furthermore, following Thorpy & Roth's classification, there are non therapeutic but palliative medications that can help with managing symptoms instead of treating the underlying circadian phase misalignment (which is not a clinical endpoint!), such as stimulants, one freely available being caffeine, which works by being an adenosine antagonist, hence blocking temporarily sleep pressure buildup, the second sleep process besides the circadian rhythm process.
Other melatoninergic agents
Given how Tasimelteon/Hetlioz binds with a high affinity to the MT2 receptor compared to MT1, and MT2 being the receptor most often associated with circadian shifts, it's safe to assume it's a unique drug compared to others and it should work for both blind and sighted individuals. It's also the only drug out of circadian rhythm shifters that also improves cognitive abilities, likely since it binds less with the MT1 receptor and hence does not produce as much drowsiness as other melatoninergic agents. If we compare with melatonin, we could say that tasimelteon can likely produce the same or greater circadian shifting (because it's binding much more with melatonin type 2 receptors) but without the drowsiness that melatonin induces as a side effect. One poster of a multicenter study done in France claim no side effect was observed in 24 patients with non-24. A pooled meta-analysis of 6 clinical trials found only minor side effects on patients with non-24 or insomnia, concluding tasimelteon is safe. But this in theory, so whether alternative drugs are in practice more effective than melatonin (which is MUCH cheaper, remember Hetlioz/tasimelteon costs $12K to $17K a month!) remains to be seen.
Ramelteon on the other hands binds with both MT1 and MT2 receptors like melatonin, but with a 3 to 16 fold higher affinity. It also has a slightly higher preference for MT1. Contrary to melatonin, ramelteon does not bind with MT3. Because ramelteon is not metabolized directly but has a first-pass through the liver, the doses are usually higher than that of melatonin to increase bioavailability. The discovery of ramelteon's mechanism of action reinvigorated the research on melatonin.
> Agomelatine binds to MT1 and MT2 receptors with approximately equal affinity and is distinctive in that it has additional antagonist properties at the serotonin receptor subtype (5-HT2c).40 Ramelteon exhibits a 10-fold greater affinity for MT1 than MT2,59 and prolonged-release melatonin exhibits an 8-fold greater affinity for MT1 than MT2.39 Tasimelteon is the only one of these four compounds that shows a greater affinity for MT2 than MT1, with the difference being 4-fold.58
> [...] Two of these compounds, ramelteon and tasimelteon, are approved by the FDA and available commercially in the United States, and three compounds—agomelatine, prolonged-release melatonin, and tasimelteon—are approved by the European Medicines Agency and are commercially available in Europe.
From: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5108473/
According to this review, agomelatin should only be considered when the patient has both a circadian rhythm disorder and major depression, as it can treat both. Both melatonin and ramelteon were shown to be non mutagenic and non carcinogenic.
The issue with the current offer of melatoninergic drugs is that "there is still a lack of melatonergic ligands with high selectivity and specificity to precisely target any particular neuropsychiatric disorders", hence the design of melatoninergic drugs targeting more specifically a receptor's type, especially MT2 as it has a more important role in circadian rhythm shifting than MT1, could yield a tremendous advance in the treatment of circadian rhythm disorders.
Melatonin has historically been the first line of treatment for non 24 in blind people. It was shown to be effective for entrainment in up to 67% of the subjects, at least on the short term.[45] However, since this is a natural hormone that is not patentable (although low dosages of melatonin were patented at some point), no pharmaceutical industrial went through the process of validating melatonin through national institutions such as the USA Food And Drugs Administration likely due to the cost of validation with little returns since it is non-patentable, and thus melatonin is not officially recognized as a treatment for non 24, although there is good evidence this is the case.[44, 45] There is currently only one recognized drug treatment for non 24, which is Tasimelteon (commercial name Hetlioz),[44] with an entrainment rate of 20% in a randomized controlled study, which seems considerably lower than melatonin's entrainment rate.[45] Apart from these two drugs, there is currently not enough evidence for any other kind of treatment or therapies for non 24, according to the 2015 guidelines of the American Academy of Sleep Medicine (AASM).[44, 45] As pointed out by an excellent review, the AASM made their criteria more difficult in the latest revision of their guidelines, so that only treatments with reliably high confidence of effectiveness could be recommended, but that does not mean other therapies are ineffective, simply that there is not sufficient evidence.[45]
[44]: AASM CRSWD clinical practice guidelines 2015 https://aasm.org/clinical-resources/practice-standards/practice-guidelines/crswd-intrinsic/ and http://sleepeducation.org/docs/default-document-library/crswd-draft-executive-summary.pdf?sfvrsn=2
[45]: BEST: plus interpretation of these guidelines (eg, non recommendation does not mean they should not be tried) and other infos: Circadian-Based Therapies for Circadian Rhythm Sleep-Wake Disorders https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5156320/
TODO: rewrite the paragraph above.
Keep in mind it is highly unlikely there will be much research and further clinical validation to light therapy and melatonin than what we have now, because the pharmaceutical industries aren't interested in unpatentable treatments, as they shown again lately with the remdesivir for COVID-19 sold at $3120 for one course of treatment when its production costs only $0.93 per day of treatment. and despite very low R&D cost of $1.5M and public uproar when Gilead, the manufacturer, tried to pass the repurposing for COVID-19 as an orphan disease, which allows for state funding and allows to sell drugs at virtually any price (usually ludicrously high), similarly to Hetlioz/tasimelteon.
The only systematic protocol for entrainment ever published seems to be the one by Czeisler et al, using microdoses of melatonin at a fixed time, everyday. The idea is that at some point, the freerunning circadian rhythm will fall in place with the appropriate timing for entrainment relative to the DLMO. Hence, this protocol does not require the assessment of DLMO using salivary samples or any other monitoring, it simply requires patience and patient's compliance to the treatment, because forgetting the dose or changing the intake timing can render the treatment ineffective. In theory, this should work very well and they demonstrated it worked on several non-24 patients. However, in the current document's author's experience, melatonin is not sufficient for some individuals. Furthermore, the treatment would not be robust, since it would rely on a single treatment and missing one dose would make it ineffective, hence it's a too fragile entrainment for a non24 patient to continue their everyday activities under this treatment. Finally, it also temporarily increases the risk of glucose malconsumption and of developing metabolic syndrome diseases, because of the exogenous melatonin intake during the awake period, until the individual's freerunning circadian rhythm falls in phase with the melatonin treatment. It's also arguable whether such low doses of melatonin, and only using melatonin, would be enough to produce enough phase advance for severe cases of non-24 with extended circadian periods (eg, longer than 25h).
Hyper-photosensitization pharmacological therapies
A new promising class of drugs to treat circadian rhythm disorders involve molecules that increases the sensitivity to light. This includes ADHD medication such as aripiprazole (abilify) and antidepressants. The common link underlying effectiveness of these molecules seems to be whether they also are histamine agonists, since the histaminergic system is coupled with the circadian rhythm and can increase entrainment to bright light. Hence, these drugs can be good complements to increase the effectiveness of bright light therapy, whether artificial or via sunlight exposure.
Among this class of drugs are the dopaminergic agonists or pro-histaminics agents such as aripiprazole, an atypical antipsychotic, which can potentiate the magnitude of the circadian shifting effect of zeitgebers such as light and melatonin, and hence may be used to resensitize or even hypersensitisize individuals to the effect of light as was observed with DSPD individuals and one non-24 case. For the individual with non-24, a previous therapy with ramelteon 8 mg/d and melatonin 1mg/d failed to provide an effective treatment, whereas arpipripazole 3mg/d in the morning did, achieving stable entrainment for at least 6 months. In the DSPD study, patients were prescribed a dose between 0.5mg and 3mg that was progressively adapted in 0.5mg steps depending on the patient's complaints and therapy's efficacy, administered either in the morning or evening without any clue as to which time was more optimal. This holds the promise of potentially allowing bright light therapy to be effective for a wider population of people with circadian rhythm disorders, by hypersensitizing individuals who were otherwise unresponsive to this therapy, or even replace the need for artificial light therapy if the increase in photosensitivity allows the individual to be sufficiently entrained with sunlight alone. Another non-24 misdiagnosed as DSPD case found similar results and also improvements in the comorbid bipolar disorder especially in the frequency of manic-depressive episodes. Another antipsychotic, cariprazine, was reported to be effective for 3 months by a reddit member with DSPD before the effect wore off, with minimal side effects on anxiety and moderate effect on weight gain.
Reddit member evilwizzard reported about a japanese clinical trial on the usage of the dopaminergic agent aripiprazole to advance the phase of DSPD individuals, and described the effects in practice on themselves, mentioning that the drugs aripiprazole, brexpiprazole and pramipexole have similar effects but with pramipexole having less side effects and easier dosage, as partial agonists such as aripiprazole become antagonists and produce the opposite effect with increased doses. This study and another found low doses of aripiprazole to be an effective treatment to entrain individuals with DSPD. The results are not so surprising as it's known, especially by patients with RLS (restless legs syndrome) or PLMD, that dopamine interacts with the circadian rhythm and especially with melatonin, and inversely. Another reddit member with non-24 also reported that both aripiprazole and resporidone both allowed them to be entrained, especially when combined with bright light and dark therapy, but with the side effect of significantly reducing their average sleep duration by about 1 ultradian cycle (ie, 6.5h of sleep instead of 8.5h).
Beside the dopaminergic-circadian interaction, another hypothese for the circadian shifting effect of aripiprazole may be mediated by the role of histamine H1 receptors in the entrainment to bright light, as anti-histaminics suppress entrainment to bright light while histamine H1 receptors activation improves it, the latter being one of the targets of aripiprazole and is hence a hypothesis that could explain the effect of aripiprazole on the circadian rhythm according to some authors. Indeed, anti-histaminics block entrainment of the circadian rhythm to bright light, and hence can cause anyone to freerun (ie, wake up later and later, and likely sleep longer but not because of tiredness as is commonly assumed but because of the circadian rhythm progressively shifting). Anti-histaminics are hence contra-indicated for circadian rhythm disorders. But the opposite class of drugs, histamine agonists such as aripiprazole, can instead improve entrainment. Indeed, a study on animals found that histamines likely regulate or at least modulate circadian phase shifting from bright light, with injections of histamine in the SCN producing as much phase delay and phase advance as photic stimulation. However, among atypical antipsychotics, aripiprazole display one of the lowest affinity to histamine H1 receptors.
> Histaminergic activity shows a clear circadian rhythm: high levels during the active period (in rodents at night, in monkeys and humans during the day), and low levels during the sleep period. Histamine appears to be necessary for the maintenance of the circadian rhythmicity of the adrenocortical hormone release, locomotor activity and food intake, and the sleep-wakefulness cycle. In addition, a role for histaminergic neurons in the light entrainment is implicated. In phase shift studies, histamine given centrally seems to entrain the activity rhythm in the same way as light impulses and inhibition of histamine synthesis seems to block the entrainment by light.
https://pubmed.ncbi.nlm.nih.gov/11640965/
Accordingly, the reddit member, after trying for himself, reported both increased sensitivity to morning light, but also to melatonin using pramipexole of at least 0.5mg, with some other DSPD sufferers having used 2mg and 4mg succesfully to completely manage their DSPD disorder. The user also mentioned drugs producing the opposite effect, inducing photo hyposensitivity: "lithium and valproate acid both decrease light's ability to suppress melatonin" and promotes a lengthened, non-24 circadian rhythm period, in line with previous studies on animals (see also here). The issue with dopaminergic agents is their tolerance build-up, so that they can not only produce addiction, but also will likely have diminishing effect with the same dosage if taken long enough, so that usually intake of dopaminergic agents need to regularly be discontinued temporarily for the endogenous dopaminergic receptors to get restored to their initial density and to again see the effect of the drugs at the normal dosage. Other agents interacting with the dopaminergic system have been shown to potentially reduce the freerunning period, in other words to produce a phase advance, such as valproic acid, especially in mice with a deficiency of dopaminergic receptors. Another drug named Brexpiprazole overlaps with much of aripiprazole targets but with lower H1 affinity.
There are however side effects with aripiprazole, sometimes severe, such as increased insulin resistance according to anecdotal reports.
Methylphenidate (Ritalin) is a known photophobic agent, it increases photosensitivity (see also here). Since methylphenidate likely increases photosensitivity (aka photophobia), which in turns may be how it mediate inattention control via modulations of the circadian rhythm, it is reasonable to assume its effect on sleep is by increasing the responsiveness to bright light exposure and its circadian rhythm shifting effect, and hence that methylphenidate's beneficial effects for ADHD can be potentiated by the concurrent adjunction of bright light therapy. This is further strengthened by the finding from trials that people with ADHD were responsive to bright light therapy, benefitting from an advanced circadian phase and improved core ADHD symptoms simultaneously (note that DSPD is extremely common in children and adults with ADHD, see the section on comorbidities). And indeed, some scientists theorize that photophobia, including when caused by drugs such as methylphenidate, necessarily work by potentiating the melanopsin ganglion cells, aka the ipRGC cells response, since photophobia also happen in people who are visually blind (see page 31 of this compendium). A case study prescribing long lasting form of methylphenidate or bupropion (an atypical antidepressant with stimulant effects) at bedtime to people with DSPD found that this improved the 4 patients' circadian rhythm alignment and severe sleep inertia, with benefits lasting for months and up to 3 years at follow-up, which is a strong indication that this therapy may be sustainable over the long term. But methylphenidate has its downsides. In practice, a study and a systematic review found that methylphenidate increases sleep issues of children with ADHD, except for those with pre-existing sleep difficulties and only when using high dosages of methylphenidate, hence this molecule may help with sleep issues under some specific conditions that need more exploration to elucidate, potentially a complementary controlled bright light therapy regimen. A longitudinal study on a similar population of children with ADHD found that the negative effects of methylphenidate seem to disappear after a few weeks of treatment. A case study found that methylphenidate could also cause hallucinations, which resolved after discontinuation of treatment (which was tested multiple times in a kind of block design). Recent studies even suggest that d-amphetamines increase vigilance precisely by modifying the circadian rhythm. Methylphenidate was found to not be genotoxic in long-term studies of children (see also here).
There are anecdotal reports by redditors of circadian rhythm shifting effects for other dopaminergic agonists such as Rotigotine/Neupro or even food containing the L-dopa amino acid such as Mucuna pruriens and the circadian shifting effects of L-dopa seems to be supported by one study on Parkinson patients. For the latter, the redditor u/mromu claimed this definitively cured their DSPD with a product of Mucuna pruriens containing 800mg of Mucuna extract from seeds and 120mg of L-dopa per pill, with the obtained phase advance still sustained stably one year after discontinuation of L-dopa intake, however this moved their phase into an ASPD phenotype which was also uncomfortable.
ADHD medication is not an exception, there are several other drugs that can potentially increase photosensitivity, such as antidepressants and antipsychotics. In fact, it is now strongly argued by some scientists that the effectiveness of antidepressants stem from their circadian rhythm shifting capacity. Indeed, all effective therapies and drugs for major depression disorder also affect the circadian rhythm, including antidepressants such as SSRIs, SNIRs and agomelatine. A clinicial trial using agomelatin found that the degree of mood improvement in depression in young individuals was associated with more circadian shifting as evidenced by melatonin sampling, providing further support to the hypothesis that the efficacy of antidepressants is tied to their circadian shifting effect. A case study also found bupropion, an atypical antidepressant with stimulant effects, to maybe be effective at shifting the circadian rhythm to treat DSPD and sleep inertia. Bupropion can be an especially interesting therapeutic option for individuals with comorbidities such as nicotine addiction, as it is approved for smoking cessation, or ADHD. Just like ADHD stimulant medication, several antidepressants can also increase photosensitivity, and hence indirectly affect the circadian rhythm through increased responsiveness to bright light exposure. Hypothetically, the hyper photosensitization by these drugs may be by increasing the pupil dilation, which allows for more light to enter the retina, which can theoretically be combined with light therapy for a greater effect than any of these treatments alone. This is supported empirically by a 2019 systematic review which found that bright light therapy was as effective as antidepressants, and that the combination was more effective than either therapies alone (see also here), which makes sense if we consider that both antidepressants and light therapy are just different means of achieving an increased exposure of the retina to bright light. This hyperphotosensitivity effect is now suspected by some scientists as being the cause of antidepressants effects on mood, going as far back as 1996 and even 1979, which was then popularized by Rosenthal in 1984 by coining the Seasonal Affective Disorder term, and in light of the recent findings, it can be further argued that seasonal affective disorder may simply be major depression. And indeed, it was demonstrated that antidepressants do alter core body temperature, and hence the circadian rhythm. However, a 2018 Cochrane Systematic Review found no evidence that antidepressants could improve insomnia, although this review did not study the effect on the circadian rhythm nor circadian rhythm disorders specifically. A group study found that sodium oxybate (SXB), an atypical antidepressant, reduced the circadian dysfunctions associated with narcolepsy.
About safety, interestingly histamine H1 agonists lead to weight gain (see also here). Although aripiprazole is known to have several, some serious, side effects, including "extrapyramidal syndrome, hyperprolactinemia, weight gain, metabolic disorders, and sedation [...] which are typical problems with other antipsychotic drugs", it has a high tolerability that makes it safe to administer to children and adolescents and is accepted by the US FDA to treat "schizophrenia, bipolar diseases, and irritability associated with autistic disorder in children and adolescents".
Yet another class of drugs is... Viagra, or Sildenafil in its generic name. Indeed, a study on hamsters found that a single administration of Sildenafil could produce phase advances in combination with bright light therapy. The authors further note that "Sildenafil alone did not induce phase shifts", while noting that it is the increase in entrainment to bright light therapy that explained the phase shift. In other words, Sildenafil is yet another hyperphotosensitizing drug, by interference with the cGMP-related pathway. Hamsters are a great model for circadian rhythm research, as they are diurnal animals just like humans and contrary to most other rodents such as mice, and since the circadian rhythm is a highly conserved process throughout evolution, it's likely that the results can be transposed to humans, although clinical trials are needed to confirm.
Nevertheless, since these drugs are often stimulants or antipsychotics, they can have serious side effects if the individual does not have a comorbidity that justifies this treatment beyond the circadian rhythm disorder. For example, "for people who do not have ADHD, stimulants flood the brain with dopamine, causing a dopamine overload. So instead of having a calming effect as they would on people with ADHD, stimulants taken without a medical reason can disrupt brain communication and cause euphoria" which can lead to "increase blood pressure, heart rate, and body temperature; decrease appetite and sleep; cause feelings of hostility and paranoia; increase a person’s risk for addiction." Furthermore, a 2021 systematic review and meta-analysis on 16 RCTs studies on humans found that antipsychotics, and especially atypical antipsychotics, decrease cortisol, melatonin and body temperature, demonstrating an effect on circadian parameters, with typical antipsychotics dysregulating sleep-wake patterns while atypical antipsychotics regulated them. This study also strongly suggests that antipsychotics need to be administered relatively to the patient's current circadian phase, but how in practice is not yet determined as more studies are needed. In more details, the results suggest that antipsychotics appears to impair cortisol and melatonin secretion and weaken core body temperature modulations, although this would need confirmation since the data was sparse.
There are other concerns: long-term data is lacking, and according to a phase IV study of clinicial trials, the hyper photosensitizing drugs such as Vyvanse (an ADHD stimulant, generic name: lisdexamfetamine dimesylate) subside after a few months up to a year. For antidepressants such as bupropion, there are strong evidence from animal studies and epidemiological studies that they decrease male fertility (see also here, here, here) and increase cardiac malformation in fetuses (see also here), with a confirmation from a systematic review on humans that antipsychotics affect male sexual reproduction via different mechanisms, and most antidepressants causing decreased libido, ejaculatory and erectile dysfunction, especially SNRIs which appear to be possibly associated with a specific risk of erectile dysfunction (see also here). However, there are conflicting findings from a few human clinical trials (see also here), although the number of participants were low. Also, most psychotropic medications cause withdrawal symptoms that may affect sleep, although there are some exceptions such as agomelatin as it even has some hepatoprotective effect.
However, there are contradicting data points. There are two reports from two different members of the (mostly sighted) non-24 subreddit that Vyvanse (lisdexamfetamine dimesylate) worked for them over a long period of time, including a 10 month period for one of them. More precisely, for the first one, who presumably had a less severe case of non-24 around 25h/day, using Vyvanse alone allows them to entrain to a DSPD-like schedule instead of non-24. For the latter, the therapy consisted of a combination of aripiprazole and vyvanse (lisdexamfetamine dimesylate) and mirtazapine and sleep restriction, allowing for the successful entrainment to a 24h schedule over 10 months whereas they had before an extreme case of sighted non24 plus hypersomnia with an endogenous 30h period and a 12h+ sleep period, reduced down to a 9h sleep period.
For those with a deficiency, vitamin A can increase entrainment to bright light, since vitamin A is necessary to synthesize all opsins in the eyes, including the melanopsin pigment necessary for ipRGC cells. Vitamin A also increases the photosensitivity to UV light which can be harmful, hence caution is required with direct sunlight exposure when supplementing with vitamin A. Similarly, B12 vitamin is known to amplify the magnitude of the circadian rhythm shift of light therapies (see also here), so that supplementation of B12 may increase entrainment to bright light therapy if there was a deficiency.
Since the ipRGC cells in the eyes are regulating both the circadian rhythm and the pupillary light reflex in response to bright light exposure (see also here), it's likely that compounds that increase eye/pupils photosensitivity/photophobia (but not dermatological photosensitivity) can also increase entrainment to bright light therapy. Some scientists even argue that this is the most likely hypothesis, that photophobia inducing drugs necessarily work by potentiating the melanopsin ganglion cells, aka the ipRGC cells response, since photophobia also happen in people who are visually blind (see page 31 of this compendium).
For more hyper photosensitizing drugs and non-pharmacological causes, see this review for a list of hyper photosensitizing drugs and this other list which is part of an excellent review on the causes and mechanisms of photophobia / hyper photosensitivity.
In summary, hyperphotosensitizing drugs need to be used in combination with bright light therapy to be effective, since their circadian shifting capacity depends on the non-visual pathway that makes bright light shift the circadian rhythm phase. Reliability with this therapy can be more easily achieved through artificial bright light therapy with light therapy glasses such as Luminette, but several cases have demonstrated robust entrainment for long periods of time (>6 months for a non-24 individual) with only sunlight exposure, so that for less treatment resistant cases (eg, likely those with a sufficiently short circadian period, maybe < 25h) daily intake of hyperphotosensitizing drugs alone may be sufficient to reach stable entrainment, although this would need confirmation especially through winter periods to observe whether the entrainment does not subside with dimmer and shorter sunlight exposure due to seasonal variations. However, due to the potential side effects such as akathisia, augmentation (especially with dopaminergic agonists) and fast tolerance build-up of stimulants and antipsychotics, it's likely not advisable to use these molecules just to treat circadian rhythm disorders, but if there is another comorbid psychiatric disorder such as depression, ADHD or schizophrenia that requires these molecules, then the circadian rhythm shifting effect through hyper photosentization that these molecules also produce can be leveraged by complementing with bright light therapy. Also, there needs to be more clarification on the optimal time of intake, since some studies used molecules such as aripiprazole in the evening while others in the morning.
Adenosinergic agents
Adenosine is the molecule underlying the homeostatic sleep pressure process S, one of the two core process with circadian rhythm process C that regulates sleep.
Adenosine antagonists, such as caffeine, have a very well established effect on the sleep homeostat. Indeed, adenosine antagonists inhibit the capture of adenosine by cells, by binding to the adenosinergic receptors. This in turn prevents feeling the fatigue effect associated with adenosine capture. But this comes at a cost: the "real" adenosine still continues to build up behind the scene, and when the adenosine antagonist molecules run out, the real adenosine comes flooding the receptors, which is colloquially termed a "caffeine crash". Caffeine (coffee and energy drinks and tea) should be avoided, especially if there is a risk of taking it in circadian misalignment. Indeed, caffeine's effects carry over up to 48h including phase delay since caffeine can modify core body temperature and is hence a zeitgeber. This long residual life and progressive circadian phase delay is potentially the reason why coffee drinkers regularly need to temporarily discontinue intake via decaffeinated drinks. Similarly, it's likely a good idea to also avoid any wakefulness inducing drug such as tea and modafinil and nootropics. If you have ADHD, prefer instead to use stimulants, which often increase photosensitivity so they may help in increasing the effectiveness of bright light therapy. There is also some evidence that caffeine, including in tea, can cause muscle cramps including nocturnal legs cramps (NLC) which is a major sleep disruptor.
A similar stimulant is modafinil. Modafinil is a wakefulness promoting agent primarily indicated for narcolepsy, although it was used in combination with light therapy and melatonin to force entrainment for an individual with sighted non-24 and is hence regularly prescribed off-label (way before this study) to individuals with non-24. But a study on night shift disorder found that modafinil can produce or worsen insomnia (ie, "made it more difficult for participants to fall asleep when opportunities for sleep arose"). However, modafinil was found to be effective in improving vigilance and feelings of tiredness for people with hypersomnia according to a 2021 Cochrane Systematic Review of clinical trials and by the AASM practice guidelines for narcolepsy and hypersomnia (summary here). Although modafinil's mechanism is not fully elucidated yet, controlled trials have demonstrated it does not affect the circadian rhythm as monitored through core body temperature, but only the sleep homeostat (see also here), which strongly suggests it somehow inhibits adenosine capture, similarly to adenosine antagonists. Past studies have reported a possible effect of modafinil on core body temperature, but later more strictly designed studies found no effect, and rather suggest that the observed core body temperature increase was caused by sleep deprivation alone, which is often improperly controlled in studies. Another study formally proved this by controlling ambient temperature, finding that modafinil had no effect on core body temperature under thermoneutral conditions, but found an insignificantly faster change in core body temperature when under modafinil. Furthermore, modafinil does not affect melatonin nor cortisol levels compared to placebo. However, modafinil has a much lesser safety profile than caffeine, with risks of tolerance buildup (ie, diminishing effects over time for the same dosage), without producing more stimulation than caffeine after sleep loss according to a clinical trial in typical sleepers, which concluded that there is no significant advantage in using modafinil over caffeine, especially given that "modafinil is less readily available, more expensive" and less safe. In summary, prefer to use caffeine if a stimulant is sought. It's also worth noting that modafinil does not increase physical performance. Although their end result on wakefulness promotion appears to be similar as demonstrated by these few studies, their biological pathways are different, so it may be possible to be more responsive to Modafinil than Caffeine, as anecdotally reported by individuals with DSPD.
While the effect of adenosinergic antagonists the sleep homeostat are very well known, the effect of the opposite class of drugs, adenosine agonists, are not well studied, but are nonetheless highly promising. Indeed, adenosine analogs such as cordycepin were found to be the strongest circadian rhythm resetters (type-0) when combined with bright light / dark therapy compared to other molecules including melatonin analogs, as they can thoroughly disrupt the molecular clocks in cells throughout the body and cause a near instantaneous realignment to the new light/dark pattern the subject is exposed to. In this study, the researchers screened several molecules, and found that cordycepin was able to drastically speed up the circadian clock realignment to a shifted timezone (under 4 days instead of 10 days) of bright light/dark exposure in vivo in mice and ex vivo in human cells (see also this journalistic vulgarization article and my informal review on dosage and safety (archive here)). In practice, such a drug could be used to help in the management of non-24, by allowing users to "skip" the "nightwalking" phases where they are in complete misalignment with the day-night cycle: instead of waiting weeks to months for their circadian rhythm to slowly and painfully freerun until it realigns with the day-night cycle (temporarily, as it will unpreventably continue to freerun if entrainment therapies do not work for the individual), the patient could instead take a cordycepin prescription to speed up this process and become realigned in a matter of days, hence minimizing the nightwalking phase which can be considered as one of the major detrimental consequences in the quality of life of an individual with non-24. Unfortunately, the drug was not yet used for humans, and the dosage used in this study is way too high for humans, and there is no study showing such high doses would be safe for humans. Interestingly, but unsurprisingly given the established links between mood disorders and circadian rhythm disruption, cordycepin was also found to produce antidepressant effects similar to imipramine. However, as of November 2023, it is not known whether the consumption by humans of cordycepin at the dosage required to reset the circadian rhythm according to this study would be safe, and consuming the c.militaris or any other mushroom that naturally contains cordycepin can only provide a dosage magnitudes lower than what was found effective in this study (but a dosage lower than this study may be effective, but it's unlikely it can be this low as the concentration in natural food). It often happens in medicine that effective molecules are shunned away because they are too dangerous for use, such as weight loss and anti-obesity drugs as they can also cause sudden cardiac failure (except nicotine but then it is addictive so it is not well studied for this use).
Interestingly, adenosine agonists inhibit histaminergic neurons and hence increase the propensity to sleep and recovery time from anesthesia, whereas adenosine antagonists have the opposite effect (see also here) as caffeine does.
A new adenosinergic agent CT1500 is investigated by CircadianTherapeutics, with the Phase I trial, assessing the drug's safety on humans but not its efficacy, started in October 2021 (thanks to Psyz0me for the news!).
Update as of january 2023: CT1500 is an adenosine antagonist, similarly to cafeine and contrary to cordycepin which was adenosine agonist. A preclinical trial on mice was published in December 2022 (see also here, thanks to carvo08 for the heads up!), with very promising results, showing that the molecule can not only phase shift, but also replace bright light altogether by entraining the circadian rhythm to a 24h schedule in constant darkness, which is an impressive feat. Note however that this remains a mice study and hence with many limitations, so that it remains to be seen whether this molecule still works in humans and to what extent. The authors however mention explicitly the potential application to the non-24 circadian rhythm disorder in humans. Their study and past studies by the same authors also highlight the importance of the adenosine pathway for the regulation of the circadian rhythm by bright light, so that using adenosinergic agents can be seen as a way to "hack" directly into the circadian regulation system that is usually modulated by bright light.
It appears that adenosinergic agents can indeed modulate the circadian rhythm, as the adenosinergic system is how bright light modulates the circadian rhythm. Hence, manipulating the adenosinergic system is some sort of "shortcut" to affect the circadian rhythm directly without needing bright light exposure. They are hence a very promising new class of chronobiotics.
A member of the sighted non-24 reddit community u/ConsciousBluebird473 reported that a prolonged time-release 200mg cafeine tablet, combined with natural unrestricted indoors sunlight exposure with no curtains, and no dark therapy (they still used a bright room light all night - source: private communications) allowed them to get an instantaneous 12h phase reset. This however required them to take the tablet at wake up (in the afternoon), then stay awake the whole night well into the next day (but they did not feel sleepy anyway) to get exposed to natural sunlight with curtains opened, and then they could only sleep at 11pm, and then they slept for >24h (fragmented by short wake ups) to recover.
Other pharmacological therapies
TODO: work-in-progress section.
Afinils such as modafinil, along with ADHD stimulants and caffeine, belong to the category of eugeroics, which can promote wakefulness by targeting various neural structures, such as histaminergic, dopaminergic or orexinergic. Since these neural systems are also known to interact with the circadian rhythm, some of these drugs may also affect the circadian rhythm, although modafinil was demonstrated to not affect the circadian rhythm (see the section on adenosinergic agents).
Some individuals with DSPD claimed using 5-HTP (5-hydroxytryptophan) or L-Tryptophan helped them. Both L-Tryptophan and 5-HTP are precursors of serotonin, itself a precursor of melatonin. Hence, 5-HTP intake can stimulate both the serotoninergic and melatonin pathways. Hence, it can indeed help with circadian rhythm entrainment, but likely not more than melatonin, with the only added benefit of the mood calming effect of serotonin, but with the potential risk of the fatal serotonin syndrome (particularly for individuals with hyperserotonemia such as autistic individuals). If you try 5-HTP and feel your heart racing, stop right away from further using the product.
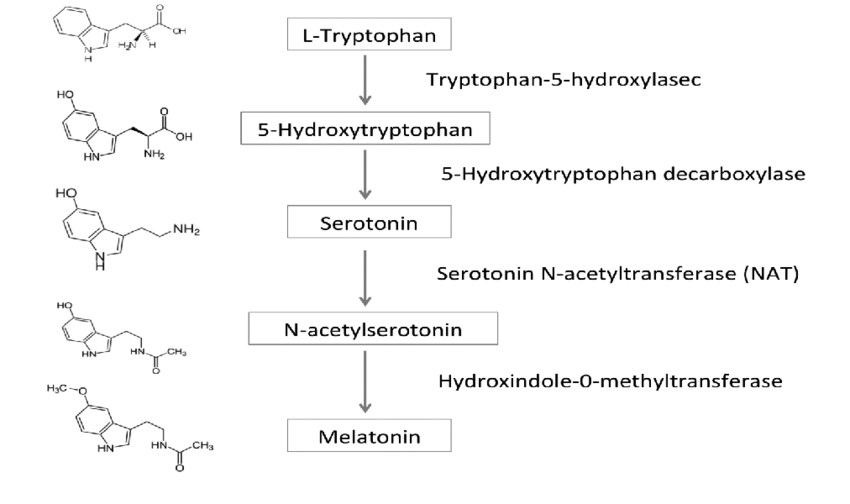
Melatonin synthesis pathway from L-Tryptophan through 5-HTP, from the figure 1 of this study under CC-BY 4.0 (thanks to Maverick on Discord)
Suvorexant/Belsomra is a new kind of sleeping pill working as an orexin antagonist, but tolerance and addiction build up fast too similarly to zolpidem and it induces a potentially important next-morning drowsiness that can cause accidents. Unfortunately, people with non-24 who tried it confirmed this does not affect the circadian rhythm, it only works as a sleep inducer.
Sleeping (hypnotic) pills and wakefulness drugs are inappropriate to treat non-24.
Glycine is anecdotally reported by several redditors to be effective in improving sleep quality, which is in line with a few studies (see also here), although measures of subjective sleep are obviously subjective measures and hence prone to bias. However, studies on a few dozens of mice suggest that glycine may be affecting the circadian rhythm, more precisely by producing a hypothermic effect that is sleep inducing, similarly to melatonin, by activation of the NMDA receptors in the SCN. Hence, glycine may be a promising complement or alternative to melatonin to induce sleep.
Epitalon is yet another alternative to melatonin, as studies on aged humans and monkeys suggest that it may restore melatonin secretion by the pineal gland. A trial on 79 coronary patients over 3 years demonstrated it may restore the circadian rhythm (see also here). Nevertheless, it seems epitalon was mostly used on older adults for now, future trials on younger people would be interesting.
Valerian is often used as a sleep aid. Although it was found to be safe, it was also found ineffective by a 2017 systematic review. An updated 2021 systematic review and meta-analysis also found valerian roots to be safe for ages 7 to 80, but could also not conclude about the effectiveness, maybe because of variable products quality used in each study and method of measurement of outcomes. A 2009 RCT trial in insomniac women, using objective sleep metrics such as polysomnography and actigraphy, did not find any significant effect of valerian on sleep. The evidence for the use of valerian for anxiety disorder is as mixed as for sleep disorders.
As of 2021, a systematic review is undergoing for Chinese patent medicine (herbal blends) to treat insomnia, so there is no result yet. Although it is necessary to be precautious as the composition can vary widely and such products can be contaminated with dangerous agents since they are mostly uncontrolled substances.
According to a case study of 3 patients, suvorexant, an orexin hypnotic, combined with ramelteon, a melatonin agonist, may be a potentially effective combined therapy for sleep initiation for individuals with DSPD, although the long term benefits remain to be seen. This is also a drug under patent, so that it is costly and studies on it may be prone to conflicts of interests. Lemborexant, an analog of suvorexant, was found to perform even better compared to the latter, zolpidem and placebo, while having fewer side effects than the other drugs according to a 2020 systematic review on insomniacs. In 2022, a mice study found that age-related insomnia was associated with hyperexcitability of the orexin/hypocretin neurons, and found that flupirtine improved sleep fragmentation and other signs of insomnia (see also this french vulgarization article), however flupirtine was banned since 2018 by the European Medical Agency due to its severe hepatotoxicity (ie, it damages the liver).
General anesthetics have been recently revisited for other purposes than anesthesia, at lower doses. Anesthetics such as ketamine have demonstrated an instant effect on depression with a novel mechanism of homeostatic synaptic upscaling by inhibiting the eEF2K protein and is FDA-approved for treatment-resistant depression since 2019 as a nasal spray and off-label in European and UK countries (treatment-resistant depression representing 30% of all major depression disorder cases), which suggests that ketamine may also be effective for circadian rhythm shifting since all other effective antidepressants affect the circadian rhythm. Although there is no repurposing for sleep issues yet, the effects of anesthetics on sleep and the circadian rhythm is well established (see also here):
> Key points: 1) GA (general anesthesia) has strong effects on the main neurotransmitter systems linked with circadian control (Gamma aminobutyric acid/N-methyl-D-aspartate (GABA/NMDA)) and may act by interfering with light-entrainment of the clock. 2) Expression of the core clock gene per2 is inhibited by GA (possibly via a NMDA/glycogen synthase kinase 3β (GSK3β) pathway). 3) GA's effect on circadian rhythms appears greatest when administered during animals' active phases 4) GA may have different effects when administered under free-running and entrained conditions. 5) Anaesthesia may mimic the mechanism involved in adaptation of the clock to changes in daylength.
>
> There is agreement that GA can strongly affect the circadian clock. How anaesthesia-induced changes in the molecular clock lead to changes in behaviour remains unclear.
More specifically, a mice study shown that ketamine at low doses may work as a type 1 resetter, which means that it acts as a relative zeitgeber with a PRC curve: "day or night administration of ketamine and pentobarbital differentially affect circadian rhythms of pineal melatonin secretion and locomotor activity in rats". A reddit user reported that low-dose ketamine already exists in the form of nasal spray and lozenges.
Here is a list of anecdotal reports of other working therapies for non-24:
- A sighted non-24 redditor reported consistent and reliable entrainment for 14 months by taking a combination of 10mg of instant release melatonin + 25mg of diphenhydramine, the user reported that the combination is necessary as taking either of the compounds alone only allowed for unreliable and inconsistent entrainment. This dosage was used by someone of about 6'4 size and 260 pounds of weight. The user furthermore clarified that the treatment required an empty stomach at least 3h before drugs intake. https://www.reddit.com/r/N24/comments/jofawh/hopefully_this_helps_somebody/
- A sighted non-24 redditor reported consistent and reliable entrainment for 7 months by following a lactose-free diet, and restart of freerunning when ingesting lactose, and is currently (November 2020) experimenting to confirm this observation: https://www.reddit.com/r/N24/comments/jnhsy3/im_fairly_sure_my_non24_is_caused_by_lactose/
TODO: add newer alternatives to modafinil that are non addictive, such as solriamfetol and pitolisant https://www.sciencesetavenir.fr/sante/somnolence_158578
Implant-based therapies
Northwestern University researchers secured a $33 million funding by the US military DARPA to make a light sensitive implant to control cellular circadian clocks of peripheral tissues throughout the body under 5 years. This means the device does not aim to resynchronize the master clock in the brain, but the other clocks that are usually very hard or impossible to entrain. The device's draft is at the time of this writing named NTRAIN (Normalizing Timing of Rhythms Across Internal Networks of Circadian Clocks). This may become the equivalent for circadian rhythm disorders of the insulin pump for diabetics.
The device aims to inject peptides to control the sleep processes. Although the exact peptide is not described, it may be the delta-sleep-inducing peptide (DSIP peptide), a very interesting molecule with seemingly very interesting potential uses such as reducing withdrawal symptoms of opioids addiction and inducing a real sleep with promoted slow-wave deep sleep stages, but there is a lack of studies and trials on its safety, or even on its efficacy for the treatment of circadian rhythm disorders, although some functional doctors (a pseudomedicine) prescribe it to treat people with DSPD, but this may have the potential to do more harm than good by disrupting the circadian rhythm. Anecdotally, a patient with DSPD who got prescribed DSIP injections found it to be ineffective. Caution is advised until more trials are conducted.
Worth noting, the circadian rhythm affects the timing of assimilation of DSIP:
> While the existence of DSIP as an endogenous peptide has been called into question [29], it does appear to cross the BBB via a nonsaturable mechanism with circadian changes in brain concentration [24].
Behavioral sleep-wake therapies for insomnia and circadian rhythm disorders
Both chronotherapy, sleep hygiene and sleep restriction are behavioral interventions aiming to forcefully modify the circadian rhythm by changing the sleep-wake pattern by the patient's will. Both induce severe sleep deprivation. Given the similarities, they can be considered as close cousins, and are hence studied here in the same section.
Chronotherapy and circadian plasticity
Czeisler is one of the major researchers in both circadian rhythm science, circadian rhythm disorders and light therapy. He pioneered the use of light therapy to modify the circadian rhythm and even went to work with NASA to help implement an automatic LED-based light therapy system for cosmonauts on the International Space Station (see also here). But Czeisler also introduced two concepts, chronotherapy and circadian plasticity, which are responsible for a lot of mistreatments of circadian rhythm disorders.
A chronotherapy is a behavioral therapy consisting of requiring the participant to go to bed and wake up at precise times, with the goal of modifying the underlying circadian rhythm through behavior. There are two types of chronotherapies: phase advance chronotherapy (sleeping and waking up earlier than usual) and phase delay chronotherapy (sleep and wake up later and later each day). Historically, phase delay chronotherapy was the first kind of chronotherapy as devised by Czeisler et al in 1981, this type could also be called "forced freerunning". The basic premisse, as stated in the eponymous seminal paper, is that some individuals such as DSPD have a circadian rhythm that is "locked up" at the wrong phase (ie, timing), and simply forcing them to freerun would allow to unlock their circadian rhythm to be able to be set in phase again to a more socially acceptable schedule. Advance chronotherapy, which consists in forcefully waking up earlier and earlier at regular intervals using an alarm clock, sometimes in combination with light therapy, was devised later.
There are two major issues that were raised about chronotherapy: are the effects sustainable or only short-lived? And is freerunning safe or can it go wrong and lock the individual into a permanent freerunning? In both cases, the core issue is whether or not the circadian rhythm can change permanently following a therapy, which is termed the circadian plasticity theory. Let's tackle this issue through the two questions outlined above, as they represent the two main lenses through which the researchers investigated circadian plasticity.
Is chronotherapy effective? Although a handful of studies shown some phase advance and phase delaying effects, they are all confounded by the change in light exposure or used in conjunction with melatonin, hence there is no evidence showing that chronotherapy alone can modulate the circadian rhythm, but instead only mask it. Given the lack of evidence in controlled trials and the high relapse rate in the few case studies published so far, with the disordered sleep patterns typically reappearing after only 1 or 2 days after chronotherapy discontinuation, the AASM concluded in their 2015 guidelines that there is no evidence of effectiveness to treat circadian rhythm disorders with chronotherapy alone. Indeed, the circadian plasticity theory was long disproved since at least 1987 since it was already observed that locomotor activity nor sleep had any effect on the circadian rhythm, and more recently with a modernly controlled study monitoring core body temperature finding that sleep-wake patterns are not a zeitgeber but at most a zeitnehmer, hence the low to no effectiveness demonstrated so far by chronotherapies. An even more tightly controlled human study monitoring not only melatonin levels, wrist skin temperature and core body temperature, but also bright light exposure, robustly demonstrated that sleep patterns had no effect on the circadian rhythm but only on the sleep homeostat / sleep pressure process S, since participants demonstrated the same circadian rhythm phase when they had sleep opportunities and when they did not (ie, pulling an all-nighter).
Is chronotherapy safe? Besides the chronic sleep deprivation induced by the procedure as the original authors noted themselves, observations on a handful of 1980s case studies claimed that a few individuals with DSPD turned into non24 after psychological stress, which led the patients to do their own unsupervised chronotherapy prior to being admitted at the clinic. The authors then amalgamated this uncontrolled self-experiment as a behavioral chronotherapy, and concluded that chronotherapy may cause individuals with DSPD to turn into non24, and is hence a far-fetched extrapolation. (Side-note: and yes, this study, the foundational case for most claims that DSPD can turn into non24, assumes the unproven hypothesis that non24 is a psychological disorder that can be solely caused by stress.) Since then, and despite widespread acceptation of this idea, there was no better controlled study demonstrating that chronotherapy can permanently cause non24. An informal survey by the Circadian Sleep Disorders Network in 2020 found that between 13% to 20% of respondents who tried chronotherapy observed their DSPD disorder turned into non-24 after using the therapy, and 95% found that it only stabilized their sleep for a month or less. Both the Circadian Sleep Disorders Network and the NORD, two well established patients associations, state that phase delay chronotherapy is not a long term solution as it involves great risks of iatrogenic complications of turning DSPD into non-24. Another possibility for these DSPD-turned-non24 cases is that of misdiagnosis, where these patients were in fact non24 all along. However, it is certainly true that it is possible for anyone to freerun, and hence phase delay chronotherapy can certainly cause a forced desynchrony with zeitgebers, akin to a constant jet lag, and hence induce DSPD into a temporary non24. But it remains debatable whether the circadian rhythm can permanently lengthen to the point of adopting a non-24 schedule, as most individuals with DSPD who try chronotherapy can still revert back to their DSPD schedule. Furthermore, the symptomatology is very different, with DSPD being still able to entrain to zeitgebers although with a delay, whereas non-24 involves a complete lack of a preferential chronotype despite exposure to zeitgebers. Finally, if such a lengthening would be possible, the opposite should also be theoretically possible (shortening the circadian period), but this was not observed with any procedure for the moment (except bright light therapy and melatonin, but only temporarily, not permanently), and especially not with phase advance chronotherapy. Given there is no biological pathway identified for such a mechanism, and no robustly controlled evidence for this switch from DSPD to non-24 (ie, by monitoring objectively the circadian rhythm via melatonin sampling or core body temperature), it is reasonable to assume that these cases may have been misdiagnosed non-24 cases all along. Future studies need to monitor the circadian rhythm before administering chronotherapy and after, for 1 week at each sampling opportunities, in order to reject the hypothesis of a misdiagnosed non-24 case.
For chronotherapy to change permanently the circadian rhythm, we would need the circadian rhythm to be plastic, meaning that it can change durably according to various factors. This is the circadian plasticity theory, which was investigated in a study by Czeisler et al in 2011 for space research. The investigators claim that shifting the circadian rhythm, eg with light therapy, affected durably the circadian rhythm beyond discontinuation of treatment! This should appear very surprising for anybody who either experienced or treated people with circadian rhythm disorders, as if it was true, these disorders would be infinitely more easy to treat. Unfortunately, this study is a perfect example of the flaws induced by the publish-or-perish culture currently reigning in research. In summary, although the study concludes that circadian plasticity exists, the authors fall short of proving it, since they show only a lasting effect of... 0.1h shortening of the circadian rhythm with a p-value of 0.02 on a sample of... 7 typical sleepers. That's barely statistically significant, and in any case wholly clinically unsignificant, because even if true, a 0.1h period shortening is not an "important finding" for the "treatment of circadian rhythm disorders" as the authors claim. See footnotes for a more detailed analysis and criticism.¹
Hence, chronotherapy so far has not been demonstrated to be either effective in the short term nor in the long term, as circadian plasticity theory is either invalid or is a very small effect that is useless for the treatment of circadian rhythm disorders. This also means that it is unlikely that chronotherapy can change DSPD into non24 permanently, as it is more likely that the freerunning will only last until the individual cycles back to their naturally delayed circadian rhythm stable phase, but nevertheless chronotherapy remains ineffective and dangerous due to the forced desynchrony and chronic sleep deprivation it inflicts on the patient, and their potential difficulties in reacquiring entrainment.
The absurdly obvious inefficiency and dangerousness of chronotherapy can be hard to grasp for the reader without the non-24 disorder. Transposed to a typical sleep schedule, chronotherapy would require the individual to sleep and wake up 1h earlier willfully and by using an alarm clock. No nap allowed. This short description should make apparent how absurd this protocol is, and how stringent and unsustainable it can be, likely not more than a month as the rebellion of the highly trained NASA crew for Mars mission monitoring demonstrated. The circadian rhythm, and sleep, simply does not work like that, they cannot be shifted or initiated at will, there are biological homeostatic processes that underlie their rythmical function, and we are forced to follow them, the ultimate cost of disregarding them (ie, extreme sleep deprivation) being death.
Given the certain risk of temporary freerunning, and the potential risk of permanent freerunning (ie, DSPD turning into non-24), individuals with DSPD should NEVER attempt any kind of chronotherapy, whether it is the most destructive phase delay type of chronotherapy (ie, sleeping a few hours later and later everyday) or the phase advance chronotherapy (ie, sleeping 15-30min earlier and earlier).
Anecdotally, in the present article's author's experience, having tried several chronotherapy schemes of both types, the author's circadian period (length) remained remarkably stable and constant, never reduced nor increased despite multiple experimented approaches and additional tools: various schemes ranging from the flexible to the extremely rigorous (to illustrate: the author eats everyday the same meal with the same composition and similar quantity everyday and properly timed according to his circadian rhythm since a year to factor out diet composition and timing as a confusion factor), so sleep hygiene or rigor was certainly not an issue. There is only one exception being the strict ketogenic diet which multiplied the author's daily phase delay by 2 (ie, daily phase delay was 1h instead of 30min), but this is not a chronotherapy and the biological changes induced by this diet are way beyond what a behavioral intervention can achieve.
Not only chronotherapy have never shown any effectiveness to treat circadian rhythm disorders, but they are also dangerous because they cause circadian misalignment: forcing someone to be awake and eat at times when their body should naturally be asleep can cause metabolic disorders (themselves potentially causing life-threatening cardiovascular diseases such as strokes over the long-term), partly due to inducing them to eat when endogenous melatonin levels are still elevated which confers increased insulin and glucose intolerance and hence risks of diabetes type 2. In fact, the links between metabolic disorders and circadian misalignment are so strong that some researchers suggest to change the name of these diseases to "circadian syndrome".
As a reminder, attempting chronotherapy is not necessary to develop non-24.
Note that a book by Michael Terman and Ian McMahan uses the term "chronotherapy" with another meaning, without behavioral intervention but rather by using properly dosed and timed zeitgebers, which are nowadays more commonly termed "chronobiotics". See here for another interesting discussion on DSPD turning non24 because of chronotherapy.
In the future, nudging may be attempted on circadian rhythm disorders, although being woefully unethical and with ambiguous benefits.
Chronotherapy also goes by other names, such as interpersonal and social rhythm therapy for bipolar disorder.
It is worth noting that more recently, other authors have redefined circadian plasticity in terms of phase shifts induced by photic inputs, ie, light therapy. This definition is very different from the original one that is discussed above.
Partially from my own post: https://www.reddit.com/r/N24/comments/gycc26/inverting_non_24/ftavh7o?utm_source=share&utm_medium=web2x
Footnotes:
¹ The study focused on healthy volunteers who were strictly monitored weeks prior and during the experiment, and who followed very strict sleeping schedules, alternating between freerunning periods in a lab environment devoid of timecues, and periods of non-24 entrainment to light (either 24.65h or 23.5h day). Each participant did both lengths of entrainment, but in a random order, before being re-entrained back to a 24h normal sleep schedule at the end of the experiments. The authors found that not only all participants could entrain to both sleep schedules thanks to light therapy, but that the participants retained a modified circadian rhythm, which remained shorter after the 23.5h day than the 24.65h! Impressive result, but is it really? There are several issues with this study, despite its marvelous design, it suffers from glaring basic statistical flaws: there were only 7 participants, which is a way too low sample size for classical statistics (such as the t-test used here), at least 50 participants would be required for valid inference, having less than 10 subjects hugely inflate the size of any effect found and the likelihood of a false positive; the circadian plasticity was found significant with a t-test finding a difference of... 0.1h, which is clinically unsignificant; the 0.1h circadian plasticity difference was furthermore found using a composite measure of circadian rhythm changes, which included mixing "plasma melatonin, plasma cortisol, and core body temperature data", which can be a way to boost the results and does not make much sense given the core body temperature is a much more reliable marker of the circadian rhythm; if we look at the circadian plasticity result using only core body temperature and not the composite measure, the p-value is 0.02, which means that there is a 1 chance in 50 that the result is a false positive, and given there are less than 10 subjects in the study, the effect size is almost certainly hugely inflated, so the false positive rate is certainly underestimated ; given that the sample size is so low, if the effect really existed, because of the low-sample-size induced inflation, the size of the effect should be much bigger than a mere 0.1h with a p-value of 0.02 (core body temperature), such a low value hints that the real effect is either negative or so small it's completely unsignificant ; and finally the duration of the phases was too short, as the aggregate metric used to measure circadian plasticity includes DLMO and hence is prone to the delayed DLMO effect, so that this result may reflect a temporary delay in circadian rhythm reentrainment and not necessarily permanent circadian plasticity.. The study could have benefited from using non-parametric statistics to reduce a bit the risk of false positives, although with such a low sample size this is unavoidable. Keep in mind that it's not a pathological population, the participants were healthy volunters, so the authors could have enrolled much more people. In conclusion, this paper clearly has many statistical flaws that would not pass peer reviewing with modern statistical standards. Given that the paper's abstract oversold the result by promising an "important finding" for the "treatment of circadian rhythm sleep disorders", it unfortunately looks like a product of the publish or perish pressure culture, which caused the production of lots of low-quality studies that nevertheless get published because of their innovative, although incorrect, findings.
Sleep hygiene
Summary: sleep hygiene is a set of tips to optimize the bedtime setup to purposedly improve sleep quality. Despite being the oldest treatment for insomnia, empirical evidence especially from controlled clinicial trials remain inconclusive for its effectiveness for the general population and for the treatment of insomnia, which is evidence of absence of efficacy of sleep hygiene, and prompted the AASM in 2021 to recommend clinicians to avoid the use of sleep hygiene with their patients, to avoid demotivating them with an ineffective therapy and delaying adequate treatment, and rather redirect them to effective therapies. Poor sleep hygiene was also never demonstrated to be a contributor of insomnia, which led to the retraction of the "Inadequate Sleep Hygiene" diagnosis from the ICSD-3. Furthermore, sleep hygiene was never demonstrated to be effective to shift the circadian rhythm. The term itself is problematic, as it implies that sleep disorders are due to "bad sleep hygiene", with insomniac patients having a "dirty" or "poor sleep hygiene". This translates the onus of therapeutic improvement on the patient instead of the clinician, so that if insomnia does not improve, it will always be blamed on the patient's uncompliance (ie, "the patient did not cooperate fully with the treatment or dropped out due to lack of rigor"). This is because the very concept of sleep hygiene stems from the fundamental misunderstanding that sleep is a flexible process that can be willfully manipulated, which is nowadays known to be incorrect, especially since the discover of the circadian rhythm and the sleep homeostat processes. Since it is ineffective on its own, there is also no demonstration of its usefulness in complement to other therapies.
Sleep hygiene consists of a set of tips mostly geared at creating good conditions for sleep, with some items inspired by conditioning theory. Other authors define sleep hygiene more generally in reference to its original conception by Kleitman, which was not a set of tips:
> Sleep hygiene refers to the notion that specific kinds of behavior are conductive to or incompatible with sleep and that modifying behavior may alleviate insomnia.
Hence, it targets improvement or manipulation of the conditions around bedtime. However, as shown by observations on the seasonal variations of the human circadian rhythm, the bedtime is independent from the wake up time (see figure 6), and the wake up time is a better predictor of the circadian rhythm. That's why sleep scientists consider sleep hygiene and sleep habits as a weak zeitnehmer (very weak zeitgeber) or even not a zeitgeber at all, which was later demonstrated empirically, with another excellently designed lab-controlled study on humans showing no effect of sleep nor sleep pressure on the core body temperature and hence the circadian rhythm. Likewise, sleep deprivation has no incidence on the core body temperature, whether in a comfortable ambient temperature nor after cold air exposure (see also here and here). Note however that sleep deprivation does mask proximal skin temperature, but not core body temperature. The authors also decry previously published research for their lack of rigor:
> [...] the evidence for a thermoregulatory role of sleep in humans is surprisingly weak. Some studies have demonstrated that sleep propensity can be modulated by circadian and behavior-induced changes in cutaneous temperature (for review, see Ref. 47). However, most studies that show that correlations of CBT decline with slow-wave sleep have not been carried out under controlled conditions, particularly posture: subjects usually lie down just before lights off (16, 43). Although this may appear to be a minor detail, for thermoregulation, it is not. Such a change in body position alone decreases CBT and increases skin temperatures for at least 2 h (27). This masking phenomenon has been entirely neglected in interpreting prior data on thermoregulation and sleep. Thus, for understanding the relationship between the thermoregulatory system and sleepiness (sleep) regulation, studies under controlled unmasking conditions before, during, and after sleep episodes are needed.
Furthermore, several studies have demonstrated that "alertness does not determine core body temperature", in other words cognitive (hyper)activity has no effect on the circadian rhythm.
Sleep doctors as well as profanes often recommend strict sleep hygiene as the first treatment for circadian rhythm disorders and sleep disorders. For example, it's not uncommon to get prescribed a very strict sleep schedule, such as sleeping everyday at the same time, avoiding any screen use 3-4h before bedtime, and waking up with an alarm clock at the same time or sometimes 15min earlier every day (which is phase advance chronotherapy).
Sleep hygiene does not work and has no scientific evidence base.
Indeed, the human body doesn't work like a clock... Humans biological processes, including sleep, follow not only a circadian rhythm (ie, a rhythm over 24h) but also an ultradian cycle (1.5h-2h), which is why when we feel a feeling of tiredness and are able to sleep, if we force ourselves to remain awake for more than 20min, the feeling will usually subside only to come back 1.5-2h later: these periods of high tiredness feeling are called the gateways to sleep (see the relevant subsection elsewhere in this document). Like all biological processes, these cycles have some natural variability every day, and hence it makes sense that the exact timing of this ultradian cycle, and the gates to sleep where you can sleep, varies a bit everyday, just like any biological process. Hence, requiring a patient to sleep at a strictly set time is not just difficult, it's impossible and goes counter to how the human body works biologically.
Not only is this not achievable, explaining the high rates of "patients uncompliance" or "laziness" which are in fact just natural and expected consequences of a non-physiologically founded sleep procedure, but as explained in the previous section on chronotherapy, sleep patterns have no effect whatsoever on the circadian rhythm (see also here), but only on sleep pressure. Hence, the "sleep consistency" items of sleep hygiene measures are simply inadequate to treat circadian rhythm disorders.
If the sleep doctors prescribing these inadequate sleep consistency measures were to wear an actigraphic device to track their exact sleep timing, they would certainly see that they themselves never sleep exactly at the same time every day, they also experience a similar variability in their sleep onset timing (although the magnitude would arguably be smaller compared to DSPD due to social jetlag and non24 due to the intrinsic freerunning component). For them to ask of the patients to follow such an overly strict sleep schedule that is not humanly possible to follow is only setting the patients to fail, and is hence a guaranteed but unfair opportunity for some of them to unduly blame their patients' low motivation or lack of self-control.
Despite being one of the oldest treatments for insomnia, the AASM guidelines since 2008 and in a 2021 systematic review state that empirical evidence does not support the effectiveness of sleep hygiene as the sole therapy (ie, single-component therapy), as it is not sufficient to improve sleep disturbances, which is in line with a previous 2014 review finding "inconclusive" evidence for recommending sleep hygiene to the general population, as there was little empirical support for sleep hygiene items. This should not come as a surprise given how the circadian rhythm orchestrates sleep as detailed above. The Task Force of the AASM, composed of the top psy* researchers who actively used and created several of the behavioral therapies analyzed here, also states that clinicians need more training to avoid prescribing sleep hygiene as the sole treatment for sleep disorders:
> There is limited research evaluating the long-term benefits of single-component treatments. Further, there is limited research examining any follow-up treatments after the delivery of a single-component therapy. Sleep hygiene is one of the oldest treatment approaches for insomnia in adults; however, recent evidence shows that it is no longer supported as a single-component therapy. Given that sleep hygiene is commonly delivered as single-component therapy in current practice, often without systematic follow-up, studies to develop and evaluate dissemination strategies for educating patients and providers about more effective approaches are needed.
In addition to explicitly qualifying it as "ineffective", the AASM experts even go as far as qualifying sleep hygiene as harmful since it can demotivate patients and delay the start of an effective treatment:
> The potential harms of utilizing a sleep hygiene intervention as a stand-alone therapy for insomnia disorder may include delayed implementation of effective therapies with continued or worsening insomnia symptoms. Patients with chronic insomnia could potentially elect not to undergo other treatments based on their experience using an ineffective intervention. As such, the Task Force did not favor the use of sleep hygiene as a stand-alone therapy for chronic insomnia.
Indeed, sleep hygiene as a medical concept was devised in the 1970s as a therapy to treat insomnia, so that up to now it's been half a decade with hundreds of studies, which could not robustly demonstrate any positive effect on any sleep parameter. Although it was medically defined in the 1970s by the psychologist Hauri, the term sleep hygiene was coined earlier, in 1939 by Kleitman, and evidence of an earlier similar concept going back 1864 in the writings of Paolo Mantegazza, and may even originate in the medieval beliefs about anguish. Even in 2011, clinicians knew there was no empirical evidence for sleep hygiene effectiveness, but its necessity just "stood to reason" (see also here), which is of course not evidence-based medicine. Indeed, although there are epidemiological correlations between poor sleep hygiene and chronic insomnia, there never was any demonstration of causation by clinical trials or animal studies, with on the contrary no empirical evidence that poor sleep hygiene could be a contributor to insomnia, suggesting that "poor sleep hygiene apparently is neither necessary nor sufficient for the occurrence of insomnia" since "patients with primary insomnia do not necessarily engage in more poor sleep hygiene practices than good sleepers, and monotherapy with sleep hygiene instructions not reliably producing significant benefit". Hence, it should rather stand to reason that "what can be asserted without evidence can also be dismissed without evidence" per Hitchens' razor, although we nowadays have several clinical trials demonstrating no effect, so that there is even more grounds to reject sleep hygiene as a pseudomedical therapy.
It's worth noting that the International Classification of Sleep Disorders (ICSD) added in its 1990 edition the "Inadequate Sleep Hygiene" as a diagnostic subcategory to insomnia but later retracted it in the ICSD-3, which was a systematic issue of the ICSD before its revision, with critics arguing against the "uncertain validity of several concepts" including inadequate sleep hygiene and the premature subcategorization as "a form of pseudo-precision" (mirror , pages 11-12). Furthermore, the poor reliability and validity of insomnia subclassifications in both the DSM-IV and ICSD-2 were confirmed by a study, finding unreliable site-varying support for "Inadequate sleep hygiene" and "Psychophysiological insomnia", and poor support for "Paradoxical insomnia" also called "sleep state misperception". Paradoxical insomnia is purposefully vaguely defined as sleep complaints without objective signs observed which can explain them, which of course bear the circular reasoning bias that if the cause of the complaint is not tested in the first place, such as the circadian rhythm, it obviously won't be observed. The same diagnosis of "Inadequate Sleep Hygiene" was also present in WHO ICD-9-CM and ICD-10-CM but seems to have been removed in ICD-11. The DSM-IV followed a similar evolution, with the various subcategories of insomnia being replaced by a single, primary insomnia diagnostic category, and the distinction between secondary and primary insomnia being removed later in the DSM-V in favor of a single and simple "Insomnia" category:
> It is important to note that despite sleep disturbance sometimes being considered a symptom of another disorder, there has been a shift away from simply considering sleep disturbances as a symptom of other problems – and ‘primary’ insomnia was removed from the most recent version of the DSM (DSM‐5, American Psychiatric Association, 2013) reflecting the current thinking that insomnia should not be dismissed as secondary to other disorders where comorbidity occurs (for a comprehensive discussion, see Harvey, 2001).
The magnitude of this paradigm shift cannot be understated, as most cases of insomnia used to be misdiagnosed and mistreated as secondary insomnia to a mental disorder less than a decade earlier, which depended on the clinical institutions, with institutions either overdiagnosing secondary insomnia, and others diagnosing mostly with primary insomnia, and interestingly with no significant difference between the diagnoses pattern of specialists and non-specialists, demonstrating how much this paradigm affected sleep diagnoses throughout medical fields:
(TODO: See also the file-drawer/publication bias issue of scientific research. Sleep hygiene is a clear example of that.)
Furthermore, it is worth noting that in its original form as devised by Kleitman, sleep hygiene was not a rigid set of tips, but rather a chapter pondering on what conditions and factors could influence the propensity to sleep, with no definitive answer. It is hence not unreasonable to consider that Hauri's sleep hygiene do's and dont's is a misinformed misdirection of the original purpose of this concept, lacking the original context in a certainly benevolent effort to convert a theoretical sleep research concept into an actionable medical practice, but in doing so he bypassed the evidence-based process since he did not first attempt to validate his recommendations. That one clinician offers suggestions to improve the medical practice is fine, but that others massively integrate it for over 40 years without any evidence backing its effectiveness is concerning.
> The earliest systematic reference to sleep hygiene can be found in Kleitman's Sleep and Wakefulness, which includes a chapter entitled, "The Hygiene of Sleep and Wakefulness." Kleitman reviews evidence regarding factors such as sleep duration, bedtime rituals, sleep surface, ambient temperature, sleep satiety, and body position. The chapter is discursive and in no way resembles the list of do's and don'ts of good sleep that exist today as sleep hygiene instructions. https://doi.org/10.1016/B0-72-160797-7/50067-7
One often used argument is that since sleep hygiene is not standardized and its composition and delivery varies widely with essentially every studies on sleep hygiene using different items (see also here), it cannot be studied in a standardized way, but the items could be. This is certainly a sound approach, but even then, either the items are too loosely defined and too numerous to by systematically studied (ie, the AASM 2021 review which discarded the network analysis because of too much variance), or there is inconclusive empirical evidence for each single item. Furthermore, this argument actually strengthens the case that sleep hygiene is not even wrong as a concept, in other words that it fails the falsifiability criterion and hence that it is pseudoscientific: if we can't study the validity of a concept, it is not scientific. Actually, the lack of standardization should have increased the likelihood of finding a positive result, since the more approaches tested, the more chances to find a positive effect with at least one of the approaches. But even despite that, no properly controlled study found any significant beneficial effect of sleep hygiene on sleep.
But even some of the most standard items, that are common to pretty much all implementations of sleep hygiene tips, are ineffective or even inadvisable in practice. For example, a 2020 systematic review found that most shift workers practiced daytime napping and caffeine consumption, "in line with best-practice fatigue-management strategies, but contrary to existing sleep hygiene recommendations". In other words, some of the sleep hygiene items can be in contradiction with best-practice fatigue-management strategies. Although avoidance of caffeine is indeed recommendable given its well established disturbances on both the sleep homeostat and the circadian rhythm, the avoidance of napping is hardly justifiable using empirical evidence. The fact that night shift workers, who are forced to find the most effective strategies to manage their sleep and fatigue on a daily basis, ignore these items is not a negligible fact.
Another argument is that since dark therapy (ie, the avoidance of blue light in the evening) is often recommended as part of a good sleep hygiene, then sleep hygiene works. This could not be further from the truth. The influence of bright light on the circadian rhythm was only discovered in the 2010s with the discovery of the ipRGC cells in the eyes, whereas (medically practicable) sleep hygiene was devised in 1977 by Hauri. Hence, sleep hygiene predates dark therapy. Further proof is that the original sleep hygiene tips indicated the opposite in its 10th item: "Rather than trying harder and harder to fall asleep during a poor night, switching on the light and doing something else". Further updated variants in the 2000s did not include anything about dark therapy either. Finally, even though nowadays blue light exposure and screens exposure in the evening is often advised to be avoided, this is, yet again, incorrect. First, because bright light affects the circadian rhythm much more than blue light, so that there is no point in turning off all screens if the user still leaves ambient room lighting on. What matters more is the light intensity of the light sources, not the color (although it has a role too in circadian rhythm shifting). Secondly, because screens can and are preferable to use, instead of using bedside lamps to read books, as screens that are properly dimmed and blue light filtered with softwares actually emit much less lux, often less than 1 lux, which means they have virtually no effect on sleep then, contrary to bedside lamps. In other words, even when dark therapy is included in sleep hygiene, it's done wrong since it is not founded on evidences but on "common sense". Filtering blue light is not enough, light intensity of ALL light sources must be dimmed down and this must be done according to the user's circadian rhythm, not the day-night cycle. This demonstrates that the very approach of sleep hygienism is misguided and the root of its ineffectiveness, as once a chronobiological view is adopted, it becomes clear that what matters is the stimulation of ipRGC cells in the eyes, and this allows clear and robust predictions about why some approaches work and some don't for circadian rhythm (non-)shifting, such as using properly dimmed and filtered screens being more adequate than reading books, contrary to what modern sleep hygienists advise. Hince, light-dark therapy is not considered part of sleep hygiene, and neither is it considered a psychological therapy, but an "alternative-complementary" therapy.
Finally, the effect of sleep hygiene, when it works, is always confounded with uncontrolled zeitgebers exposure, such as uncontrolled bright light exposure. They are also often of poor quality using subjective sleep metrics and with no control group. There is actually one study that strongly supports this (see a summary here). This study on insomniacs compared sleep hygiene, consisting of a constant sleep schedule, versus sleep hygiene with physical exercise, and sleep hygiene with light therapy. Only the group using light therapy saw any benefit as demonstrated by both subjective measures and actigraphy (objective measure), showing that sleep hygiene and physical exercise did not improve the sleep disturbances of insomnia whereas light therapy did.
There is another argument about whether using sleep hygiene in combination with another therapy/components would allow for a synergistic effect leveraging benefits for sleep hygiene that do not appear when using sleep hygiene alone. Indeed, even the AASM claims that sleep hygiene MAY still be useful as part of CBT-i (although the 2014 review makes no such claims), and, in its (not yet updated) 2008 guidelines on insomnia treatments, that "all patients with chronic insomnia should adhere to rules of good sleep hygiene" (see also these other guidelines). Note that this is a consensus-based recommendation, which according to the authors, mean this recommendation is not based on empirical evidence but on consensus from clinicians experience. The answer is: maybe, but there is no evidence. Since there is no evidence, sleep hygiene should not be considered as a primary therapy to try on insomniacs, just like we don't consider eating carrots as a therapy for insomnia, nor looking at cute cats pictures, or reading the latest sci-fi novel as a treatment for depression, etc. As Hitchens' razor states: "What can be asserted without evidence can also be dismissed without evidence." Furthermore, there is no reason to think that there would be any kind of synergistic effect. A synergistic effect happens when the components already have an effect on their own, and the combination increases the magnitude. Since sleep hygiene has no robustly reproducible effect on its own, it's unlikely to have a synergistic effect when combined with anything else. Sleep hygiene is likely just redundant.
Yet another criticism is that the AASM 2021 meta-analysis drawn conclusion on sleep hygiene based only on 3 RCT studies, and hence only concludes that more research is needed. This is not the case, and the AASM Task Force explicitly took this into consideration. For younger therapies, they state that there is a lack of studies and further research is needed. Whereas for sleep hygiene, they decided to explicitly conclude that it is ineffective. This is because they took into account that 1) as they state, sleep hygiene is half a century old, hence there was plenty of time for studies to be conducted and reveal a robustly positive effect. Since they could not, despite insomnia affecting up to 10% of the population at any given time, this is evidence of the absence of positive effect. 2) there is a large body of studies on sleep hygiene, which is to be expected due to its age, but only 3 studies were of adequate quality to be included, which shows that most studies were not properly designed or controlled and this strongly hints at a publication bias/file-drawer effect, preventing studies with negative results (ie, sleep hygiene has no effect) from being published.
Another criticism fueled by moving the goalpost is to claim that despite sleep hygiene being ineffective for insomnia, it may still be sound and helpful for typical sleepers. First, sleep hygiene was devised for insomnia, it was never meant for good sleepers. Secondly, there is no evidence of that either, with a review of controlled clinicial trials on good sleepers finding no empirical support for sleep hygiene recommendation for good sleepers. There were serious reasons to doubt that sleep hygiene could be effective for good sleepers since it was not effective for insomniacs. Indeed, good sleepers can only have small and/or transient sleep issues per definition, otherwise they would be insomniacs. If sleep hygiene could not be shown to be effective for insomnia, where any improvement on sleep will show up as a huge effect, it would be reasonable to assume that sleep hygiene could lead to any improvement for much lesser sleep issues. For example, increasing by 10 min the sleep of an insomniac who usually sleep only 2h is huge, whereas 10min for a good sleeper sleeping 7-8h is a negligible effect. If sleep hygiene cannot increase the already very short sleep of insomniacs, why would it increase the sleep of good sleeper with a much smaller margin of improvement? In summary, there is no evidence for sleep hygiene to be helpful for good sleepers either, and due to the issue of underpowering, it's unlikely there will ever be, even if sleep hygiene had a (tiny) effect for good sleepers.
Even where sleep hygiene could be useful, it fails to be accurate enough to be helpful with regards to the current knowledge of circadian rhythm science. For example:
- "drinking coffee before bed is bad" is inaccurate, as it should be "drinking coffee at all is bad for sleep" since its effects on the circadian rhythm and the sleep homeostat can last up to 48h.
- "avoid screens before bed, prefer to read a book" is inaccurate, as what matters is the light color and light intensity. Avoiding screens but allowing to light up bedroom lamps which can be much more intense and most likely of an inadequate color temperature that affects the circadian rhythm is not an improvement. Rather, filtering blue-green light content by using red light emitting room lamps and screens, as well as dimming their illuminance is the adequate method to minimize circadian disruption from light emitting sources. This particular item may stem from a misunderstanding of Crary's critique of the modern 24/7 capitalistic society, which uses screens as one of the various means of light control to regulate and extend labour worktime. But this constitutes only one mean, and this item confuses the tool, which can be harmless or even positive (avoiding boredom, allowing enriching cognitive activities at night that can contribute to cognitive and personal development) with the root cause (capitalism).
- "getting exposed to bright light in the morning using an alarm clock" has a very high risk of exposing the patient with DSPD or non-24 to bright light before their core body temperature minimal point, which is the pivot point in the light PRC curve where bright light therapy switches from phase delaying to phase advancing, worsening the patient's sleep-wake schedule by further delaying their circadian phase. This is especially problematic for workers, as they are likely to get exposed only to bright sunlight in the morning, and minimal dim artificial light or indirect sunlight in offices, hence getting maximal phase delay due to morning sunlight and minimal phase advance due to office environment. In fact, there are anecdotal reports (private communications) from individuals with DSPD who report being able to sleep and wake up earlier up to 3 hours earlier by wearing blue blocking glasses in the objective morning.
- "Avoiding exercise close to bedtime": Other studies on cohorts found no evidence that physical exercise impaired nor improved sleep, even when done close to bedtime or at night. It is now established that physical exercise does shift the circadian rhythm but only weakly, by modulating the BMAL1 clock gene expression in muscles through muscle contraction.
- "Avoid staying in bed for anything else than sleeping and sex": empirical observations demonstrate that individuals with DSPD, the most common intrinsic circadian rhythm disorder, actually spend less time in bed than typical sleepers. In addition, they do not require more extra sleep hours during the weekend than weekday compared to the general population, meaning that they suffer as much social jet lag as anyone else, but not more, which means that their insomnia difficulties during the workweek are not caused by a hypothetically too different sleep pattern during the weekends.
This is the intrinsic issue of non evidence based medicine therapies. Since it's not evidence based, there is no ground truth to fact check its implementation. This could be fixed by devising a standardized, evidence-based sleep hygiene set of tips, but there is no such standard yet, so there is no way to systematically update the tips according to the latest evidence (and they were not devised based on evidence in the first place), and it appears there is no will from the research or medical community to do that. In the end, sleep hygiene is just an informal set of tips with no empirical evidence support from properly controlled trials.
Sleep hygiene relies on a fundamental ableist assumption, the assumption that good sleepers have a good sleep behavior, whereas bad sleepers such as insomniacs have a bad sleep behavior. In reality, bad sleepers often tried a lot of things to improve their sleep, and have a much more stringent routine than good sleepers, since their sleep disorder prevents them from having the flexibility to play around their sleep patterns without severely detrimental consequences as good sleepers do. Clinicians never prescribe sleep hygiene to good sleepers. This is a profound misunderstanding of the fact that sleep disorders are disorders, not simply a bad habit. Hence, it is unsurprising that when sleep hygiene fails to treat a sleep disorder, and this always happens since sleep hygiene is ineffective, the common ableism trope of blaming the victim/patient is the only offered explanation by clinicians prescribing this pseudo-therapy.
TODO: more infos and thoughts on this issue: https://www.reddit.com/r/N24/comments/m25uyr/sleep_hygiene_does_not_work_there_is_no/ (mirror: https://archive.is/ouyYi ) and https://www.reddit.com/r/DSPD/comments/m25yo4/sleep_hygiene_does_not_work_there_is_no/ (mirror: https://archive.is/ZqVey ) and https://www.reddit.com/r/N24/comments/putoif/comment/henn38l/?utm_source=share&utm_medium=web2x&context=3 and https://www.reddit.com/r/insomnia/comments/p8vcls/does_sleep_hygiene_really_make_a_difference/
Sleep hygiene is furthermore a very inadequate word, as it implies that a sleep pattern can be "dirty" or unhealthy, and is understood as such for example by this study which claims that evening chronotypes can be caused by an "unhealthy lifestyle", obviously without any proof. This is because the word hygiene implies a role for the patient in the pathogenesis of their own pathology: somehow, they caused their sleep disorder, and that they can just as well "decide to fix it" with just some tips. The evidence of ineffectiveness of sleep hygiene is another proof this assumption is all but unfounded.
Avoiding screens altogether is unnecessary and extreme, all that is needed is during the biological evening and night to install a blue light filter app and reduce the brightness of the screens to the minimum and dim or turn off or dim down all environmental lights. It's surprising that some doctors focus only on screens, because they forget that if the user doesn't use any screen but keeps the room lights on, or even just use a white light bed lamp to read a book, this will delay the circadian rhythm much more than just looking at a screen due to environmental lamps often emitting much more lux than screens. To avoid the unwanted circadian phase delays induced by light and indirectly by screens, what matters is to avoid the stimulation of the ipRGC cells in the eyes during the biological evening and night, and all that is needed to achieve that is to filter blue light and reduce the intensity of all environmental light sources (not just screens).
On the other hand, more flexible recommendations of sleep conditioning can be helpful, although not a solution to shift the circadian rhythm as they cannot help with circadian shifting nor entrainment. The basic idea is that just like diabetics need to be stricter with their diet, individuals with a sleep disorder need to be stricter with their sleep than those without these afflictions, despite the afflictions not being their fault. This can be expressed as follows: always put one's sleep first, and avoid sleep disturbances.
For example, alcohol is known to be a strong sleep and circadian rhythm distubance. Hence, alcohol should be avoided all the time for people with a sleep disorder.
Another good tip from good sleep hygiene recommendations is to avoid staying in bed when not being able to sleep under 30min, as to avoid losing time. Sleep hygienist recommend that as there is a hypothesis that staying in bed too long will make the brain or body behaviorally learn to associate laying down in bed with other activities than sleeping and hence make sleeping difficult, but there is no evidence. Rather, this tip is useful only to free yourself from unnecessarily losing time trying to sleep when you cannot due to trying to sleep in circadian misalignment.
Some molecules that can affect sleep quality should be avoided: avoid caffeine (coffee, energy drinks, tea) 6h at least before bedtime. Caffeine was shown to phase delay the circadian rhythm up to 40 min over 49 days of experiments if a double expresso was ingested 3h before habitual bedtime of typical sleepers, with the effect likely being greater for individuals with a circadian rhythm disorder such as non-24 or DSPD since they have a weaker entrainment to bright light and other zeitgebers that could counterbalance caffeine's effect. Caffeine should be avoided even early in the day, as there is evidence it can still delay the circadian rhythm on the following nights and prevent napping even during the next day, showing caffeine's effects carry over at least 48h after intake, affecting the sleep quality with as little as 200mg of caffeine in the morning being sufficient to modify the brain's activity as observed by EEG. Interestingly, caffeine increases core body temperature, which is known to increase wakefulness and demonstrates that caffeine is indeed a zeitgeber, it can modify the circadian rhythm. Furthermore, there is evidence that caffeine can reduce melatonin blood levels.
Another issue with sleep hygiene is that it often includes sleep restriction since the original 1977 tips. The widespread but unsupported idea that oversleeping is as bad as undersleeping also stems from the original sleep hygiene tips. Just like dietary restriction is an inappropriate treatment and detrimental for malnutrition, sleep restriction strategies are inappropriate and detrimental for sleep insomnias and will only worsen circadian rhythm disorders. Restriction is never a solution to the chronic lack of a vital need. For someone already severely and chronically lacking sleep, sleep restriction is obviously not going to help and will only create further sleep deprivation. And often the individual is already doing some kind of sleep restriction, often due to work, and obviously didn't get any benefit since they feel chronically sleep deprived and tired. Several common therapies such as sleep restriction (sleep hygiene only), cognitive therapy or meditation relies on requiring "efforts" from the patient over their sleep, which only lead to further chronic sleep deprivation and dopamine buildup (forbidden sleep zone). Some strategies also require believing, a positive mindset, or other subjective capacity rooted in ableism and psychosomatism such as stress management. The VLiDACMel therapy does not require any such "effort" from you, and no therapy should ever do. The only thing required is your commitment to follow the instructions regularly and at the adequate timing for your circadian rhythm. All steps are done when you are naturally awake, without requiring you to wake up or sleep earlier forcefully. A positive mindset is a plus as it allows to stay motivated and committed, but it's not necessary, and the author actually was not believing the therapy would work since he had to iterate several times with varying the parameters until it did eventually, despite not believing if it was even possible to be entrained at all. In other words: an effective therapy will work reliably and reproducibly regardless of whether you believe or stay positive about it.
If you get entrained, you may feel like your days are shorter. This is because you are used to longer days than 24h. It's important then to remember your commitment to improve your sleep by entrainment, and so to put your sleep first: if you still have work to do but you feel tired, then write down the remaining tasks or ideas you have left but can't fulfill now, then postpone to tomorrow your activity (eg, work) and go to sleep asap. Interestingly, night shift workers get the same feeling of a too short day, and they are also advised to put their "sleep first, not an afterthought".
Going to bed too early is a waste of time of course, plus it worsen insomnia. If your body is not ready to sleep, laying down in your bed isn't gonna help. So yes don't lose your time like that, do stuff when you're not feeling sleepy. This is not unconventional. Ask any typical sleeper to systematically lay down at noon in their bed because someone else decided this should be the time they should sleep at. They simply will be driven mad by their inability to sleep at the required time and the loss of time spent at trying to sleep (as opposed to actually be sleeping). Individuals with non-24 and DSPD have the same human activities, they just are active at a different times. There is nothing unconventional or shameful in doing and enjoing activities when you are awake, regardless of when others are awake. That does not mean one should disrespect others sleep, so if you are active during the night please be mindful of others, but this does not mean you should lose your life waiting because others are sleeping.
There is another major issue with sleep hygiene, which is that it is often used as a functional control condition, with very imprecise description of its actual content and delivery. Hence, this is a major confound for lots of studies, as for example the alleged efficacy of sleep restriction may in fact be a null effect compared to a negative effect caused by sleep hygiene.
Sometimes it can be read, even from certified sleep medicine physician, that conditioning, ie via alarm clocks, can change the circadian rhythm. Obviously, this is not the case: in addition to the empirical evidence that sleep patterns do not affect the circadian rhythm, we can simply reason that if this was true, then the prevalence of circadian rhythm disorders would be almost null, affecting only those who have no access to an alarm clock. Instead, alarm clocks can have an indirect effect on the circadian rhythm by allowing the individual to get exposed to bright light (artificial or sunlight) a bit earlier in their PRC curve and hence get more phase advance. In other words, it's not conditioning, but simply earlier exposure to zeitgebers that affect the circadian rhythm.
---
TODO: integrate the paragraphs below.
The lack of evidence for using sleep hygiene as a primary, standalone therapy has now been noted and is interpreted in guidelines as a sign of a lack of efficacy. However, it is still recommended as a complementary, optional therapy, in addition to other therapies. But there is no evidence either for the efficacy of sleep hygiene as an adjunct therapy!
One argument is that it is an innocuous process, especially because it is drug-free. For the latter, there are plenty of counter-examples: recommending someone to not sleep, even just 24h, signicantly increase their risk of death and guarantees it after a few days/about a week. There is also the example of behavioral chronotherapy, which has been reported to cause DSPD disorders to turn into non-24, a more severe circadian rhythm sleep-wake disorder. Hence, I would argue that sleep hygiene can in fact be harmful, and can very well worsen sleep and general health, especially when applied to people with physiological sleep disorders, and more specifically can increase sleep deprivation, cause more circadian phase misalignment, and ...
There is often an argument of intuition and benevolence to support sleep hygiene. But intuition and benevolence are no indicator of truth! Throughout history, several other pseudotherapies were devised on the same principles, and ended up being harmful, such as arsenic used to "cure" several ailments during the Victorian era in England: indeed, it was logical to think that a compound that could clean things outside of the body could also be used to clean the insides from illnesses. Intuition and benevolence are not evidence, and modern evidence-based medicine should never rely on these, what we need is (objective) data to show whether therapies work or not.
Sleep restriction
Sleep restriction is a behavioral intervention that requires the patient to restrict their time asleep, as to increase their propensity to sleep at the desired time by increasing the buildup of adenosine (homeostatic sleep process S).
This study describes the typical sleep restriction procedure:
> The total time in bed allowed in the sleep prescription was equal to the average total sleep duration plus 50% of the total time spent awake in bed (therefore reducing the total additional ‘wake time’ by half), with a minimum time in bed of 5 hours. Actual bedtime and wake-up times were negotiable. Participants were asked to continue with a sleep diary until the next visit.
> Participants attended a second visit 2 weeks later. If participants were sleeping for <85% of the time they spent in bed (according to their diary), the time allowed in bed was further reduced to total sleep duration plus 30 minutes. If patients felt excessively sleepy, they were advised to spend 30 minutes more in bed each night. Wherever possible, wake-up time was kept constant, and any changes required were made to bedtime. Participants were also given a written flowchart summarising change options (available from authors on request) and asked to self-adjust their sleep every 2 weeks thereafter. Control participants also attended a second visit after 2 weeks where their general sleep progress according to sleep hygiene guidelines was discussed. Scripts were used for the delivery of instructions to both groups.
What theory underlies the assumption (but not proof) of efficacy for sleep restriction to treat insomnia and circadian rhythm disorders? Here is what the same study describes:
> The sleep restriction component of CBT-I consolidates fragmented sleep by reducing the time allowed in bed (the sleep opportunity); thereby inducing mild sleep deprivation to enhance the endogenous sleep drive.
Indeed, previous studies demonstrated that sleep patterns have no effect whatsoever on the circadian rhythm (see also here), but only on sleep pressure.
This theory stems from the following study:
> Treatment of chronic insomnia by restriction of time in bed, 1987 https://pubmed.ncbi.nlm.nih.gov/3563247/
Unfortunately, this study only used subjective measures of sleep. And all subsequent studies finding any significant effect only did so on subjective measures, again. According to this 2014 review, there is no study with a significant effect of sleep restriction on improving objective sleep measures (actigraphy, polysomnography, melatonin, core body temperature), especially without a confounding of other potential factors such as changes in bright light exposure. Furthermore, the study found that the vast majority of studies done on sleep restriction were of inadequate methodological design (ie, poor quality). Out of 22 studies matching the criteria for this review, only 4 could be included.
So is sleep restriction viable or not? It's not, and it's detrimental. And this can be inferred very simply: sleep is a vital need for all living creatures, just like food and drinking (ref). Just like malnutrition can not be treated with dietary restrictions, nor thirst be quenched by restricting water drinks, insomnia and chronic sleep deprivation can not be treated with more sleep restriction/deprivation.
The 1987 theory founding the rationale for sleep restriction is very bogus and doesn't account for basic sleep mechanisms, especially that the homeostatic sleep process S is mostly uncoupled from the circadian process C (for context, Borbély's model of these 2 processes was published in 1982, just 5 years prior, and has only been confirmed empirically in the last 2 decades) and that the sleep-wake schedule does not affect or only weakly the circadian rhythm. Hence, the sleep restriction theory is an old theory that was interesting to investigate at the time but is clearly deprecated given current knowledge of how sleep and its underlying mechanisms work.
Proponents of sleep restriction therapies argue that there is evidence of an association between daytime fatigue and napping, and poor sleep and health outcomes. While this is true, association is no causation. They herein assume that daytime napping is causing, or at least contributing, to the poor sleep and health outcomes. However, daytime napping and fatigue is an unambiguous sign of an already present severe sleep disorder, with napping being merely a compensation mechanism for the body's homeostasis to recover a sustainable sleep balance. As such, there is no properly controlled study showing a direct improvement on objective sleep measures by simply restricting naps, and likely the opposite would be shown if negative results studies were as published as positive ones. Restricting nap is hence focusing on a consequence of sleep dysregulation, and worsening it further. Napping should never be prevented forcefully, rather the underlying sleep dysregulation causing these naps should be treated.
Furthermore, empirical evidence show that the chief complaint of intrinsic circadian rhythm disorders such as DSPD is insomnia and fatigue, but not excessive daytime sleepiness, and they spend less time in bed than typical sleepers. Hence, it is illogical to expect sleep restriction, which only adds more sleep deprivation over the endogenously generated sleep deprivation by the circadian rhythm disorder, to produce any beneficial effect.
Finally, beyond the fact that sleep deprivation cannot change the circadian rhythm and instead can reduce the effect of zeitgebers such as bright light, sleep deprivation also impairs interoception, and hence the ability to feel sleep pressure. Hence, sleep restriction is counterproductive for the intended goal of manipulating or stabilizing either sleep process. However, sleep deprivation does affect sleep pressure obviously by increasing the adenosine buildup, hence using sleep restriction as a therapy rests on the sole hypothesis of treating a too low buildup of adenosine, which is a hypothesis for the ethiology of sleep disorders that remain to be empirically demonstrated.
Cognitive behavioral therapy for insomnia (CBT-i)
Cognitive behavioral therapy for insomnia (CBT-i) can be seen as a mix of multiple behavioral based interventions for sleep, with sleep hygiene is being a core element of CBT-i and always including sleep restriction or even chronotherapy sometimes. Nevertheless, CBT-i has not been shown to be effective for circadian rhythm disorders so far, as night owl chronotypes had less benefits from CBT-i interventions than insomniacs, and the AASM stating in its guidelines that only indirect evidence exists for both chronotherapy (or Prescribed Sleep-Wake Scheduling as they call it) and CBT-i.
While there is no evidence chronotherapy/sleep hygiene can shift or entrain the circadian rhythm, there is in fact some evidence of the opposite, that it doesn't work, but it's the change in the (uncontrolled) timing of light exposure that shifts the circadian rhythm, as the authors of this study state: "The study showed that the direction of circadian phase change is determined by the light-dark exposure, not by the fixed sleep schedule, and that both morning and evening light exposures need to be controlled to shift circadian phase." Accounts from individuals with non-24 report that CBT-i had no effect on their circadian rhythm at all, with in the end the therapist recognizing their powerlessness and providing no alternative.
Furthermore, the sleep restriction required by CBT-i is actually counterproductive, as it decreases the effectiveness of light therapy. So the CBT-i is not only detrimental to health due to the increased sleep deprivation, but also reducing the effectiveness of the really effective procedures.
CBT-i is not even effective to treat insomnia, as there is no empirical demonstration of its effectiveness on improving objective measures of sleep, it only affects subjective measures which are nothing more than the placebo effect. (TODO: rewrite this part to extend with refs etc).
In February 2021, the AASM conducted a systematic review and meta-analysis on behavioral therapies for insomnia, including but not limited to CBT-i and sleep restriction. Several points are interesting to note:
- They support the use of behavioral therapies, especially CBT-i, just like most health institutions, to treat insomnia.
- With objective measures such as polysomnography and actigraphy, CBT-i and sleep restriction both show a reduction of total sleep time and sleep efficiency (ie, more sleep fragmentation). This is the opposite to what is sought. The only improvements observed with these therapies are either with subjective/behavioral metrics, or in the increase of the duration of the wakefulness periods. Which is double dipping, since these therapies aim to reduce the time spent in bed, hence of course they increase the time spent awake.
- All authors are practitioners of these therapies, as is apparrent by their frequent mentions of the "TF's experience", which creates a conflict of interest but a necessary one since of course only experts of the field can review and assess the work in this domain. This is why dogma exists in science, and why ineffective therapies can only be rejected by other scientists finding and demonstrating more effective therapies. Science is an iterative process, with demonstrably better models progressively replacing older inaccurate (or less accurate) models over time. In the author's experience, it's the first time he observed such subjective comments inside the results, not the discussion, of a systematic review, that's very peculiar.
- There are also some inexcusable biases for a systematic review, such as this sentence which is inaccurate given current evidence and is, of course, not sourced at all: "In addition, unhelpful behaviors can have a direct impact on the physiological systems controlling sleep. For example, variability in the timing of sleep-wake behaviors can create circadian dysregulation, and excessive time in bed can diffuse the homeostatic drive for deep sleep and can also lead to conditioned arousal."
- Zeitgebers such as bright light exposure were not controlled in this review. It is the present document's author's conviction that when the behavioral therapies work, a change in the exposure to zeitgebers is the key factor.
- Sleep diaries are now a standard assessment for insomnia too, not just for circadian rhythm disorders. They further considered that only sleep diary measured outcomes should be considered critical in determining a therapy's efficacy, not behavioral scales, similarly to what the present document's author proposes.
- They state the following reasons: "In the study of insomnia treatments, nighttime sleep and insomnia symptoms are most commonly measured with daily sleep diaries,29 which capture information about the timing of sleep (bedtime, rise time) in addition to individual sleep parameters, such as sleep latency (time to fall asleep initially), wake after sleep onset (WASO; duration of nighttime wakefulness), and early morning awakenings (waking in advance of the desired rise time) that are commonly the primary symptoms targeted in insomnia treatments. Additional summary metrics commonly derived from daily sleep diaries include total sleep time and sleep efficiency (total sleep time/time in bed*100%). Daytime napping/sleeping behaviors are also commonly tracked in daily diaries when delivering treatment. The primary advantage of sleep diaries is that they allow for the daily collection of information on nighttime symptoms, making them less subject to recall bias than questionnaires. Treatment effects are most commonly assessed with aggregated mean-level changes in individual sleep diary parameters across time, generally every 1 or 2 weeks, but increasingly, the variability of these parameters across days is also being viewed as clinically important."
- Actigraphy is becoming a standardized assessment for insomnia, but remains optional for now.
- The limitations section is arguably the most interesting, as they state that:
- there is a lack of studies about the adverse side effects of behavioral therapies. Despite this lack, the authors consider these effects to be negligible or minimal for most therapies "based on their clinical experience".
- there is a lack of follow-ups on the long-term efficacy of most behavioral therapies, especially single-component therapies (eg, using only sleep hygiene).
- the drop out rates are never reported (so that we can assume that a lot of the clinically significant effects are inflated due to the undue rejection of patients who failed to see improvements in their sleep following behavioral therapies, and could have been rejected as drop-outs due to their "lack of compliance").
- the content of CBT-i is highly varying, so that no two CBT-i therapy is the same. To the point where they considered to do a network analysis to quantify what are the most effective content, but finally decided not to as the variability was too great to even permit such an analysis. As other studies have shown, CBT-i also often includes the regulation of exposure to bright light, or even sometimes directly a bright light therapy + dark therapy component. Yet, the task force still strongly recommends such a multiform therapy with no definitive nor reproducible content.
- the old age and small sample size of most studies for single-component therapies such as sleep restriction.
- they recognize that in current standards, insomnia is seen as a primary disorder, not secondary to psychological disorders, which fits with previous reviews (see here and here): "some treatments (eg, biofeedback, relaxation therapy) emerged decades ago and thus reflect clinical conventions of those times, such as a focus on sleep-onset insomnia and conceptualization of most insomnia as a symptom of another disorder; therefore, they do not reflect current diagnostic or assessment standards."
- they consider sleep hygiene alone is not supported by evidence as the sole treatment for insomnia: "Sleep hygiene is one of the oldest treatment approaches for insomnia in adults; however, recent evidence shows that it is no longer supported as a single-component therapy."
- By extension, a difference must also be made between all old behavioral therapies that lack evidence despite the length of time that they were (or could be) studied, versus newer behavioral therapies that lack evidence due to the short timeframe since their inception.
- they are aware of the backlash against sleep restriction therapy, but can not comprehend it and commit a circular reasoning fallacy: "Among the available psychological treatments themselves, it seems that patients may initially believe sleep restriction therapy to be undesirable; however, those who improve with this treatment rate it positively." → obviously, for those for whom it works, they will have a positive experience, but what about those who did not benefit? How many are there? How many drop-outs?
The results of this review led to this 2021 guideline on behavioral therapies, with a strong recommendation for CBT-i, and conditional for the rest, such as sleep restriction therapy, but does not recommend sleep hygiene as a single-component therapy.
It's worth noting that a previous institutional review found cognitive behavioral therapies (CBT) to also be effective for a wide range of mental afflictions, such as anxiety related disorders.
In the present document's author's view, this systematic review and widespread position about CBT-i and sleep restriction (as well as the absurd "paradoxical intention" and "intensive sleep retraining" therapies) shows how archaic the current management of insomnia is, disregarding most of the current knowledge about sleep's mechanisms and especially the circadian rhythm (all the behavioral therapies being based on manipulating solely the homeostatic sleep process). That is not to say that CBT-i is a scam, but that it is likely not directly affecting insomnia per se but other ailments that indirectly improve insomnia, as can be suspected from the low, but still significant, success rate. The bottom line is that we need more specific, more systematic interventions for insomnia, or otherwise the insomnia pandemic will continue to rise.
Mindfulness meditation therapies
An excellent study by Soomi Lee et al (see here for a summary) found that contrary to the well held, but incorrect and baseless, assumption that mindfulness therapies can shift sleep patterns, this study found on the contrary that the effectiveness of mindfulness therapies are dependent on sleep duration and quality, which demonstrates the opposite causal relationship.
Conclusion on behavioral therapies
In conclusion, given the findings of this excellent study with well controlled (light and position were controlled) and measured (core body temperature, melatonin and sleep schedule) conditions, and the findings from this slightly biased but nonetheless interesting 2021 AASM meta-analysis and systematic review, behavioral therapies can modify the sleep-wake schedule and the circadian rhythm, but with only a small effect. These behavioral therapies for insomnia can be especially good for individuals with both insomnia and a co-morbid psychiatric disorder, as these therapies can often treat both.
The main issue, in this document's author's view, with behavioral therapies is that they are awfully unoptimized, as they target the sleep-wake schedule to treat insomnia or the circadian rhythm. Hence, they are treating a symptom, instead of the root cause. Insomnia can be caused by a variety of intrinsic and extrinsic conditions including physiological illnesses, each optimally treated with a specific treatment: sleep apnea requires a CPAP, Parkinson a motor regulator, epilepsy an anti-seizure drug, viral infections require to wait or an antiviral, circadian rhythm disorders are better treated with light-dark therapy rather than behavioral therapies according to a systematic review. Pretty much anything that can impair the immunological system and the core body temperature can impair sleep. Optimal sleep disorders therapies would target these root causes rather than the symptom, as insomnia is a symptom of multifactorial origins: not two insomnia are the same.
This issue is especially present for multi-components therapies such as CBT-i, as their content remains unstandardized and hence highly variable and is poorly reported, which makes it impossible to pinpoint what components are beneficial or detrimental and how they should be optimally delivered. This is especially worrying since several core components of CBT-i, such as sleep restriction and sleep hygiene, were found to be unsupported by empirical evidence, in other words that they were ineffective to improve insomnia, and other components are either highly variable in their inclusion or their effectiveness remain unstudied. If the components of the CBT-i therapy are ineffective on their own, then the little effectiveness found with CBT-i must come from somewhere else, an unaccounted factor, and until this factor is accounted for, the mechanisms for CBT-i effectiveness will remain elusive and the therapy's content and delivery unoptimized.
For instance, although we cannot at this stage state it with certainty since this factor is rarely monitored in psychiatric studies, it is possible that light therapy may actually be the root cause of most if not all of the effects observed with behavioral therapies. If correct, then the other components of behavioral therapies such as sleep restriction and chronotherapy are actually reducing the main effect of light therapy because of the increased sleep deprivation, which reduces the patient's circadian response to bright light and lack of timing relatively to the circadian rhythm. There is actually some empirical evidence from one study comparing the effect of structured sleep hygiene versus structured sleep hygiene and bright light therapy for insomniacs, which found that the patients only significantly benefitted from the latter group. Furthermore, behavioral therapies can even worsen the sleep disorder due to mistiming, as exposure to bright light before the core body temperature minimum (CBTmin), which happens around the middle of the circadian night, delays the circadian rhythm and hence sleep schedule. A sleep restriction or chronotherapy intervention actually puts the patient at the risk of mistiming light therapy by getting prematurely exposed to sunlight or artificial light therapy too early in their circadian night, before the CBTmin point, which would then delay their circadian rhythm phase and hence worsen their sleep issues.
(Side-note: The idea that a combination of ineffective therapies can make the whole effective is incorrect and stems from the pseudoscientific holistic medicine movement. Combining slightly effective therapies can have a synergistic effect, but each component must have an effect on their own in the first place)
Nevertheless, modern behavioral therapies are a huge step forward, as the advent of CBT-i allowed to target treatments specifically on insomnia, regardless of co-morbid psychological disorders, whereas just 2 decades earlier the clinical practice was focused on treating the co-morbid psychological disorders and disregarded insomnia altogether, by assuming the latter would remit by treating the co-morbid condition, which is not the case, since there is strong evidence showing that psychological disorders are independent from insomnia and circadian rhythm disorders (or rather actually insomnia is a risk factor that precedes psychological disorders), and hence should be treated separately.
One other main issue with behavioral therapies in the author's opinion, as also stated in the AASM review, is that there is a lack of studies on their adverse effects. Some therapies such as relaxation therapy are certainly harmless and hence can only have a positive or null effect on sleep or psychological issues, but other therapies such as sleep restriction have a very harmful potential although not widely recognized by the medical profession currently: increased risk of cardiovascular disease such as strokes, increased rate of accidents such as car accidents, etc. This needs more investigation, especially since similar if not greater effects can be achieved with much less harmful therapies such as light-dark therapy and melatonin, the latter becoming an accepted standard treatment for insomnia in recent years.
A general rebuttal of the psychological theories of sleep disorders etiologies and treatments
Stress-induced heart attacks that are in most cases not even caused by any prior stressful events in most cases, a pseudoscientific belief in psychologically induced death that is medically accepted, the father of stress weaponizing his invention for Big Tobacco against public health measures, an imaginary factor supposed to cause all mental illnesses, epilepsy and parkinson being diagnosed as imaginary mental illnesses and treated with electroshocks, a well established and still renowned professor training ruthlessly his children to match the behavior he wanted and driving most of them to suicide: welcome to the dark side of psychology, which pervaded most of the modern psychological practice on sleep.
This topic merits a deep and careful inspection, which would be arguably be a very fascinating enterprise, but very time consuming, as the production of this field is dantesque, given the threshold for evidence is much lower and that theoretical (ie, hypotheses based on no data) are legion and well accepted for publishing. Hence, there is a propensity to propose hypotheses first, and verify them rigorously much later down the line, or never, since randomized clinical trials only represent a small proportion of the publications in this field.
If you are interested in reading a very preliminary and disorderly, or even partially wrong, document, here is a draft with randomly placed bits of information: SleepDisordersPsychoRebuttal.html . This document may or may not ever be finished, as it is not a priority of mine to debunk an approach to sleep medicine as it is to search for actually effective approaches. This work was only done so far to elucidate which approaches were promising, or just sound, and which were not, to guide future work. It is hence provided with no guarantee of any kind.
Cannabis
Contrary to common beliefs, marijuana (cannabis/THC) is not a hypnotic, it does not help with sleep disorders, and it has a lot of psychological/neurological minor but invasive side effects such as a drastic loss of motivation.
However, according to a 2019 review there is some evidence that cannabinoids may be capable of shifting the circadian rhythm, and more anecdotally, some individuals with DSPD claimed that specific formulations may be more effective such as by oral ingestion.
Furthermore, cannabis may be helpful to treat co-morbid disorders that can impair sleep quality, with a study showing that long-term use of cannabis improves symptoms of PTSD. A 2022 systematic review found that while CBD and THC can improve anxiety, they have negligible effect on sleep.
Various other treatments
- Probiotics and parabrobiotics were not found to improve objective sleep parameters, only PSQI subjective parameters, according to a 2020 systematic review.
Core body temperature monitoring for circadian phase assessment
Introduction and history of core body temperature measures
Imagine being able to monitor your circadian rhythm. You could know if you had a circadian rhythm disorder while continuing attending to your obligations, without having to use a sleep diary while disabling any alarm clock and cancelling appointments for weeks. You could track when is your circadian night and hence when you're more likely to fall asleep and sleep long with a high quality sleep. Or also if it's currently your circadian day, when you can expect to be at your peak productivity with the highest vigilance level. You also would not need to guess anymore the optimal timing to use bright light therapy and melatonin. That's what core body temperature (CBT), and potentially electrocardiograms (ECG), promise.
Monitoring pertinent biomarkers is essential for the robust management of any disease. Insulinotherapy for diabetes is only effective when the user knows how to monitor their glucose levels. Similarly, without a reliable way to monitor the circadian rhythm, it is likely impossible to robustly manage circadian rhythm disorders, even if we have efficient therapies, because we simply don't know when to use them. Biology is per definition variable, even with a strict compliance to the therapy, there will be variability in the circadian rhythm, which requires adaptations in at least the timing but potentially the dosage of the therapy on a day-to-day basis. It is hence not sufficient to simply define a therapy and ask for patient compliance: the patient needs to be able to adapt it flexibly depending on how their circadian rhythm reacts and varies on a daily basis.
However, current methods of estimating the circadian rhythm are unreliable. A 2021 study published in the Sleep journal described a human trial where shift workers were monitored with urinary melatonin, behavioral sleepiness and task vigilance scales (Karolinska Sleepiness Scale and Psychomotor Vigilance Task) with and without light exposure monitoring. When using both the behavioral scales and light exposure monitoring, they could only accurately estimate the circadian phase for 65% and 35-47% (as measured by the urinary melatonin) for respectively day-shift and night-shift workers, which is arguably unsatisfactorily unreliable, especially since endogenous circadian rhythm disorders likely lead to a more variable circadian rhythm phase than night shift workers and certainly more than day-shift workers who are just typical sleepers (and for which the accuracy was only 65%). Although it is worth noting that melatonin levels are only an imperfect proxy to measure the circadian rhythm (see also here), and hence may limit the conclusions drawn from the previous study, this is still a better target measure of the circadian rhythm than behavioral scales or actigraphy. Hence, there is a clear need for newer technologies that can more accurately model the circadian rhythm and individually estimate the circadian phase.
Thermoregulation is a core bodily function that governs alertness and sleep, as theorized by Krauchi in 2006 and pioneered by the Mammoth Cave study of sleep and the circadian rhythm by Nathaniel Kleitman in 1938, who was the first to use a rectal thermometer to study the circadian rhythm in human physiology and especially sleep, and later developed further by the works of Aschoff et al in the 1950s. Core body temperature is the internal bodily temperature, the temperature in organs. Humans are homeothermic and even endothermic animals, for which a highly stable and robust internal temperature is necessary to survive. Hence, core body temperature is highly robust against environmental, ambient temperature changes. This is in contrast with skin temperature, which is in direct contact with the environment and does not reflect the same robustness. Core body temperature represents about 80% of body heat content. Core body temperature is the primary messenger to synchronize local clocks throughout all cells in the body with the central circadian rhythm clock in the SCN. For all purposes, core body temperature is the best proxy of the circadian rhythm, as the circadian rhythm involves modulation of the core body temperature by vasodilatation and vasoconstriction of distal skin (ie, respectively heat gain and heat loss/dissipation at the hands faster and feet to a slower pace) (see also here). Core body temperature is not only highly coupled with the circadian rhythm, but also with subjective alertness and sleepiness levels (see also here).
The coupling between core body temperature, sleep and activity/alertness is present in all organisms:
> Under entrained conditions all organisms exhibit relative inactivity during their circadian temperature nadir, which in higher species, is coupled with the sleep period. The phylogenetic comparison, together with a large body of experimental data, indicates that the nocturnal decline of CBT in humans is functionally linked to reduced alertness which in turn can lead to initiation of sleep.
Even for nocturnal animals such as mice, their core body temperature is low when they sleep and high during wakefulness periods, with bright light signaling sleep periods and melatonin levels during wakefulness periods: essentially, melatonin and bright light are wired inversely to the noradrenergic vasoconstrictor system in nocturnal animals compared to diurnal animals, but the circadian rhythm and its linear coupling with core body temperature remain, which shows how conserved, generic, robust and essential the thermoregulatory and circadian rhythm system is.
To summarize, core body temperature is the circadian rhythm and alertness:
> In conclusion, the results from our constant routine studies confirm Aschoff's early experiments showing a constant phase angle between the circadian rhythm of heat production and heat loss which in turn determines the circadian pattern of CBT. Thus, this phase relationship itself represents the circadian regulation of the endogenous rhythm in CBT.
> The circadian rhythms of CBT and alertness are not just separate outputs of the circadian pacemaker in the SCN. The relationship between alertness and CBT are more than correlative. Heat loss per se, and hence reduction in CBT, either induced by homeostatic or circadian thermoregulation or pharmacologically, leads to sleepiness. In humans, melatonin can be considered the hormone that prepares the body physiology for sleep.
Hence, any compounds that increase core body temperature can increase alertness and phase delay the circadian rhythm, whereas drugs that decrease core body temperature will induce sleep. Bright light therapy is however a counter example, as although its acute effect is only to increase core body temperature, due to photic history notably through melatonin, it can also increase sleep induction.
Compared to melatonin which requires saliva or urinary samples, body temperature can be monitoring 24/7 using wearables. Furthermore, melatonin can be decoupled from the circadian rhythm, hence it is not always a reliable predictor, whereas temperature has much stronger links.
Unfortunately, measurement of core body temperature is difficult. Since it is shielded by design from environmental factors, the most accurate methods are only invasive, requiring to insert a probe into an organ, such as rectal thermometry, oesophagal thermometry and oesophagal thermometry. The most accessible organ is the colon, with rectal electronic thermometers being an inexpensive, reliable, minimally invasive and easy to use device to acquire core body temperature. However, for the purpose of circadian rhythm monitoring, it's necessary to acquire core body temperature evolution all the time, including when sleeping. Usually, circadian rhythm studies achieve that by measuring CBT with a probe inserted 10 cm into the rectum or even 15cm, since the depth of insertion influences the measurements, which is uncomfortable and not achievable for the untrained patient at home, this can only be done under medical supervision. In any case, circadian rhythm monitoring for circadian rhythm disorders would ideally require a lifetime 24/7 monitoring, which is unrealistic with rectal probes, as wearing such as probe is fraught with bacterial and dermatological complications.
Although core body temperature is often used in the singular, it in fact encompasses several sites, as each organ has its own slightly different temperature. For this reason, some circadian rhythm researchers recommend to use the "brain core temperature", assuming this will reflect the temperature of the pineal gland and the suprachiasmatic nucleus, which are the main regulators of the circadian rhythm. However, there is nothing that suggest that the temperature of these structures reflect the thermoregulatory changes effected by these structures throughout the rest of the body. And even if this was the case, there is no reason to believe that there would be any difference in measuring the brain temperature versus other sites.
Over time, various technologies have been devised to acquire temperature, most often skin temperature, but also some devices to acquire core body temperature. Including the recent zero heat flux and dual heat flux methods, which allow to non-invasively acquire core body temperature at 2-5cm below the patch of skin where the device is positioned. However, these devices are still considered experimental. Unfortunately, such devices have only been produced in limited quantities. One of the first zero-heat-flux commercial thermometers is the 3M SpotOn, now rebranded and packaged inside the much more expensive BearHugger. Despite its clinical-grade accuracy being scientifically demonstrated, it suffered from several issues such as non portability (a wired receiver station was required) and single use electrodes consumables with patented technology (which made the device much more expensive to use and not durable) prevented its use for consumers, as it was anyway targeted at clinical staff and hospital settings. These issues, especially the need to be wired, stemmed from the technology used, as the zero-heat-flux method is particularly energy consuming and hence cannot run on battery.
GreenTEG, a company specialized in conceiving components such as heat flux sensors technology for building works and scientific applications, released their CORE line of products in 2021, which is a portable thermometer using a variant of dual-heat-flux-method, which allowed to significantly reduce energy consumption and hence make a portable, battery run sensor. This product was initially manufactured as one of many by several manufacturers in the wave of COVID-19 temperature screeners, which were sensors with the goal of en-masse screening for COVID-19 infected at public venues by detecting if their temperatures was high enough to qualify as fever. Unfortunately, the CORE (with an offshoot called CALERAresearch) was found in an independent validation study to not reflect core body temperature accurately under the standards (indeed, there are international standards to qualify a sensor as being an accurate thermometer for core body temperature, since this is very sensitive and can change medical decisions between life and death especially for children and critical care patients). This is thus very different from other zero heat flux (but unwearable) thermometers such as 3M SpotOn which are validated and can be medically used. In the current document's author's experience, the device can be partially useful, as it sometimes can show the drop in core body temperature when transitioning from the circadian day to the circadian night, but it does so unreliably, and other measures (such as the transition between the circadian night and day, the circadian siesta, etc) are highly unreliable to do. This may be because the sensor always filter all the data through a closed-source AI algorithm, and since the company up to now refused to open the access to the heat flux channels (the real raw data), there is no way to know if this issue can be improved.
An alternative circadian rhythm monitoring sensor could potentially be made by measuring the wrist skin temperature. Indeed, since the pioneering work of Czeisler in 1978 on wrist skin temperature, later research has shown that distal skin temperature especially at the hands reflects the bodily thermoregulation and correlates strongly with sleepiness and vigilance and also melatonin secretion (see also here). One notable researcher, Antonio Martinez Nicolas, conducted the most extensive suite of studies on the applicability of such device for circadian rhythm monitoring, by conceiving a simple DIY device combining a cotton wrist band with an iButton thermistor using a velcro as an attachment system (see also here), and the new measure "Wrist skin temperature increase onset" (WTiO) (see also here), which were then validated quantitatively as reliable metrics of the circadian rhythm, more precisely being the inverse of the circadian component of the core body temperature (as wrist skin temperature captures when the core body changes energy flux to dissipate energy from the limbs, so there is an increase in distal temperature when the core body temperature drops, and inversely). But skin temperature is more subject to environmental noise, eg, washing hands, going outdoors, etc, whereas core body temperature is not influenced. Furthermore, it appears there is a maximum threshold, with distal skin temperature reaching its maximum much faster than core body temperature reaches its minimum, so that a few dozens of minutes are required for the first whereas several hours for the latter, combined with the fact it starts rising about 90min before core body temperature starts dropping, this means that distal skin temperature may be less robust as a single metric to monitor continuously the circadian rhythm. Finally, and likely the main hurdle, is that Czeisler in 1978 already found that wrist skin temperature, and likely other distal skin sites temperatures, can dissociate from the CBT rhythm when subjects freerun, which is the case for individuals with non-24, and would hence preclude the use of wrist skin temperature for circadian rhythm monitoring in individuals with the non-24 disorder. However, since distal skin temperature reflects much more closely the variations of melatonin levels, and the proportion in distal temperature changes reflect the effect of exogenous melatonin — with a more optimal timing before DLMO associated with greater distal temperature changes —, distal skin temperature can likely be a reliable monitor of endogenous melatonin secretion and effectiveness of exogenous melatonin administration, a potentially invaluable tool to design optimal individualized melatonin therapeutic plans (including optimized timing and dosage).
Finally ECG, including heart rate and heart rate variability, is a potential other tool to monitor the circadian rhythm, as the cardiac system is deeply coupled with the thermoregulatory system: when the body dissipates energy via vasodilatation, the heart rate also slows down (see also here and here).
Although temperature wearables are arguably one of the least developed wearable technology compared to ECG, PPG and actigraphy, there is already a significant body of research and technologies such as deep temperature sensors, including zero-heat-flux and dual-heat-flux, are highly promising, although seldom studied and even more rarily available in industrial products, they are mostly DYI by lab researchers. Nevertheless, the COVID-19 sanitary crisis led to a welcome surge of interest in temperature monitoring wearables, and hence we are seeing new products emerging in the market, such as they very promising heat flux sensors that promise to capture core body temperature directly, as well as old timers that are re-explored such as the Maxim Thermocron iButton. UPDATE as of April 2021: FitBit now implemented continuous temperature sensing during nights with a minute-by-minute sampling resolution in their FitBit Sense product line (see also here). This is significative and a confirmation of the predicted trend since FitBit is a major actor in wearables. These products are however not sufficiently accurate to be of any value for circadian rhythm monitoring, and especially if they do not monitor at all time but only during nights (because otherwise we cannot know when the circadian night starts and stops if we do not monitor the whole circadian 24h period!).
For more information, please consult the Wearadian project which aims to repurpose or produce a set of sensors to monitor the circadian rhythm and sleep pattern continuously, all the time and under all circumstances (out of lab as well as in lab).
How to use core body temperature to estimate the current circadian night's phase (timing)
The circadian night can be robustly estimated by monitoring the minimal point in the core body temperature graph, this should represent the midpoint of the circadian night. Note that both body posture and the circadian rhythm affect the core body temperature, so that the real minimal point can only be reached when lying down and under the circadian night.
Sometimes, the circadian night may not appear clearly, as there may be multiple phases during the same day with a low temperature, but the temperature should be markedly lower when under the circadian night.
For example, for the current document's author, core body temperature may decrease down to just above 36.5°C when sleeping under the siesta or lying down without sleeping, but can only reach below 36.5°C down to about 36.2°C when sleeping during the circadian night. This 36.5°C threshold will different between different individuals, but it should always be the same for one individual (ie, it never changed for the document's author).
Hence, in practice, when using a core body temperature sensor for the first time, first it is necessary to acquire some data over several nights to estimate the threshold that clearly indicate a circadian night. One way is to pay attention to the lowest core body temperature readings you can get each day. This will help define a threshold, under which this indicate a circadian night for sure, or is indecisive when above. Indeed, the temperature can remain above the threshold even when sleeping during the circadian night. So the idea is that when the low values below the thresholds are attained, this is a robust indication the circadian night is happening at this time, but if above, it does not indicate anything.
Note that various factors can also affect the core body temperature (but not that many), for example fever due to illness. But a lot of factors that may affect skin temperature do not affect core body temperature, especially external factors such as ambient temperature or stress, as core body temperature is designed to be robust to external influence since its stability is necessary for humans survival (see endotherm).
Beyond direct instantaneous estimation of the circadian night without lenghty trial-and-error as with a sleep diary, core body temperature sensor may also correlate with energy levels and mood.
Research for circadian rhythm assessment by core body temperature monitoring
Core body temperature is likely the most promising approach for continuous circadian rhythm monitoring, but as of 2024 there is currently no reliable and validated such tool available to consumers. This section explores the current knowledge on the links between core body temperature and circadian rhythm monitoring, as well as the various technological implementations and limits for various technologies of thermometers.
This section will describe both the physiological links between temperature and the circadian rhythm, and then the methods to both acquire and analyze temperature data to estimate the current state of the circadian rhythm, and finally how to use this information to adapt the therapies.
TODO: the rest of the section is WIP
Core body temperature monitoring:
- Only the rectal temperature is both non-invasive and medical-grade (reflecting core body temperature accurately), although deep sensing wearables such as zero-heat-flux and dual-heat-flux may become good alternatives in the future: https://www.gssiweb.org/en/sports-science-exchange/Article/monitoring-internal-body-temperature#articleTopic_3
- Another review showing infrared and oral temperatures are not reliable: https://doi.org/10.1097/01.NURSE.0000390678.95642.7f
- Digital thermometer in the axillary is a good relative predictor of core body temperature: https://doi.org/10.1016/j.ijnurstu.2010.11.003
- «In an individual with DSWPD (B), DLMO occurs later than in a normal sleeper (A) and is potentially influenced by hypersensitivity to evening light, increased evening light exposure, and decreased morning light exposure. A delay in the phase of the circadian pacemaker and sleep-wake timing has been reported in patients with DSWPD, manifesting as a longer interval between CBTmin and sleep offset time, compared with normal sleepers (A). [...] In healthy sleepers, the temperature minimum during sleep occurs ~2 hours before sleep offset, whereas in DSWPD the temperature minimum has been reported to occur ~4 hours before sleep offset.» https://doi.org/10.1016/j.jsmc.2016.05.004
- Evaluation of Commercial, Wireless Dermal Thermometers for Surrogate Measurements of Core Temperature, 2019 (comparing iButtons with others) https://pubmed.ncbi.nlm.nih.gov/30882250/
- "The analysis indicates that the distribution of sleep onsets during free run is bimodal, with one peak at the temperature trough and, contrary to previous reports, a second peak 9-10 h later." https://www.ncbi.nlm.nih.gov/pubmed/3605382/
CBTmin is often assessed in circadian rhythm disorders study to quantify the circadian period tau. https://aasm.org/resources/clinicalguidelines/crswd-intrinsic.pdf
Links between core body temperature and circadian rhythm:
- Review to understand the technologies available for temperature monitoring, but don't trust what is said about specific devices, most are dead or not medically validated: Current Developments in Wearable Thermometers, 2018 https://www.jstage.jst.go.jp/article/abe/7/0/7_7_88/_pdf
> The core temperature is regulated by the thermoregulatory system with its center being the hypothalamus. By means of vasomotor, sweat and evaporation, this system is capable of maintaining the core temperature within a narrow range. Fluctuation of the core temperature obeys the circadian rhythm and shows the lowest value in the early morning and a peak in the afternoon or early evening with a difference about 1°C.
- Evaluation of wireless determination of skin temperature using iButtons, 2006
In other words, it seems most of the core body temperature variations are due to the circadian rhythm.
- DLMO is on average 7/8h (sleep duration) before Tmin: https://www.ncbi.nlm.nih.gov/pubmed/14667152
Dataset of 362 clinical-grade wearable devices for continuous monitoring, done in 2019 and published in 2020: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6931114/ + https://pubmed.ncbi.nlm.nih.gov/31778885/ + https://dataverse.no/dataset.xhtml?persistentId=doi:10.18710/QXMY88
> Another interesting rhythm that is controlled by the biological clock is the cycle of body temperature, which is lowest in the biological night and rises in the biological daytime. This fluctuation persists even in the absence of sleep. Activity during the day and sleep during the night reinforce this cycle of changes in body temperature, as seen in Figure 9.
https://science.education.nih.gov/supplements/nih_sleep_curr-supp.pdf
> The circadian sleep propensity rhythms had two apparent peaks (afternoon and nocturnal peaks) and a trough (nocturnal sleep gate). The timings of the nocturnal sleep gate and the nocturnal peak were correlated exclusively with temperature and melatonin rhythms (P < 0.05), while that of the afternoon peak was significantly correlated with habitual wake time and melatonin rhythm.
https://pubmed.ncbi.nlm.nih.gov/10459703/
> Although most models of sleep regulation include a circadian component, the actual mechanism by which the circadian timing system promotes--in addition to homeostatic pressure--transitions between sleep and wakefulness remains to be elucidated. [...] A review of the literature shows that increased brain temperature is associated with a type of neuronal activation typical of sleep in some structures (hypothalamus, basal forebrain), but typical of wakefulness in others (midbrain reticular formation, thalamus). Not only local temperature, but also skin temperature are related to the activation type in these structures. Warming of the skin is associated with an activation type typical of sleep in the midbrain reticular formation, hypothalamus, and cerebral cortex (CC). The decreasing part of the circadian rhythm in core temperature is mainly determined by heat loss from the skin of the extremities, which is associated with strongly increased skin temperature. As such, alterations in core and skin temperature over the day could modulate the neuronal activation state or "preparedness for sleep" in arousal-related brain structures. Body temperature may thus provide a third signaling pathway, in addition to synaptic and neurohumoral pathways, for the circadian modulation of sleep. [...] Finally, the model indicates that appropriately timed direct (passive heating) or indirect (bright light, melatonin, physical activity) manipulation of the nocturnal profile of skin and core temperature may be beneficial to disturbed sleep in the elderly.
https://pubmed.ncbi.nlm.nih.gov/10841209/
> The circadian rhythm of core body temperature (CBT) is a well-documented physiological phenomenon. Already in 1842, Gierse [6] had shown that his own oral temperature revealed a maximum temperature in the early evening and a minimum in the early morning hours with a maximum-minimum range of 0.9 °C. It had been assumed for a long time that muscular activity (exercise) and digestive processes were the most important factors for generation of the CBT rhythm [8]. Aschoff and his colleagues systematically explored the causes of this rhythm [1, 2]. He showed that the circadian rhythm of CBT is determined both by changes in heat production and changes in heat loss, and concluded that heat production undergoes a circadian rhythm which is phase advanced by 1.2h with respect to the circadian rhythm of heat loss, i. e. when heat production surpasses heat loss, CBT increases – transport of heat needs time. Therefore, when we want to explain changes in CBT we need to know the relationship between heat production and heat loss.
Ref: How is the circadian rhythm of core body temperature regulated? Kurt Krauchi 2002 editorial http://www.chronobiology.ch/wp-content/uploads/publications/2002_02.pdf
> In humans, melatonin contributes to the body temperature rhythm since it is responsible for vasodilatation of the skin of the extremities through its activation of thermosensitive neurons present in brain areas involved in sleep regulation. The melatonin secretion schedule is closely related with the propensity to sleep and coincides with a fall in the central body temperature, arousal level and performance. Indeed, since 1992 we know that the circadian rhythms of melatonin and body temperature are inversely coupled. The hypothermic properties of melatonin are accountable for the generation of at least 40% of the amplitude of the circadian body temperature rhythm. Manipulation of melatonin levels might be clinically useful to resynchronize the body temperature rhythm under conditions of body temperature rhythm desynchronization.
Ref: https://www.ncbi.nlm.nih.gov/pubmed/25719796 — it was shown that exogenous melatonin but only with higher doses > 1mg can produce hypothermia as also shown here for >3mg.
CRITICAL: Circadian clock changes are propagated throughout all cells of the body via changes in core body temperature, which is modulated by bright light exposure through the SCN (or just the ipRGC cells as shown below by another study):
From the press release:
> The SCN responds to light entering the eye, and so is sensitive to cycles of day and night. While light may be the trigger, the UT Southwestern researchers determined that the SCN transforms that information into neural signals that set the body's temperature. These cyclic fluctuations in temperature then set the timing of cells, and ultimately tissues and organs, to be active or inactive, the study showed.
> Scientists have long known that body temperature fluctuates in warm-blooded animals throughout the day on a 24-hour, or circadian, rhythm, but the new study shows that temperature actually controls body cycles, said Dr. Joseph Takahashi, chairman of neuroscience at UT Southwestern and senior author of the study.
From the study's abstract:
> Environmental temperature cycles are a universal entraining cue for all circadian systems at the organismal level with the exception of homeothermic vertebrates. We report here that resistance to temperature entrainment is a property of the suprachiasmatic nucleus (SCN) network and is not a cell-autonomous property of mammalian clocks. This differential sensitivity to temperature allows the SCN to drive circadian rhythms in body temperature, which can then act as a universal cue for the entrainment of cell-autonomous oscillators throughout the body. Pharmacological experiments show that network interactions in the SCN are required for temperature resistance and that the heat shock pathway is integral to temperature resetting and temperature compensation in mammalian cells. These results suggest that the evolutionarily ancient temperature resetting response can be used in homeothermic animals to enhance internal circadian synchronization.
Ref: https://www.sciencedaily.com/releases/2010/10/101014144314.htm and http://dx.doi.org/10.1126/science.1195262
→ This means that temperature (including peripheral temperature) monitoring is likely the best marker of the circadian rhythm, even more so than melatonin (melatonin being a precursor to change the temperature, the latter being the final signal for cells to change their clocks).
CRITICAL: Another similar result, but showing that the SCN is not even necessary for body temperature modulation, the ipRGC cells are sufficient:
> Here we show that body temperature and sleep responses to acute light exposure are absent after genetic ablation of all ipRGCs except a subpopulation that projects to the SCN. Furthermore, by chemogenetic activation of the ipRGCs that avoid the SCN, we show that these cells are sufficient for acute changes in body temperature. Our results challenge the idea that the SCN is a major relay for the acute effects of light on non-image forming behaviors and identify the sensory cells that initiate light’s profound effects on body temperature and sleep." https://doi.org/10.7554/eLife.44358
CRITICAL: Furthermore, the circadian clock and the cell cycle are coupled, which means that the circadian clock is a core regulator of all cells cycles throughout the body. By accounting for the other discoveries, this means that body temperature controls the cells cycles through the circadian clock modulation. This is another strong supporting evidence for the hypothesis that circadian rhythm disorders are bodily disorders, not just brain disorders.
Ref: Droin C, Paquet ER, Naef F. Low-dimensional Dynamics of Two Coupled Biological Oscillators. Nat Phys. 2019 Oct;15(10):1086-1094. doi: 10.1038/s41567-019-0598-1. Epub 2019 Aug 5. PMID: 32528550; PMCID: PMC7289635. https://pubmed.ncbi.nlm.nih.gov/32528550/
> Patients with DSWPD and N24SWD had significantly longer melatonin and temperature taus compared to controls. Circadian non-delayed DSWPD had normally timed temperature and melatonin rhythms but were typically sleeping at relatively late circadian phases compared to those with circadian-delayed DSWPD.
Ref: Circadian tau differences and rhythm associations in Delayed Sleep-Wake Phase Disorder and sighted Non-24-Hour Sleep-Wake Rhythm Disorder, 2013 https://doi.org/10.1093/sleep/zsaa132
CRITICAL lead: minimal core body temperature can be used to optimally time bright light therapy, but not for melatonin (for which only DLMO matters):
> As the CBTmin serves as the “inflection point” between delaying and advancing effects for light, the DLMO serves as the approximate inflection point for advancing and delaying effects of melatonin.
> [...]
> No improvements in actigraphically determined sleep parameters were observed, and our analysis demonstrated no group difference with respect to the timing of DLMO27 (Appendix, Table S2). As the present review did not analyze outcomes relative to the timing of melatonin administration, however, it is important to note that the authors reported an inverse relationship between the timing of melatonin administration (irrespective of dose) and the magnitude of DLMO phase advance, such that earlier timing of the former (in relation to DLMO) resulted in greater phase advances. No such correlation was identified with respect to CBTMin (assessed only within the active treatment groups).
Ref: AASM 2015 guidelines on circadian rhythm disorders: https://aasm.org/resources/clinicalguidelines/crswd-intrinsic.pdf
Also same thing in British guidelines: https://doi.org/10.1177/0269881119855343 — see also: https://www.guidelines.co.uk/sleep-disorders/consensus-statement-on-evidence-based-treatment-of-insomnia-parasomnias-and-circadian-rhythm-disorders-an-update/455239.article
> Uchiyama et al. had earlier determined that sighted non-24 patients' minimum core body temperature occurs much earlier in the sleep episode than the normal two hours before awakening. They suggest that the long interval between the temperature trough and awakening makes illumination upon awakening virtually ineffective,[22] as per the phase response curve (PRC) for light.
Ref: https://en.wikipedia.org/wiki/Non-24-hour_sleep%E2%80%93wake_disorder from https://pubmed.ncbi.nlm.nih.gov/11058797
Posture, sleeping and light exposure in the evening can all mask the circadian rhythm from core body temperature profile:
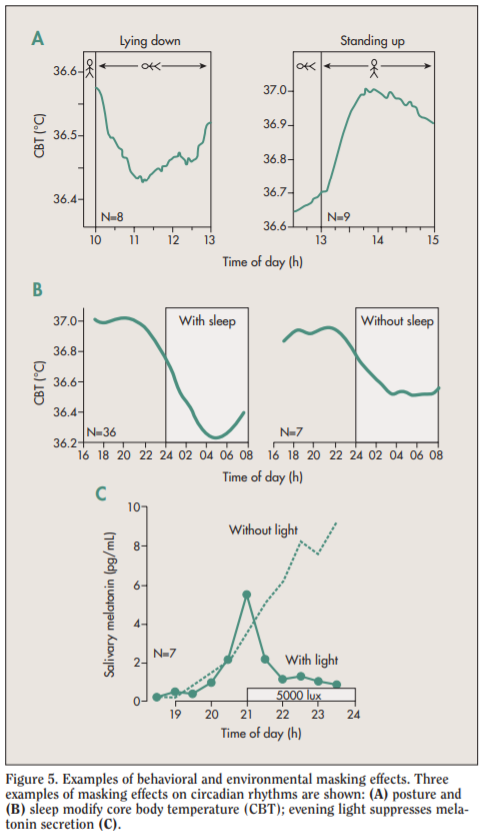
Ref: Figure 5 of https://web.archive.org/web/20181221161338/http://www.chronobiology.ch/wp-content/uploads/publications/2007_02.pdf
Causal link between core body temperature and circadian rhythm:
> The 24-h rhythm in core body temperature is the result of differential 24-h rhythms in heat production and heat loss. It has been demonstrated that under strictly controlled so-called ‘constant routine’ conditions, in which subjects remain awake in a fixed semi-supine condition without physical activity and with food and drinks taken in small portions throughout the day and night, the core temperature rhythm remains. Furthermore, the core temperature rhythm has been shown to result mostly from the circadian rhythm in heat loss, and to a lesser extent from changes in heat production [12]. Dry heat loss is caused by increased skin blood flow, allowing the dissipation of heat from the warm blood to the cooler environment. Although the iButton is not suitable for core body temperature assessment in humans, the assessment of temperature at multiple sites of the skin provides a reliable estimate of heat loss [12,13], suggesting a role for the iButton in long-term studies on the circadian variation in skin temperature and heat-loss which is responsible for an important part of the circadian variation in core body temperature.
> Body temperature can be divided into core and skin surface temperature (Fig. 1). Mammals, including humans, are homoeothermic; i.e., they require an almost constant internal body temperature. Core temperature is defined as the temperature of the hypothalamus, which is the regulatory center of the body.
> The core temperature is regulated by the thermoregulatory system with its center being the hypothalamus. By means of vasomotor, sweat and evaporation, this system is capable of maintaining the core temperature within a narrow range. Fluctuation of the core temperature obeys the circadian rhythm and shows the lowest value in the early morning and a peak in the afternoon or early evening with a difference about 1°C.
> Researchers estimate core temperature by taking measurements in the auditory canal, esophagus, and stomach, but rectal temperature is a more accurate method of estimating hypothalamic temperature.
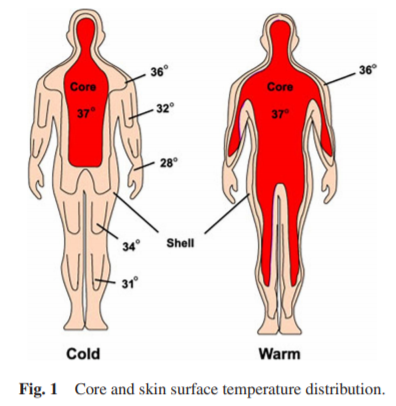
Heat distribution depending on room temperature, from a human in a cool (20°C, left) and warm (35°C, right) ambient temperature. Modified from Aschoff, 1971.
Ref: https://doi.org/10.14326/abe.7.88
> In the cardiovascular system, melatonin seems to regulate the tone of cerebral arteries; melatonin receptors in vascular beds appear to participate in the regulation of body temperature. Heat loss may be the principal mechanism in the initiation of sleepiness caused by melatonin. The role of melatonin in the development of migraine headaches is at present uncertain but more research could result in new ways of treatment. Melatonin is the major messenger of light-dependent periodicity, implicated in the seasonal reproduction of animals and pubertal development in humans.
Ref: https://pubmed.ncbi.nlm.nih.gov/9730580/
Proximal versus distal and distal-to-proximal skin temperatures:
Ref: https://doi.org/10.1016/j.physbeh.2006.04.026
Distal wrist temperature circadian profile is delayed according to the chronotype (Figure 2) + High variability range between individuals (Figure 3 — meaning the difference between tempmin and tempmax throughout 24h is 1-3 degrees smaller for some as compared to others who have a more stable temperature profile — hence per subject feature scaling of temperature profile is necessary):
Ref: https://doi.org/10.4108/eai.20-5-2019.2282879
Brain temperature vs rectal temperature (with both thermocouples and dual-heat-flux method): Temperature Monitoring With Zero Heat Flux Technology In Comparison With Thermocouple Needle Probe During Selective Hypothermia, Mohammad Fazel Bakhsheshi et al, 2018. https://doi.org/10.1115/DMD2018-6930
> In addition to the sleep-promoting effects, melatonin completely suppressed the normal diurnal rise of core body temperature.
Ref: Sleep-Promoting and Hypothermic Effects of Daytime Melatonin Administration in Humans, 1997 https://doi.org/10.1093/sleep/20.2.124
Sleep deprivation increases body temperature, hence more difficulty to sleep! And melatonin can help, particularly supraphysiological doses.
> "Minimum rectal temperature, calculated from smoothed temperature data from 2300 to 0515 h, was greater in bright-light sleep deprivation, resulting in suppression of melatonin, than in conditions of sleep deprivation in dim light or sleep in the dark. An exogenous melatonin infusion in bright light returned the minimum temperature to that seen in dim-light sleep deprivation. A nonsignificant elevation in mean and minimum temperature was noted in all conditions of sleep deprivation relative to sleep. We conclude that melatonin secretion contributes to the lowering of core body temperature seen in the early morning in humans."
Ref: https://pubmed.ncbi.nlm.nih.gov/1778910/
CRITICAL: all studies by Antonio Martinez Nicolas at University of Murcia on wrist skin temperature (WT), especially:
- The following 2 studies for how to attach the iButtons (1 sample per 10min) using Velcro on cotton sports wristband to measure wrist skin temperature reliably and comfortable: https://pubmed.ncbi.nlm.nih.gov/18761026/ and https://www.ncbi.nlm.nih.gov/pubmed/25813804
- BEST CRITICAL: WTiO (Wrist skin temperature increase onset) anticipates melatonin secretion. Ref: Circadian phase assessment by ambulatory monitoring in humans: correlation with dim light melatonin onset, 2014 https://pubmed.ncbi.nlm.nih.gov/24164100/
- BEST VALIDATION: WT = wrist skin temperature: "Although the overall circadian pattern of WT was similar regardless of the masking effects, its amplitude was the rhythmic parameter most affected by environmental conditions. The acrophase and mesor were determined to be the most robust parameters for characterizing this rhythm. In addition, a circadian modulation of the masking effect was found for each masking variable. WT rhythm exhibits a strong endogenous component, despite the existence of multiple external influences. This was evidenced by simultaneously eliminating the influence of activity, body position, light exposure, environmental temperature and sleep. We therefore propose that it could be considered a valuable and minimally-invasive means of recording circadian physiology in ambulatory conditions." Uncovering Different Masking Factors on Wrist Skin Temperature Rhythm in Free-Living Subjects, 2013 https://www.ncbi.nlm.nih.gov/pubmed/23577201
- "Our results show that the WT rhythm exhibits an inverse phase relationship with OT, and it is phase-advanced by 60 min with respect to OT. WT started to increase in association to bed time and dropped sharply after awakening. A secondary WT increase, independent of feeding, was observed in the early afternoon. In conclusion, WT wireless recording can be considered a reliable procedure to evaluate circadian rhythmicity, and an index to establish and follow the effects of chronotherapy in normal living subjects." Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system, 2008 https://pubmed.ncbi.nlm.nih.gov/18761026/
- BEST CRITICAL: PhD Thesis: Crosstalk between Synchronizers and the Human Circadian System, D. Antonio Martinez Nicolas, 2014, PhD Thesis http://hdl.handle.net/10201/40027
- Summary: modern circadian rhythm science introduction + whole PhD thesis aim was validation of wrist skin temperature as a reliable method for circadian rhythm monitoring, and methods to analyze!
- CRITICAL: core body temperature versus proximal and distal (wrist) skin temperatures. This shows why proximal temperature such as on the trunk cannot reliably be used to estimate the circadian rhythm. But core body temperature and distal (wrist) skin temperature both can.
- "The cardiovascular system is the major effector of thermal changes in thermoneutrality. In thermoregulatory terms, blood means heat and cutaneous circulation is the variable heat insulator underneath the skin, which determines, depending on the skin proximity, the heat transference velocity. The cutaneous circulation is regulated by vessels patency that is controlled by the autonomic nervous system. Ambient temperature changes are translated into blood redistribution, if weather becomes cool, blood will be stored in the “core” (trunk) to diminish heat loss by the “shell” (extremities), whereas in warmer conditions blood is redistributed toward the periphery to dissipate heat from the core (Figure 8), which is the same that occurs in wake and sleep conditions (Krauchi, 2007). This blood redistribution is controlled by sympathetic nervous system, which dilates and constricts peripheral vessels in general, and arteriovenous anastomoses more specifically (which are abundant in glabrous skin and are widely innervated by sympathetic nerves)." → heart rate variability HRV (high frequency or low frequency can't remember) may be able to detect the biological night too by detecting when the heat redistribution is active???
- "In addition, cardiovascular system shows, as it has long been known, circadian modulation in blood pressure and heart rate (Blazquez et al., 2012; Kräuchi et al., 2012; Veerman et al., 1995). Recently, circadian rhythms have also been discovered in vascular tone and cardiac output (Veerman et al., 1995). All these rhythms have a similar pattern with high values during daytime and low values during nighttime. Nowadays, it is suggested that all these rhythms are a consequence of a circadian pattern in the sympathetic tone instead of the sleep-wake or rest-activity cycle dependence (Furlan et al., 1990; Yamasaki et al., 1996). The sympathetic activity pattern is reflected in the heart rate variability rhythm with an inverse pattern, that it is, variability is higher during rest phase (lower sympathetic activity) and lower during activity phase (higher sympathetic activity) (for review see Guo & Stein, 2003)."
Before-bedtime passive body heating by warm shower or bath to improve sleep: A systematic review and meta-analysis, 2019 https://doi.org/10.1016/j.smrv.2019.04.008
Effects of thermal environment on sleep and circadian rhythm, 2012 https://www.ncbi.nlm.nih.gov/pubmed/22738673
> Body temperature depends on the balance between heat production and loss. Heat production usually varies circadianly, since a major source of variation is muscular rest and activity; but even in subjects at strict rest and fasting this circadian variation persists, with the lowest values in the small hours of the morning (25), in fair correspondence with the familiar rhythm of body temperature. This rhythm in metabolic rate is, however, unlikely to contribute significantly to the temperature rhythm, since the amplitude of the temperature rhythm is commonly as large in people confined to bed, and even in the paralyzed, as in those with much larger metabolic swings due to diurnal activity. The rhythm in metabolism is therefore more likely to be a consequence than a cause of the temperature rhythm, especially as the quoted amplitude of the metabolic rhythm is that which the van’t Hoff-Arrhenius equation would predict from a circadian temperature oscillation of around 1°C. The cause of the rhythm of body temperature probably lies in variations in the heat-loss mechanisms, such as cutaneous blood flow; in fact, the diurnal rhythm of finger or skin temperature is nearly a mirror image of that of rectal temperature (94, I I I). However, the amplitude of the internal temperature rhythm is very similar in a temperate or in a humid tropical climate (I) ; it seems, then, that the rhythm is in the temperature-regulating mechanism, rather than in any single contribution to it, such as metabolic rate or cutaneous blood flow (9). The ease of measuring body temperature must have contributed to the enormous accumulation of data, both by assiduous workers in this field such as Kleitman, and by people who in the course of easterly or westerly journeys have taken the opportunity to make personal observations.
Source: Mills 1966 https://doi.org/10.1152/physrev.1966.46.1.128
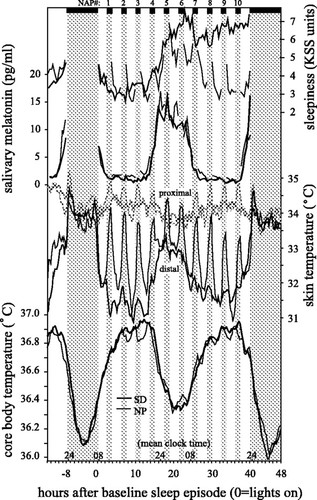
> The main finding of the study was that accumulation of sleep pressure increased subjective sleepiness and slow-wave activity during the succeeding recovery night but did not influence the thermoregulatory system as measured by distal, proximal, and CBT. The circadian rhythm of sleepiness (and proximal temperature) was significantly correlated and phase locked with CBT, whereas distal temperature and melatonin secretion were phase advanced (by 113 ± 28 and 130 ± 30 min, respectively; both P < 0.005). This provides evidence for a primary role of distal vasodilatation in the circadian regulation of CBT and its relationship with sleepiness. Specific thermoregulatory changes occur at lights off and on. After lights off, skin temperatures increased and were most pronounced for distal; after lights on, the converse occurred. The decay in distal temperature (vasoconstriction) was significantly correlated with the disappearance of sleep inertia. These effects showed minor and nonsignificant circadian modulation. In summary, the thermoregulatory system seems to be independent of the sleep homeostat, but the circadian modulation of sleepiness and sleep inertia is clearly associated with thermoregulatory changes.
> [...]
> A general relationship between thermoregulation and sleep regulation has been long hypothesized, e.g., sleep as an energy conservatory state (23, 49). In humans, sleep is typically initiated on the declining portion of the CBT curve when its rate of change is maximal (11, 21, 38, 50, 51). We have shown that body heat loss via distal skin regions (measured by the distal-proximal skin temperature gradient; DPG) is the variable most closely linked to subjective sleepiness and predicts sleep onset latency (30, 31). More recently, we have also shown that distal vasoconstriction after a nap (between 4:00 and 8:00 PM) or after night sleep (between 11:00 PM and 7:00 AM) is correlated with disappearance of sleep inertia after lights on (34).
> [...]
> A multiple regression analysis for repeated measures revealed that the intraindividual time course of subjective sleepiness was significantly correlated with distal skin temperature [standardized coefficient (std b) = +0.293, P < 0.0001] and CBT (std b = −0.132, P < 0.005) but not with proximal skin temperature (std b = +0.055, NS) and melatonin (std b = −0.041, NS).
> [...]
> Residuals of distal and proximal skin temperatures and CBT followed a significant circadian pattern, and CBT and proximal skin temperature had inverse phase relationships to distal skin temperature (see below), all being similar for both protocols. Although sleepiness was significantly detrended (as indicated by nonsignificant statistical ANOVA terms for time and protocol × time), the protocols remained different [protocol: F(1,7) = 36.6, P < 0.0005] and nap × protocol [F(9,63) = 15.7, P < 0.0001]. In NP, sleepiness clearly follows a circadian pattern with maximal values at naps 5 and 6. In SD, the time course of sleepiness shows a circadian pattern, which is overlapped with an increasing trend on the second day (for detrending of this effect, see below).
> [...]
> In NP and SD, no phase differences between KSS and CBT curves were found, indicating inverse phase-locked patterns. Similarly, no phase differences were found between proximal skin temperature and CBT. In contrast, distal skin temperature and melatonin compared with CBT showed similar significant phase advances by 113 and 130 min, respectively. Similarly, distal skin temperature and melatonin were also phase advanced with respect to KSS.
>
> Compared with the time segment at the same circadian phase without sleep (night 2), an 8-h sleep episode increases distal and proximal skin temperatures and conversely reduces CBT.
>
> This study supports our previous hypothesis that the circadian modulation of sleepiness is primarily related to the circadian regulation of distal vasodilatation (and hence to heat loss and CBT reduction), whereas the homeostatic-regulated increase of sleepiness is nearly independent thereof (32). The findings also suggest that this close relationship between distal vasodilatation and sleepiness also holds for the process of sleep inertia, thus confirming and extending our published data (30). This means that both the evening increase in sleepiness, which leads to maximum sleep propensity in the middle of the night, and the exponential decline of sleepiness upon awakening can be described as a function of changes in distal vasodilatation. In contrast, the homeostatic increase in sleepiness related to duration of prior time awake is not related to a thermoregulatory function. This homeostatic buildup process of sleepiness has been related to topographic EEG correlates in the frontal cortex (10). From these different physiological correlates, two “kinds” of sleepiness can be postulated (thermoregulatory related and unrelated).
>
> The finding that the circadian pattern of all measured body temperatures did not differ between the two protocols indicates that they were independent of the homeostatic buildup of sleepiness (sleep pressure). This allows the conclusion that all the measured circadian patterns are not influenced by a masking process via the sleep homeostat, i.e., counteractions taken by a subject to keep awake. The buildup process of sleep pressure over 40 h has in fact no, or only minor, thermoregulatory consequences. This finding confirms a previous CR study in which our group (36) found nonsignificant changes in body temperatures (proximal, distal, and CBT) at the same circadian phase 24 h after a sleep-deprivation episode. This does not preclude the possibility that, with longer than 40-h SD protocols, the thermoregulatory system may no longer be independent of sleep pressure.
>
> the falling limb of the CBT rhythm in the evening is steeper than the rising limb in the morning. Similarly, the rising limb of the distal skin temperature and DPG (data not shown) rhythm in the evening is also steeper (Fig. 1) than the falling limb in the morning. This indicates an asymmetrical regulation of heat loss and heat production in the evening and morning. Heat loss seems to be dominant in the evening, and heat production seems to dominant in the morning (36). We could also define the temporal relationship between the circadian rhythm of sleepiness and the thermoregulatory system. Sleepiness was significantly phase delayed by −96 min and −114 min with respect to distal skin temperature and salivary melatonin and phase locked to CBT with a negative correlation coefficient. Because the circadian rhythm of distal skin temperature precedes both sleepiness and CBT, this could be the reason why distal skin temperature is a better predictor for sleepiness (and hence for sleep-onset latency) than CBT (30, 31). Interestingly, sleepiness shows not only a close phase relationship to distal skin temperature and CBT but also a similar asymmetrical circadian pattern, with a faster rise in the evening than decline in the morning.
>
> As found in earlier studies (5, 29, 30, 34, 37), thermoregulatory changes induced by lights off do influence CBT but slowly. In contrast, distal and proximal skin temperatures increase immediately after lights off and before the onset of sleep stage 2 (5, 29, 30, 34, 37). This indicates that redistribution of body heat from the core to the shell occurs shortly after lights off via relaxation.
>
> The well-known circadian modulation of sleep onset latency (SOL to sleep stage 1 or 2; Ref. 16) yielded the shortest values near the CBT trough in naps 5 and 6 and longest values in naps 4 and 10 (42) just before distal skin temperature increased and CBT decreased. Similarly, an analysis of the time awake during a nap revealed maximal time awake values in naps 4 and 10 and minimal values during naps 5 and 6 (42). However, independent of the differences in SOL and time awake in each nap, the skin temperatures increased at a similar rate (see Fig. 1), indicating that the subjects relaxed after lights off, although without necessarily falling asleep. An important factor for the relaxation-induced effects could be eye closure occurring before sleep onset, which deserves to be investigated separately.
>
> The extent of the increase in distal skin temperature exhibited a trend toward circadian modulation; the nonsignificance can be explained by a ceiling effect. When distal skin temperature reaches its circadian maximum, it is difficult to increase distal skin temperature above proximal skin temperature. The large and rapid increase in skin temperatures after lights off (0.7°C in proximal and 1.6°C in distal skin temperature) only led to small decreases in CBT (∼0.027°C/75 min), with a time lag such that the lowest value actually occurred 75 min after lights on (Fig. 2). Therefore, it is not possible to extrapolate the reduction of CBT from a short sleep episode (75 min) to that during an 8-h dark (sleep) episode (see below). Furthermore, had subjective ratings of sleepiness after lights off until occurrence of sleep been measured, one could speculate that it would increase in parallel with relaxation, withdrawal of the sympathetic nervous system, redistribution of heat from the core the shell, and increase of distal skin temperature. Thus these changes after lights off can be interpreted as an inverse process of sleep inertia (see below). What we see are thermoregulatory effects mainly induced by relaxation, most probably via a decrease in sympathetic tone, and not by the occurrence of sleep per se (33). In real life situations, it can be assumed that many effects on sleepiness and sleep, e.g., induced by exercise, hot or warm bathing, intake of food, hot or cold fluids, may occur via redistribution of heat from the core to the shell or vice versa.
>
> Redistribution of blood from the shell to the core occurs via vasoconstriction, mainly in distal skin regions. However, this takes awhile, in the hands faster than in the feet (data not shown). It is most rapid in proximal skin regions.
>
> The extremities cooled at a rate very closely parallel to the decay of sleepiness. The symmetry between the thermoregulatory processes related to the increase in sleepiness and those related to its dissipation is striking. However, to directly test these relationships, further studies with thermophysiological interventions (e.g., cooling the extremities) are required.
>
> If we compare the same circadian phase awake and asleep, the maximum reduction of 0.31°C occurs after 5 h of sleep. In a similar CR study, a reduction of 0.46°C was found (5). The discrepancy can be explained by the higher light intensity level during the wake situation (40 vs. <8 lux in our study), leading to a suppression of melatonin secretion and consequently to higher CBT values.
>
> This leads to the conclusion that onset of sleep stage 2, and herewith onset of increased SWA, does not have additional thermoregulatory effects (see also Ref. 35). We have also shown that after lights off, when distal vasodilatation increases, heart rate falls in parallel, indicating a decrease in cardiac output, which could explain why the increase in distal vasodilatation does not induce an efficient heat loss with a consequent decrease in CBT (35). Furthermore, after the acute hyperemic response to lights off, proximal and distal temperatures remained at a comparable high value, indicating a loss of the core-shell dichotomy (4) of the body during sleep. This one-compartment state makes the body more vulnerable to heat loss or heat uptake, which could be a reason that a thermoneutral environment is preferred for sleep as a protection against external cooling and warming.
>
> We have disproved the long-held belief that sleep, or more precisely NREM sleep, causes CBT to decline. Because lying down and relaxation after lights off evoke an increase in skin temperatures and a decline in CBT, these major masking effects have confounded prior studies and rendered their conclusions doubtful. This is not to deny the importance of such masking in real-life conditions. Thus it appears that the circadian pacemaker drives the circadian propensity for sleep via a circadian rhythm in heat loss (vasodilatation). A similar but inverse mechanism is responsible for the sleep inertia upon awakening. These thermoregulatory mechanisms underlying circadian sleepiness and sleep inertia are not related to changes in “homeostatic sleepiness” resulting from being awake over longer periods.
>
> The corollary of these findings has important consequences in sleep medicine. Independent of circadian phase, sleepiness is augmented by conditions of warming and diminished by cooling of distal skin regions (leading to increased and reduced convective body heat loss, respectively). This knowledge supports the simple old-fashioned methods to speed up falling asleep (e.g., warm bath) or to rapidly dissipate sleep inertia (e.g., cold shower). In contrast, sleepiness related to extended episodes of prior wakefulness cannot be thermally manipulated and require a different strategy, such as a short nap, to diminish sleep pressure (a “power nap” that does not induce subsequent sleep inertia).
Summary: this lab-controlled study did not find sleep pressure (nor naps) alter any kind of body temperature, whether core body temperature or skin temperature or limbs (distal) temperature. However, light exposure directly modulated the core body temperature. And subjective sleepiness is definitely correlated with distal skin and core body temperature, hence to the circadian rhythm, on top of sleep pressure. The circadian rhythm is what affects more the subjective sleepiness than the sleep homeostat. Both sleep, naps and sleep deprivation have no effect on the circadian rhythm (ie, no difference during recovery night post sleep deprivation). CBT and distal skin temperature can monitor or predict sleepiness. Distal skin temperature is affected by sleep and rises up much faster than CBT. This study strongly supports the hypothesis that circadian rhythm disorders are in reality thermoregulatory disorders.
Source: Challenging the sleep homeostat does not influence the thermoregulatory system in men: evidence from a nap vs. sleep-deprivation study, 2006 https://doi.org/10.1152/ajpregu.00381.2005
- GREAT REVIEW about how heat loss and heat production works and how the circadian rhythm and core body temperature work: The cold sensations after prolonged sleep deprivation cannot be due to a decrease of CBT since it is not influenced by the sleep homeostat and hence not by sleep deprivation, but there is some evidence suggesting that sleep deprivation can delay sweating and hence may "shift the sensitivity of the thermoregulatory system to store heat more efficiently". + this review explains how the capillaries work for the thermoregulatory system in the context of the circadian rhythm (see arteriovenous anastomoses (AVAs) in addition to capillaries as they are larger and allow for 10K times more blood flow). AVAs seem to be independent of local temperature. Distal skin regions, the major sites for vasomotor heat loss, include ear lobes, fingertips, toes, eyelids and lips, and are rich in not only capillaries but also AVAs, contrary to the "core" of the body which include the head, thorax and abdomen.
- Cold hands syndrome = sleep-onset insomnia? https://www.researchgate.net/publication/6075422_The_thermophysiological_cascade_leading_to_sleep_initiation_in_relation_to_phase_of_entrainment
- > This article reviews circadian thermoregulation in relation to sleep induction and phase of entrainment in the light of the comprehensive thermophysiological and chronobiological concepts of Jürgen Aschoff. The idea that temperature and sleep are interrelated is based on evolutionary history. Mammalian sleep developed in association with endothermy, and all species, independent of temporal niche, usually sleep during the circadian trough of their core body temperature (CBT) rhythm. The circadian pattern of CBT results from the balance between heat production and heat loss, the latter being relevant for sleep induction. Sleep under entrained conditions is typically initiated on the declining portion of the CBT curve when its rate of change and body heat loss is maximal. Body heat loss before lights off, via selective vasodilatation of distal skin regions, promotes sleepiness and the rapid onset of sleep. This thermophysiological effect represents the cement between the circadian clock and the sleep-wake cycle, and in turn determines phase of entrainment (Psi) and sleep onset latency (SOL). These interrelationships have been recently studied in a particular subset of the general population, mainly women, who suffer from cold hands and feet (the so-called vasospastic syndrome, VS). Women with VS exhibit not only a lower capacity to lose heat during the daytime but also a prolonged SOL, a disturbed Psi of the circadian clock with respect to the sleep-wake cycle and psychologically, a disposition to turn experienced anger inwards. This naturalistic model leads us to a more general conclusion that regulation of distal skin blood flow may have clinical relevance for insomnia, in particular sleep onset insomnia.
- Although it was hypothesized that warming/cooling extremities could force a modulation of the core body temperature, and hence of the circadian rhythm, as an alternative to bright light therapy and melatonin, a 2019 study found that periocular warming could increase limbs skin temperatures, but without affecting proximal nor core body temperature, which strongly suggests that moduling the temperature of extremities is not a viable procedure to modulate the circadian rhythm. https://doi.org/10.1038/s41598-019-42116-x
- But here is another study finding an opposite result: Manipulation of Core Body and Skin Temperature Improves Vigilance and Maintenance of Wakefulness in Narcolepsy https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2225580/
- BEST: circadian rhythm link with reduced sleep onset (hence link between sleep onset insomnia and circadian rhythm disorders): Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol. 2000 Mar;278(3):R741-8. doi: 10.1152/ajpregu.2000.278.3.R741. PMID: 10712296. https://pubmed.ncbi.nlm.nih.gov/10712296/ (mirror)
- BEST GRAPH cbt and heart rate and distal-proximal temperature:
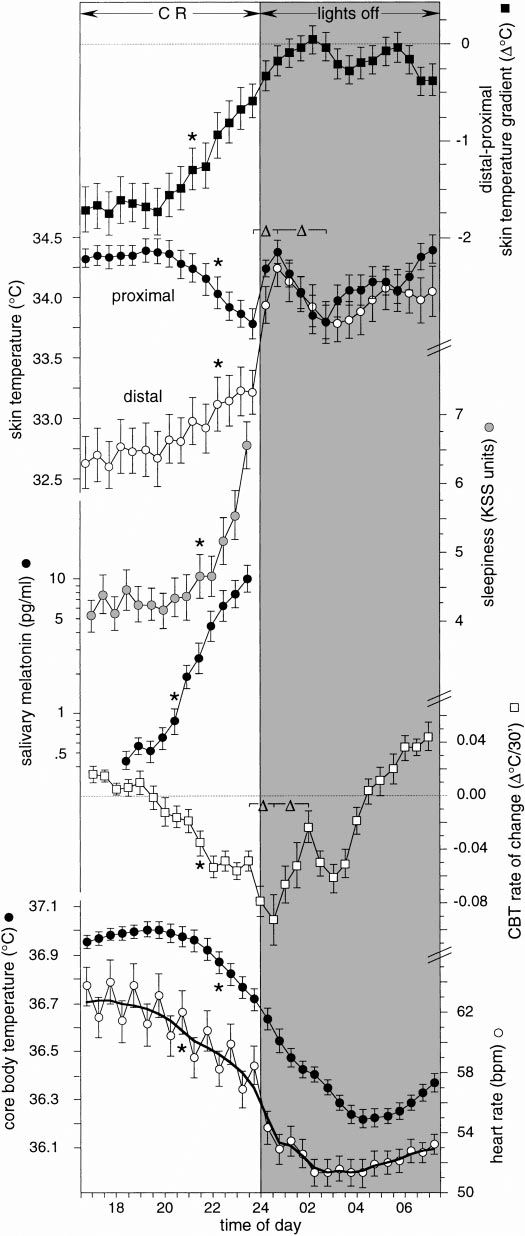
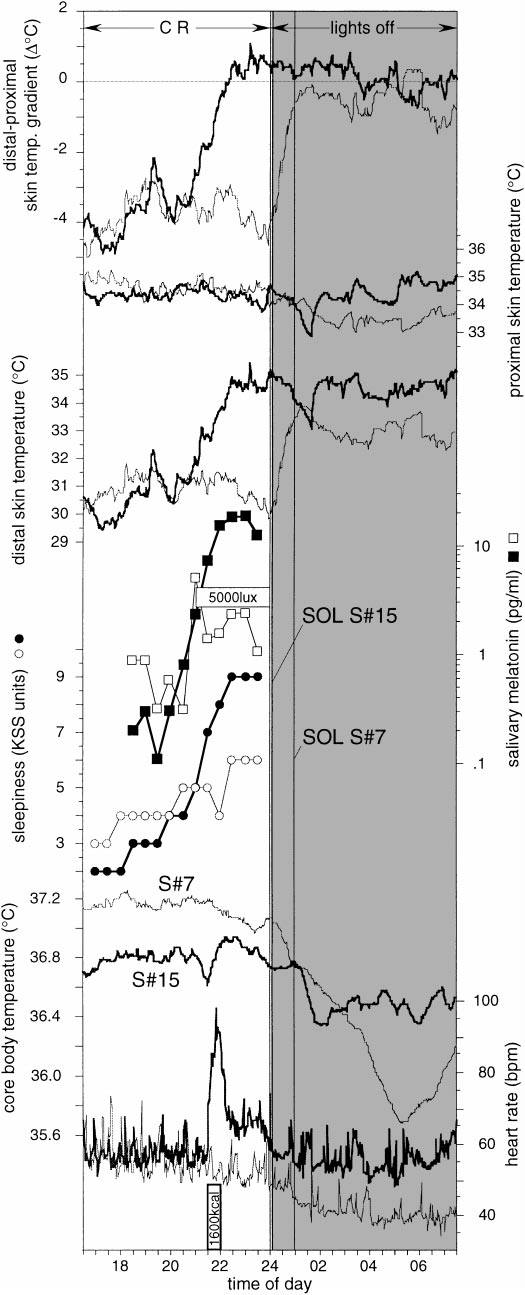
- BEST CRITICAL: sleep patterns have no effect whatsoever on the circadian rhythm :
- Challenging the sleep homeostat does not influence the thermoregulatory system in men: evidence from a nap vs. sleep-deprivation study, 2006 https://doi.org/10.1152/ajpregu.00381.2005
- Is sleep per se a zeitgeber in humans? 2003 https://pubmed.ncbi.nlm.nih.gov/12693871/
- One thing to keep in mind is that the core body temperature is lagging behind feelings, this is because by the time the core body temperature drops, the process to make this temperature drop already started 1-2h before. Temperature is decreased by the body by increasing the temperature of the extremities such as hands and feet, so that it dissipates faster, by increasing the blood flow to them. So if you measure the wrist skin temperature for example, you can see a rise in temperature that precedes core body temperature's decrease. I am testing wrist skin temperature too (I have data published online over 6 months if you'd like to try some analyses, I plan on collecting at least 1 year of data before starting to investigate!), but it's more variable, less reliable than core body temperature. (TODO: add ref about wrist skin temperature preceding core body temperature).
- Example of a flawed study: Life-style regularity and core body temperature phase in delayed sleep phase syndrome, 2016 https://doi.org/10.1046/j.1446-9235.2003.00059.x
- "The time of core body temperature minimum was negatively correlated with the SRM score (primary end-point) and the activity level index (ALI) score (secondary end-point). This means that lower regularity in social activities contributes to a later circadian phase." → the conclusion does not follow the premisses. The authors assume a causality between the observed metrics AND a directionality of causality without testing either of these hypothesis. They also disregard previous research that do not fit with their hypothesis over the previous decades. By accounting for previous research on social cues not being zeitgebers,these results can be more accurately reinterpreted as observing that the circadian rhythm regulates social activity (instead of the other way around), which is a bit obvious... And less sensational, it wouldn't have been published.
- Dual heat flux technology (using the Tcore device, which is not a wearable but a clinical monitor) was already used by several studies on astronauts, and was found to be accurate! (Although they placed the sensor on the forehead, measuring core brain temperature, not body temperature): Long-Term Bed Rest Delays the Circadian Phase of Core Body Temperature, 2021 https://pubmed.ncbi.nlm.nih.gov/34040542/
- However, this also another example of a flawed study, as they claim that position modifies the core body temperature, while acknowledging that bright light can be a confound that they did not properly control:
- "Given that HDBR studies employed standard indoor light levels and followed strict 24-h day/night (sleep/wake) cycles, we attribute the observed phase delays to bed rest rather than to the effects of altered light levels and/or non-photic cues."
- And the use of old references to support their hypothesis while disregarding newer, better controlled evidence is not a good practice... "External cues in the present experiments such as strict sleep-wake/rest-activity cycles, regular day-night cycles [participants were exposed to daylight illumination corresponding to standard indoor light levels (i.e., 100–500 lux)], and regular mealtimes are all well-known critical “zeitgeber” to synchronize and entrain the circadian rhythm to the 24-h day-night cycle (Mistlberger and Skene, 2005). Non-photic behavioral “zeitgeber” have the potential to preserve the temporal adaptation of circadian rhythms to the 24-h day irrespective of light levels (Klerman et al., 1998; Wright et al., 2001). For instance, in a study employing light levels as low as 1.5 lux, the entrainment to a 24-h day could be maintained by scheduled sleep/wake (rest/activity) cycles (Wright et al., 2001)."
- However, this also another example of a flawed study, as they claim that position modifies the core body temperature, while acknowledging that bright light can be a confound that they did not properly control:
- Use of melatonin sampling, core body temperature and distal-proximal temperature monitoring for DSPD in ADHD: https://pubmed.ncbi.nlm.nih.gov/23952346/
- Axillary and Thoracic Skin Temperatures Poorly Comparable to Core Body Temperature Circadian Rhythm: Results from 2 Adult Populations, 2004 https://doi.org/10.1177%2F1099800403260620
- "In both studies, temperatures were recorded continuously for 24 h while subjects carried out normal activities. Axillary and thoracic probes were insulated purposely to prevent ambient effects. [...] In addition, correlations between temperatures at various measurement sites were calculated and agreement determined. The circadian timing of axillary and skin temperatures did not closely approximate that of rectal temperature: the mean acrophase (clock time) for study 1 was 18:57 h for axillary temperature and 16:12 h for rectal; for study 2, it was 03:05 h for thoracic and 15:05 h for rectal. Across individual subjects, the correlations of axillary or thoracic temperatures with rectal temperatures were variable. Results do not support the use of either axillary or skin temperature as a substitute for rectal temperature in circadian rhythm research related to adult women."
- "the thermoregulatory system seems to be independent of the sleep homeostat, but the circadian modulation of sleepiness and sleep inertia is clearly associated with thermoregulatory changes." https://doi.org/10.1152/ajpregu.00381.2005
- BEST CRITICAL: "As previously noted (Ref. 36 and Fig. 1), the falling limb of the CBT rhythm in the evening is steeper than the rising limb in the morning. Similarly, the rising limb of the distal skin temperature and DPG (data not shown) rhythm in the evening is also steeper (Fig. 1) than the falling limb in the morning. This indicates an asymmetrical regulation of heat loss and heat production in the evening and morning. Heat loss seems to be dominant in the evening, and heat production seems to dominant in the morning (36)." → ie, sleep fatigue appears suddenly, whereas sleep inertia can take a long time to clear up (this study also correlates sleep inertia with the delay for core body temperature to increase). https://doi.org/10.1152/ajpregu.00381.2005
- BEST CRITICAL: This also means that core body temperature sensors can be used to monitor sleep inertia (very useful for assessing whether the individual can drive or do other potentially dangerous attentional tasks). https://doi.org/10.1152/ajpregu.00381.2005
- Concise description of the two major protocols for studying circadian rhythms: https://pubmed.ncbi.nlm.nih.gov/22877674/
- BEST CRITICAL: influences of the circadian rhythm on the heart rate: https://pubmed.ncbi.nlm.nih.gov/22877674/
- "A circadian rhythm in resting heart rate has been reported by different research groups in healthy humans, with a broad peak occurring during the middle of the biological day and a trough during the biological night (Figs. 2 and and3;3; Burgess et al., 1997; Kräuchi and Wirz-Justice, 1994; Scheer et al., 2010; Shea et al., 2011). On the other hand, the reactivity of heart rate to standardized exercise and postural changes is not influenced by the circadian timing system (Fig. 3; Hu et al., 2011; Scheer et al., 2010). Considering that the circadian peak in heart rate does not occur during the biological morning and that heart rate reactivity to behavioral stressors is not under circadian control, it is unlikely that the circadian timing system’s influence on heart rate contributes to the morning peak in adverse cardiovascular events. In rats, there is also a circadian rhythm in resting heart rate under constant dark conditions, thus even independent of the circadian rhythm in behavioral activity (Scheer et al., 2001). After lesioning the SCN, the circadian rhythm is abolished and the level of resting heart rate is intermediate between that during the biological day and night in intact animals. This suggests that the SCN has an inhibitory and excitatory influence on resting heart rate during the biological day and night, respectively. This is reminiscent of the regulation of melatonin and corticosteroids for which the SCN uses both inhibitory (e.g., GABA) and excitatory neurotransmitters (e.g., glutamate; Kalsbeek et al., 1996; Perreau-Lenz et al., 2003)."
- e-pills (gastrointestinal core body temperature measurements) methods: https://www.gssiweb.org/en/sports-science-exchange/Article/monitoring-internal-body-temperature
- "Importantly, in exchange for the convenience of wireless measurement, the use of an ingestible thermistor pill requires prior planning to ensure proper placement of the pill within the GI tract. The ingestion of the pill should be done at least 3 h prior to the exercise to minimize the chance of prematurely measuring gastric temperature and within 8 h to minimize the chance of pill passage (Casa et al., 2015; Ganio et al., 2009; Hosokawa et al., 2016). Furthermore, if not timed correctly, cold-fluid ingestion may also influence the temperature reading (Savoie et al., 2015), which can be further impacted by individual variations in gut motility. Therefore, close attention to the athlete’s ad libitum fluid ingestion behavior is warranted when interpreting the data."
- Also: Infrared skin temperature is -1°C compared to core temperature using an ingested e-pill! https://doi.org/10.3945/ajcn.2009.27567
- Skin includes an intrinsic clock feature for temperature compensation. https://tel.archives-ouvertes.fr/tel-01220534/document
- BEST CRITICAL: temperature affects all biological organisms, with a higher temperature making cycles faster/shorter, including the circadian rhythm, see works on neuronal resilience under different temperatures simulating climate change by the legendary Pr. Eve Marder: https://www.quantamagazine.org/eve-marder-on-the-crucial-resilience-of-neurons-20210517/
Genetics and circadian syndrome
TODO: Work-in-progress section, will be expanded and rewritten in the future. See the Food section for a more detailed account until then.
It is estimated that ~40% of sleep disorders are inherited, and a study in twins shown that heritability of the circadian rhythm is between 46% to 70%, suggesting that the circadian rhythm is mostly endogenously defined, with minor environmental influence.
The inheritability of specific circdian rhythm disorders are not currently estimated, but there are at least some portion that are of genetic origin. Indeed, the current document's has evidence of the non-24 disorder affecting his lineage over at least 2 generations of direct ancestors above. Interestingly, the ancestor two levels above was born in Vietnam, which isn't a country that lacks sunlight exposure, but it is unknown when the symptoms started, although they were clearly present when they moved to Europe. The two ancestors have both one male and one female, which suggests this is not a X nor Y chromosomal inheritance.
This study screened for lots of genetic mutations associated with circadian rhythm disorders, including DSPD and non-24, including MTNR1B.
This study also finds mutations in MTNR1B but in DSPD (although I suspect the carriers of the GG alleles were maybe undiagnosed non-24).
To summarize these findings, there is now a whole body of work demonstrating that melatonin type 2 receptors mutations MTNR1B in various alleles (MTNR1B rs10830963 and rs10830962 and rs1387153) are strongly associated or even predictive (causing?) both metabolic disorders including diabetes and obesity (see also this systematic review and this review), as well as circadian rhythm disorders including DSPD and non-24. See the Food section above for more detailed information about these alleles.
genetics, early bird vs night owl (DSPD): Jones SE, Tyrrell J, Wood AR, et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet. 2016 Aug 5;12(8). https://www.ncbi.nlm.nih.gov/pubmed/27494321 + Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms, Jones et al, 2019, Nature Communications https://www.ncbi.nlm.nih.gov/pubmed/30696823 + Circadian Polymorphisms in Night Owls, in Bipolars, and in Non-24-Hour Sleep Cycles https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4225198/
Book summarizing latest findings (2020) in the genetics of chronotypes such as DSPD: Neurological modulation of sleep, 2020. Does not mention MTNR1B however. See section Genetics of Sleep, also explain that genetic analyses and public biobanks have helped make tremendous progress! + GWAS of Chronotype (summarizes all big studies but says should be interpreted with caution) + page 62 interactions of both genetic and environment cause sleep disorders (first sentence of page 62) + link between sleep duration and depression on same page + no link between shift work and type 2 diabetes
translin, linking sleep disorders and metabolic syndromes in fruit flies: https://www.sciencedaily.com/releases/2016/03/160324133839.htm
The AG variant in FUT2 rs602662 location can predispose to vitamin B12 deficiency, with the latter causing peripheral neuropathy. This mutation seems hereditary since it was observed in the present document's author along with his father's DNA. B12 vitamin is known to amplify the magnitude of the circadian rhythm shift of light therapies (see also here) and B12 supplementation entrained a few individuals with non24 (see also here and here).
Clinical-grade (30x) whole-genome sequencing (WGS) of DNA is now available in the consumer market with Nebula Genomics and Dante Labs. Genome sequencing differs with exome sequencing (the coding part of the DNA, 0.02% of the whole DNA — which is now considered an incorrect assumption as more and more non-coding regions are increasingly revealed to in fact code important genetic features). Clinical-grade (30x) differs from non-clinical grade sequencing (0.4x) in that the DNA needs to be read multiple times to ensure the readout is correct, as there is a probability that each readout is incorrect due to noise, hence by reading 30 times (30x) it's much more likely to reach a stable and correct readout. Since 2022, Nebula Genomics also offer a research-grade 100x sequencing for a slightly increased price compared to 30x, although this is not necessary for most purposes unless one wants to potentially leverage future genomic technology that may benefit from a higher sequencing, although 30x is more than sufficient for all current research purposes. Nebula Genomics offer raw data download (which is unfortunately rare with WGS providers) and online viewers based on IGV. The author of the present document sequenced his DNA with Nebula Genomics services, the data is published under CC BY SA 4.0 on FigShare. Other providers of consumer-grade exome-sequencing such as 23andMe doesn't sequence the parts of the genome that allows to check some of the most interesting variants such as in the CRY1 gene. (See this for conversion of Dante Labs data to genealogical formats)
Mutation PER3 rs228697 allele G found in 67 patients with non-24. Furthermore, the mutations rs908078 (C - minor - allele), rs34883305, rs34870629, rs74439275, and rs3750275, rs11130215, rs1104976, rs2271566, and rs6790630 in BHLHE40 were identified in 4 people with non-24, in addition to rs2482705 GG and rs3828057 GG for DSPD. See also this layman summary, and this one from a non-24 author. Out of all these genes, the author of the present document found only a half-positive result of rs2482705 AG for both his DNA and his father's, although it's rs2482705 GG that was associated with DSPD.
Patke A, Murphy P, Onur E, et al. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017 Apr 6, Volume 169 , Issue 2 , 203 – 215.e13.
https://news.ucsc.edu/2020/10/night-owls.html
However, current genetical studies are limited by dogma, which led researchers to underestimate the extent of regulatory control of the circadian rhythm over all cells:
> To understand the study, Hurley explains, one needs to know that the “central dogma of biology” is that DNA (our genes), makes RNA (gene transcripts) and proteins (the “actors” of the cell).
> “As a field, we were making inferences about what the circadian clock did in the body by looking at the transcripts as a proxy for which proteins had a circadian rhythm,” Hurley says.
> “However, as a protein biochemist, I know that a lot happens when transcripts are made into proteins and that by only looking at the transcripts, we would miss a lot of what was going on in the cell.”
> Specifically, the study team examined how macrophages’ levels of RNA and proteins changed over two days — finding 80 percent of the circadian proteins did not have a transcript that also had a circadian rhythm. Subsequent analysis also revealed the interplay of the circadian rhythm and metabolism essentially times the macrophages’ immune functions — a process that results from the splitting and fusing of mitochondria.
This bias compounds on others, such as the lab work hours, and they all hinder our collective progress towards the discovery of more aspects of the circadian rhythm:
> Variety is the spice of life
> Although early birds are typically viewed as more productive, likely because their natural rhythm fits more closely with a 9-5 job, having a range of chronotypes in the workplace can be beneficial. In the laboratory, for example, the discovery of one protein was thrown into doubt when two opposing chronotypes performed the same experiment. When the ‘owl’ postdoc did the experiments in the evening, the protein was there, but when the ‘lark’ PhD student performed the same experiments in the morning, the protein was nowhere to be found. The answer? It was rhythmic! By chance, the difference in the lab’s working patterns led to a key discovery."
Another issue is that, despite the enormous literature, most genetic-wide association studies (GWAS) results before 2013 failed to reproduce due to statistical shortcomings, such as underpowered too small sample size and too many genetic tests. However, in recent years, "the resulting cultural shift has rapidly transformed our understanding of the genetic architecture of complex traits and, in a few years, has produced many hundreds more reproducible findings than in the previous 15 years75. Routine sharing of single-nucleotide polymorphism (SNP)-level statistical results has facilitated routine use of meta-analysis, as well as the development of novel methods of secondary analysis76." Nevertheless, imaging genomics, or brain genomics, tend to still be flawed with small sample size and too many researchers degrees of freedom even up to 2017, which makes genetic results associated with neuroimaging results to be less robust and reproducible than their counterparts without neuroimaging, due to being constrained by the difficulty and hence lower sample size of neuroimaged subjects. In summary, it seems that genetic studies pre 2010 were mostly underpowered, but now most are fine since they are done on big cohorts. However, genetic studies associated with neuroimaging results (eg, such as the genetic basis of psychological disorders) are less robust and less reproducible due to the smaller sample size, which is due to the difficulty in neuroimaging subjects (you can't put 100K people in a MRI, whereas you can sample the DNA much more cheaply).
A genetic study shown that the circadian rhythm rely on a network of multiple clocks, providing redundancy and hence resilience in face of circadian disruptions. We can hypothesize that disruptions of this network may underlie some of the circadian rhythm disorders.
TODO: the author of the present document strongly suspects that a dysregulation in histaminergic H1 receptors may be a cause of circadian rhythm disorders such as non-24. If true, then demographic studies should demonstrate that people with circadian rhythm disorders have a high chance of comorbid allergies. It would be interesting to study if there is any common genetic mutation in the H1 receptors in people with circadian rhythm disorders.
Insomnia and genetics: https://pubmed.ncbi.nlm.nih.gov/30804565/
Circadian and Homeostatic Regulation of Human Sleep and Cognitive Performance and Its Modulation by PERIOD3, 2009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7644166/
Lack of association between PER3 variable number tandem repeat and circadian rhythm sleep–wake disorders https://doi.org/10.1038/s41439-018-0017-7
JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets, 2010 https://pubmed.ncbi.nlm.nih.gov/20876817/
"In addition, a great number of genes have been identified that are thought to have a significant role in the circadian gene network such as the orphan nuclear receptors REV-ERB (α and β) and ROR (α, β, and γ) [27, 28].The CLOCK:BMAL1 dimers initiate the transcription of the second feedback loop. The Bmal1 promoter contains Retinoic acid-related Orphan receptor Response Element (RORE) that can be bound by REV-ERB and ROR proteins. REV-ERB proteins repress transcription by competitively binding to BMAL1 promoter whereas RORs activate Bmal1 transcription [26–28]." https://pubmed.ncbi.nlm.nih.gov/33520862/
- "Molecular studies also revealed phase synchronization and desynchronization in short and long days, respectively (Hazlerigg et al., 2005; Inagaki et al., 2007; Naito et al., 2008; Sosniyenko et al., 2009). These changes in synchrony underlie the photoperiodic modulation of circadian waveform as expressed at the tissue level, in different clock genes (Nuesslein-Hildesheim et al., 2000; Sumova et al., 2003; Johnston et al., 2005). Importantly, the molecular studies have shown that in long photoperiods, the neurons of the rostral and caudal SCN desynchronize. For instance, period 1 bioluminescence reporters revealed that period 1 expression in the rostral SCN shows a bimodal activity pattern in long days, with one component that follows dawn and the other component that follows dusk (Inagaki et al., 2007). In the caudal SCN on the other hand, period 1 expression is locked to dusk, under all photoperiods (Inagaki et al., 2007). Hazlerigg and coworkers (2005) observed an advance of the peak in period 2, rev-erb a and dbp in the caudal SCN in long days, relative to the rostral SCN. Naito et al. (2008) showed multiple peaks in period 1 bioluminescence in the rostral SCN in long days. In short days, the expression profiles of all genes were in synchrony in all studies. Importantly, Naito et al. (2008) showed that the single-cell expression profiles were not different between long and short days, consistent with the electrophysiological studies, and that the changes in waveform are thus a circuit property that is based on phase changes among neurons. A particular subtype of neurons in the retinorecipient area of the hamster SCN could mediate the reorganization of the SCN neuronal network (Yan & Silver, 2008). This group of cells is activated when days get longer and inactivated when days get shorter. Activation of this population may alter the strength of the intercellular connections in the SCN network, and thereby affect the synchrony among neuronal oscillations (Yan & Silver, 2008)." https://pubmed.ncbi.nlm.nih.gov/21143668/
> At the molecular level, the basis of the mammalian circadian clock is self-sustained circadian oscillations of core clock genes arranged in an autoregulatory transcription-translation feedback loop, with transcription factors Clock and Bmal1 driving the circadian expression of Period (Per) and Cryptochrome (Cry) genes that mediate negative feedback within the clockworks (Takahashi, 2017). Light stimulation results in acute induction of Per1/2 (Takahashi, 2017). Conventional time-point clock gene expression profiling from animals under different light cycles has provided a snapshot of how the clock gene rhythms in the SCN are influenced by various lighting conditions (Messager et al., 2000; Schwartz et al., 2011), but with limited temporal resolution that does not fully capture dynamic changes in the clock gene rhythms induced by different light cycles.
>
> The advent of clock gene reporters (e.g. Per1::GFP, PER2::LUC) (Kuhlman et al., 2000; Yoo et al., 2004) enabled assaying the motion of the circadian clock in real time and with low variability. However, to date the fundamental question of how clock genes in the SCN encode different lighting conditions has been primarily approached by manipulating light exposure in vivo and subsequently explanting the SCN into slice culture in constant darkness to retrospectively infer the in vivo entrained state, due to technical challenges in mimicking retinal light input ex vivo. While much progress has been made with this paradigm, this approach can have significant limitations. The SCN activity observed in a free-running condition reflects relaxation of the explanted SCN network back toward baseline, rather than active encoding of entrainment (Rohr et al., 2019), and explantation can disrupt expression of the in vivo state (Pendergast et al., 2009).
>
> Here, combining long-term organotypic explant culture, cyclic red light optogenetic stimulation, and the PER2 bioluminescent reporter, we assess how the clock gene rhythms in the ex vivo SCN change in real time to achieve entrainment to light cycles.
https://doi.org/10.7554/eLife.70137
The Circada Study is a new genetics investigation by Jackie Lane et al, which is not yet including non24 but may be in the future. Here is a link (as of September 2022) to join the waiting list for participation.
Additional informations
This document is a short version of the key practical points that can be useful for the treatment (entrainment) of treatment-resistant non24. A longer document is available at: https://lrq3000.github.io/non24article/SleepNon24.html
The author is also active on reddit: https://www.reddit.com/user/lrq3000 (see here for hidden/deleted posts by moderators or other users).
Primary bibliography of major reviews
Here is a selection of major reviews to get introduced to the key topics necessary to understand circadian rhythm science. All these references are already linked in context in the rest of this document, but if you want to know more about circadian rhythm research, here is where to start with these dense but concise reviews. If you do not have an academic account that allows you to access these documents, try to search for an alternative open link with scholar.google.com or on biorxiv for a preprint publication or through sci-hub.tw (you may have to use DNScrypt to access the website).
Circadian rhythm disorders clinical guidelines
USA guidelines
- Highly recommended summary: Auger, R. R., Burgess, H. J., Emens, J. S., Deriy, L. V., Thomas, S. M., & Sharkey, K. M. (2015). Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 11(10), 1199-1236. https://doi.org/10.5664/jcsm.5100
- Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015 Oct 15;11(10):1199-236. doi: 10.5664/jcsm.5100. PMID: 26414986; PMCID: PMC4582061. https://pubmed.ncbi.nlm.nih.gov/26414986/
- Executive summary of the AASM CRSWD 2015 guidelines: http://sleepeducation.org/docs/default-document-library/crswd-draft-executive-summary.pdf?sfvrsn=2
- Highly recommended general overview of sleep health and sleep disorders and the essential importance of sleep for health: Ramar K, Malhotra RK, Carden KA, Martin JL, Abbasi-Feinberg F, Aurora RN, Kapur VK, Olson EJ, Rosen CL, Rowley JA, Shelgikar AV, Trotti LM. Sleep is essential to health: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2021 Jun 21. doi: 10.5664/jcsm.9476. Epub ahead of print. PMID: 34170250. https://pubmed.ncbi.nlm.nih.gov/34170250/
- All AASM guidelines and reviews for all sleep disorders: https://aasm.org/clinical-resources/practice-standards/practice-guidelines/
- Some newest guidelines are not yet listed on the AASM website, such as:
- Maski, K., Trotti, L. M., Kotagal, S., Auger, R. R., Rowley, J. A., Hashmi, S. D., & Watson, N. F. (2021). Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, jcsm-9328. https://doi.org/10.5664/jcsm.9328 (summary here)
- Some newest guidelines are not yet listed on the AASM website, such as:
UK guidelines
- Wilson S, Anderson K, Baldwin D, Dijk DJ, Espie A, Espie C, Gringras P, Krystal A, Nutt D, Selsick H, Sharpley A. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. 2019 Aug;33(8):923-947. doi: 10.1177/0269881119855343. Epub 2019 Jul 4. PMID: 31271339. https://pubmed.ncbi.nlm.nih.gov/31271339/
French guidelines
- Elisabeth Ruppert, Ulker Kilic-Huck et le Groupe consensus chronobiologie et sommeil de la Société française de recherche et médecine du sommeil (SFRMS). Republication de : Diagnostic et comorbidités des troubles du rythme veille-sommeil. 2016. doi: 10.1016/j.msom.2018.12.002. https://www.sfrms-sommeil.org/wp-content/uploads/2020/08/Republication-de-_-Diagnostic-et-comorbidite%CC%81s-des-troubles-du-rythme-veille-sommeil-_-Elsevier-Enhanced-Reader.pdf
- Damien Leger, François Duforez, Claude Gronfier, Le traitement par la lumière des troubles circadiens du rythme veille-sommeil, La Presse Médicale, Volume 47, Issues 11–12, Part 1, 2018, Pages 1003-1009, ISSN 0755-4982, https://doi.org/10.1016/j.lpm.2018.10.013.
European guidelines
- Reference educational textbook for sleep clinician trainings in Europe: Bassetti, Claudio, et al., éditeurs. Sleep Medicine Textbook. Second edition, European Sleep Research Society, 2021. ISBN: 9781119789017.
- The textbook is structured according to this updated consensus documentation: Penzel, T, Pevernagie, D, Bassetti, C, et al. Sleep medicine catalogue of knowledge and skills – Revision. J Sleep Res. 2021; 30:e13394. https://doi.org/10.1111/jsr.13394
- This is the recommended textbook material for nationwide courses across Europe such as DIU Sommeil Et Sa Pathologie in France.
International guidelines
- Palagini L, Manni R, Aguglia E, Amore M, Brugnoli R, Bioulac S, Bourgin P, Micoulaud Franchi JA, Girardi P, Grassi L, Lopez R, Mencacci C, Plazzi G, Maruani J, Minervino A, Philip P, Royant Parola S, Poirot I, Nobili L, Biggio G, Schroder CM, Geoffroy PA. International Expert Opinions and Recommendations on the Use of Melatonin in the Treatment of Insomnia and Circadian Sleep Disturbances in Adult Neuropsychiatric Disorders. Front Psychiatry. 2021 Jun 10;12:688890. doi: 10.3389/fpsyt.2021.688890. PMID: 34177671; PMCID: PMC8222620. https://doi.org/10.3389/fpsyt.2021.688890
- https://circadianhealthclinics.com/people?jr=on
Sighted non-24 disorder
- Uchiyama M, Lockley SW. Non-24-Hour Sleep-Wake Rhythm Disorder in Sighted and Blind Patients. Sleep Med Clin. 2015 Dec;10(4):495-516. doi: 10.1016/j.jsmc.2015.07.006. PMID: 26568125. https://pubmed.ncbi.nlm.nih.gov/26568125/
- Hayakawa T, Uchiyama M, Kamei Y, Shibui K, Tagaya H, Asada T, Okawa M, Urata J, Takahashi K. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep. 2005 Aug 1;28(8):945-52. doi: 10.1093/sleep/28.8.945. PMID: 16218077. https://pubmed.ncbi.nlm.nih.gov/16218077/
- Malkani RG, Abbott SM, Reid KJ, Zee PC. Diagnostic and Treatment Challenges of Sighted Non-24-Hour Sleep-Wake Disorder. J Clin Sleep Med. 2018 Apr 15;14(4):603-613. doi: 10.5664/jcsm.7054. PMID: 29609703; PMCID: PMC5886438. https://pubmed.ncbi.nlm.nih.gov/29609703/
- Okawa M, Uchiyama M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndrome. Sleep Med Rev. 2007 Dec;11(6):485-96. doi: 10.1016/j.smrv.2007.08.001. Epub 2007 Oct 25. PMID: 17964201. https://pubmed.ncbi.nlm.nih.gov/17964201/
- Abbott SM. Non-24-hour Sleep-Wake Rhythm Disorder. Neurol Clin. 2019 Aug;37(3):545-552. doi: 10.1016/j.ncl.2019.03.002. Epub 2019 Apr 30. PMID: 31256788. https://pubmed.ncbi.nlm.nih.gov/31256788/
Layman overviews of non-24
- Great layman overview of non-24 while still being accurate, by James Fadden, Vice President of the Circadian Sleep Disorders Network and who has sighted non24: http://www.sleepreviewmag.com/2015/05/need-know-non-24/
- Great layman overview of non-24 by an independent researcher afflicted with sighted non-24: https://wordthingsofjon.blogspot.com/2015/11/non-24-hour-sleep-wake-disorder.html
- Quite extensive and accurate, although with mistakes but few ones, overview of non-24 by the NORD (american National Organization for Rare Disorders): https://rarediseases.org/rare-diseases/non-24-hour-sleep-wake-disorder/
- A concise and helpful introduction to non-24 as a form of Q&A by the Circadian Sleep Disorder Network: https://www.circadiansleepdisorders.org/docs/N24-QandA.php
- and good overview of available treatments: https://www.circadiansleepdisorders.org/treatments.php
- More on the personal side than the technical or biological side, the book Sleep Misfits: The reality of Delayed Sleep Phase Syndrome & Non-24 by Sally Cat is highly recommended, being the only book currently written compiling the experience specific to patients living with non-24 and DSPD handicaps. Reviews note that this book can help in validating one's own experience, and help with acceptance and coping with the handicap.
Circadian rhythm disorders
- Magee M, Marbas EM, Wright KP Jr, Rajaratnam SM, Broussard JL. Diagnosis, Cause, and Treatment Approaches for Delayed Sleep-Wake Phase Disorder. Sleep Med Clin. 2016;11(3):389-401. doi:10.1016/j.jsmc.2016.05.004 https://pubmed.ncbi.nlm.nih.gov/27542884/
- Burgess HJ, Emens JS. Circadian-Based Therapies for Circadian Rhythm Sleep-Wake Disorders. Curr Sleep Med Rep. 2016;2(3):158-165. doi:10.1007/s40675-016-0052-1 https://pubmed.ncbi.nlm.nih.gov/27990327/
- Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med Rev. 2019 Aug;46:108-123. doi: 10.1016/j.smrv.2019.04.012. Epub 2019 Apr 30. PMID: 31108433. https://pubmed.ncbi.nlm.nih.gov/31108433/
- Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13(1):47-60. doi:10.1016/j.smrv.2008.04.009 https://pubmed.ncbi.nlm.nih.gov/18845459/
Zeitgebers and circadian rhythm science
- Roenneberg, T., & Merrow, M. (n.d.). Type 1 and Type 0 Resetting. Encyclopedia of Neuroscience, 4146–4149. doi:10.1007/978-3-540-29678-2_6170 . URL: https://doi.org/10.1007/978-3-540-29678-2_6170
- Crosstalk between Synchronizers and the Human Circadian System, D. Antonio Martinez Nicolas, 2014, PhD Thesis http://hdl.handle.net/10201/40027
- Did not watch these videos but it seems like a nice course accessible to the layman to better understand the scientific findings in circadian rhythm science: Coursera - Circadian clocks: how rhythms structure life.
- Skene DJ. Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol. 2003 Apr;15(4):438-41. doi: 10.1046/j.1365-2826.2003.01006.x. PMID: 12622847. https://pubmed.ncbi.nlm.nih.gov/12622847/
- Meijer JH, Michel S, Vanderleest HT, Rohling JH. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur J Neurosci. 2010 Dec;32(12):2143-51. doi: 10.1111/j.1460-9568.2010.07522.x. PMID: 21143668. https://pubmed.ncbi.nlm.nih.gov/21143668/
Bright light therapy and human eye physiology
- Auger, R. R., Burgess, H. J., Emens, J. S., Deriy, L. V., Thomas, S. M., & Sharkey, K. M. (2015). Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 11(10), 1199-1236. https://doi.org/10.5664/jcsm.5100
- Blume, C., Garbazza, C. & Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 23, 147–156 (2019). https://doi.org/10.1007/s11818-019-00215-x
- Tähkämö L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. 2019;36(2):151-170. doi:10.1080/07420528.2018.1527773 . URL: https://pubmed.ncbi.nlm.nih.gov/30311830/
- Brouwer A, Nguyen HT, Snoek FJ, et al. Light therapy: is it safe for the eyes?. Acta Psychiatr Scand. 2017;136(6):534-548. doi:10.1111/acps.12785 . URL: https://pubmed.ncbi.nlm.nih.gov/28891192/
- Roberts JE. Ocular phototoxicity. J Photochem Photobiol B. 2001;64(2-3):136-143. doi:10.1016/s1011-1344(01)00196-8 . URL: https://pubmed.ncbi.nlm.nih.gov/11744400/
- Martinsons C. (2017) Photobiological Safety. In: Karlicek R., Sun CC., Zissis G., Ma R. (eds) Handbook of Advanced Lighting Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-00176-0_51
- Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med Rev. 2019 Aug;46:108-123. doi: 10.1016/j.smrv.2019.04.012. Epub 2019 Apr 30. PMID: 31108433. https://pubmed.ncbi.nlm.nih.gov/31108433/
- Figueiro, M. G., Nagare, R., & Price, L. L. A. (2018). Non-visual effects of light: How to use light to promote circadian entrainment and elicit alertness. Lighting Research & Technology, 50(1), 38-62. https://doi.org/10.1177/1477153517721598
- Rea Mark S., Nagare Rohan, Figueiro Mariana G. (2021). Modeling Circadian Phototransduction: Quantitative Predictions of Psychophysical Data. Frontiers in Neuroscience, 15, 44. https://doi.org/10.3389/fnins.2021.615322
Melatonin and physiology
- Auger, R. R., Burgess, H. J., Emens, J. S., Deriy, L. V., Thomas, S. M., & Sharkey, K. M. (2015). Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 11(10), 1199-1236. https://doi.org/10.5664/jcsm.5100
- Arendt J. Melatonin: Countering Chaotic Time Cues. Front Endocrinol (Lausanne). 2019;10:391. Published 2019 Jul 16. doi:10.3389/fendo.2019.00391 . URL: https://doi.org/10.3389/fendo.2019.00391
- Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8 Suppl 3:34-42. doi:10.1016/j.sleep.2007.10.007 . URL: https://doi.org/10.1016/j.sleep.2007.10.007
- Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47(10):2336-2348. doi:10.1023/a:1020107915919 . URL: https://pubmed.ncbi.nlm.nih.gov/12395907/
- Opie LH, Lecour S. Melatonin has multiorgan effects. Eur Heart J Cardiovasc Pharmacother. 2016;2(4):258-265. doi:10.1093/ehjcvp/pvv037 https://pubmed.ncbi.nlm.nih.gov/27533945/
Food, circadian misalignment of the digestive system and metabolic syndromes
- Covassin N, Singh P, Somers VK. Keeping Up With the Clock: Circadian Disruption and Obesity Risk. Hypertension. 2016;68(5):1081-1090. doi:10.1161/HYPERTENSIONAHA.116.06588 . URL: https://pubmed.ncbi.nlm.nih.gov/27620394/
- Zimmet P, Alberti KGMM, Stern N, et al. The Circadian Syndrome: is the Metabolic Syndrome and much more!. J Intern Med. 2019;286(2):181-191. doi:10.1111/joim.12924 . URL: https://pubmed.ncbi.nlm.nih.gov/31081577/
- Duboc, H., Coffin, B., & Siproudhis, L. (2020). Disruption of Circadian Rhythms and Gut Motility. Journal of Clinical Gastroenterology, 54(5), 405–414. URL: https://pubmed.ncbi.nlm.nih.gov/32134798/
- McHill AW, Wright KP Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev. 2017 Feb;18 Suppl 1:15-24. doi: 10.1111/obr.12503. PMID: 28164449. https://pubmed.ncbi.nlm.nih.gov/28164449/
- Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014 Jul;14(7):507. doi: 10.1007/s11892-014-0507-z. PMID: 24816752; PMCID: PMC4308960. https://pubmed.ncbi.nlm.nih.gov/24816752/
- Chasens ER, Imes CC, Kariuki JK, Luyster FS, Morris JL, DiNardo MM, Godzik CM, Jeon B, Yang K. Sleep and Metabolic Syndrome. Nurs Clin North Am. 2021 Jun;56(2):203-217. doi: 10.1016/j.cnur.2020.10.012. Epub 2021 Mar 10. PMID: 34023116; PMCID: PMC8144542. https://pubmed.ncbi.nlm.nih.gov/34023116/
Health, sleep deprivation and circadian misalignment
- Foster RG. Sleep, circadian rhythms and health. Interface Focus. 2020 Jun 6;10(3):20190098. doi: 10.1098/rsfs.2019.0098. Epub 2020 Apr 17. PMID: 32382406; PMCID: PMC7202392. https://doi.org/10.1098/rsfs.2019.0098
- Colten HR, Altevogt BM, Institute of Medicine (US) Committee on Sleep Medicine and Research, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press (US); 2006. URL: https://pubmed.ncbi.nlm.nih.gov/20669438/
- Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139-154. doi:10.3109/09540261.2014.911149 . URL: https://pubmed.ncbi.nlm.nih.gov/24892891/
- Waters F, Chiu V, Atkinson A, Blom JD. Severe Sleep Deprivation Causes Hallucinations and a Gradual Progression Toward Psychosis With Increasing Time Awake. Front Psychiatry. 2018 Jul 10;9:303. doi: 10.3389/fpsyt.2018.00303. PMID: 30042701; PMCID: PMC6048360. https://pubmed.ncbi.nlm.nih.gov/30042701/
- Vetter C. Circadian disruption: What do we actually mean? Eur J Neurosci. 2020 Jan;51(1):531-550. doi: 10.1111/ejn.14255. Epub 2018 Dec 5. PMID: 30402904; PMCID: PMC6504624. URL: https://pubmed.ncbi.nlm.nih.gov/30402904/
- Harini C. Krishnan and Lisa C. Lyons. Synchrony and desynchrony in circadian clocks: impacts on learning and memory. Learn. Mem. 2015. 22: 426-437. https://doi.org/10.1101/lm.038877.115
- Rüger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009 Dec;10(4):245-60. doi: 10.1007/s11154-009-9122-8. PMID: 19784781; PMCID: PMC3026852. https://pubmed.ncbi.nlm.nih.gov/19784781/
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O'Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015 Mar;1(1):40-43. doi: 10.1016/j.sleh.2014.12.010. Epub 2015 Jan 8. PMID: 29073412. https://pubmed.ncbi.nlm.nih.gov/29073412/
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017 May 19;9:151-161. doi: 10.2147/NSS.S134864. PMID: 28579842; PMCID: PMC5449130. https://pubmed.ncbi.nlm.nih.gov/28579842/
- Guo, JH., Ma, XH., Ma, H. et al. Circadian misalignment on submarines and other non-24-h environments – from research to application. Military Med Res 7, 39 (2020). https://doi.org/10.1186/s40779-020-00268-2
Mental health, sleep disorders and circadian disruptions
- Walker WH 2nd, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020 Jan 23;10(1):28. doi: 10.1038/s41398-020-0694-0. PMID: 32066704; PMCID: PMC7026420. https://pubmed.ncbi.nlm.nih.gov/32066704/
- Harvey AG. Insomnia: symptom or diagnosis? Clin Psychol Rev. 2001 Oct;21(7):1037-59. doi: 10.1016/s0272-7358(00)00083-0. PMID: 11584515. https://pubmed.ncbi.nlm.nih.gov/11584515/
- Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med Rev. 2019 Aug;46:108-123. doi: 10.1016/j.smrv.2019.04.012. Epub 2019 Apr 30. PMID: 31108433. https://pubmed.ncbi.nlm.nih.gov/31108433/
- Matteson-Rusby SE, Pigeon WR, Gehrman P, Perlis ML. Why treat insomnia? Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00743. doi: 10.4088/PCC.08r00743bro. PMID: 20582296; PMCID: PMC2882812. https://pubmed.ncbi.nlm.nih.gov/20582296/
Genetics of circadian rhythm chronotypes and disorders
- Watson, Ronald Ross, and Victor R. Preedy, eds. Neurological Modulation of Sleep: Mechanisms and Function of Sleep Health. Academic Press, 2020. https://doi.org/10.1016/C2018-0-00689-1
Biopolitics of sleep
- The Biopolitics of Melanopic Illuminance, Magnus Eriksson and Geraldine Juárez, Scapegoat (10), 2017. https://web.archive.org/web/20210905132205/http://www.scapegoatjournal.org/docs/10/14.pdf
- Foster RG. Sleep, circadian rhythms and health. Interface Focus. 2020 Jun 6;10(3):20190098. doi: 10.1098/rsfs.2019.0098. Epub 2020 Apr 17. PMID: 32382406; PMCID: PMC7202392. https://doi.org/10.1098/rsfs.2019.0098
Videos
Gentle introductions to the science of sleep and circadian rhythm.
- Why we sleep? By Russell Foster, TED, Aug 2013. https://www.youtube.com/watch?v=LWULB9Aoopc
- Health lies in healthy circadian habits. By Satchin Panda at TEDxBeaconStreet, Dec 2017. https://www.youtube.com/watch?v=erBJuxVR7IE
Future
Recommendations for sleep studies and clinical practice for the early detection and management of circadian rhythm sleep-wake disorders
The author recommends the following should be systematically done in all sleep disorders diagnosis or study in order to improve the fast and accurate diagnosis of circadian rhythm sleep-wake disorders:
- Require the patient to sleep according to their natural sleep schedule for a few weeks before diagnosis. If not possible, take note of this issue in the diagnosis as this can hide the real disorder.
- Systematically screen for (pre) diabetes or other signs of a metabolic disorder.
- Require the patient to maintain a sleep diary for 2 weeks, whatever the sleep disorder is and not only when there is a suspicion of a circadian rhythm disorder, as misdiagnoses with other sleep disorders such as narcolepsy and hypersomnia may be prevalent.
- For clinicians and sleep researchers: use core body temperature or wrist skin temperature to measure the circadian rhythm or DLMOff (time the endogenous melatonin secretion stops) as these 3 are coupled, avoid the melatonin levels and especially DLMO (DLMOn - melatonin onset) as it is an unreliable proxy (decoupled from circadian phase shifting and delayed).
- Conversely, always report results relatively to the individuals' minimum core body temperature (or peak wrist skin temperature) or DLMOff, never in absolute time as reporting results relatively to the circadian rhythm allows for easier translation and personalization of treatment to various chronotypes and circadian rhythm disorders. For example, here is a good example, and here is a bad one on exactly the same research question.
- Use a more stringent definition of entrainment (eg, freerunning pattern break over at least 1.5 months without an alarm clock), to better assess therapeutic effectiveness by reducing the number of false positives due to natural transient entrainment and relative coordination that regularly happens for individuals with non-24 (as likely happened for some subjects - particularly those using an alarm clock - in this study).
- "Perhaps the most important implication of relative coordination and transient entrainment is in the diagnosis of this disorder. Individuals who demonstrate transient entrainment might easily be misdiagnosed as entrained if circadian phase is not assessed for a sufficient period of time. Inspection of Figure 1 indicates that it may be necessary to assess observed circadian phase for more than 3 months in some cases before a conclusive diagnosis can be made."
- The same study suggests that "visual inspection of sleep diary data is likely to have a high false negative rate" and so cannot "accurately diagnose N-24s", and propose that "a physiological assessment of entrainment status is necessary for accurate diagnosis of this CRSD whether it be directly through serial determinations of circadian phase or possibly indirectly via spectral analysis of longitudinal actigraphy data."
- Improvement of the early detection and management of circadian rhythm disorders and other sleep disturbances in children should be the top priority. Historically, sleep disturbances have been largely ignored by the psychomedical field, missing unique opportunities for improved health outcomes. Even nowadays (as of 2021), "education about sleep and sleep disorders is lacking in medical school curricula, graduate medical education, and education programs for other health professionals" according to the AASM (see also here), and the AASM recommends to put policies in place and teach sleep health at schools even as early as K-12, just like dental health is taught. Children with sleep disorders are likely often misdiagnosed, and these cases should be treated with particular care, as misdiagnosis can have long lasting detrimental effects on this vulnerable population. Indeed, even if they have comorbid diseases, clinicians should be cognizant about sleep disruption complaints, especially with children and teenagers, as sleep disruption worsen all risks of comorbidities. Here are some recommendations for children, borrowing from a previous study with further reasoning:
- Clinicians should be trained to ask parents about their children's bedtime and wake-up time (maintaining a sleep diary for at least 2 weeks if necessary), as asking parents about whether their children have sleep issues is unreliable since parents are usually unaware and/or are not educated about circadian rhythm sleep-wake disorders.
- Chronic daytime sleepiness should always be a red flag for a sleep or circadian rhythm disorder.
- Teachers should be trained to recognize and report signs of sleepiness in classroom (and avoid sanctioning sleepiness since this is a symptom of disease, not of rebellion).
- In both cases, "reports of delayed or irregular sleep-wake rhythms and daytime sleepiness should trigger referral for a circadian rhythm evaluation".
- For management, accommodations should be sought with the educational institutions to allow the child to sleep in phase with their circadian rhythm, without restriction, until the entrainment therapy works (if it does even), as research has demonstrated that it's crucial to sleep in phase with one's own circadian rhythm, especially during childhood, to prevent the development of other diseases, such as metabolic disorders.
- Chronic sleep loss impairs neurodevelopment and incurs neuronal loss, especially if from a young age, hence the necessity for accomodations of children with sleep disorders as chronic sleep deprivation can not only stunts cognitive performance, but also pave the way for the development and early onset of neurological disorders and major mental mood and psychotic disorders such as depression.
- The B-SATED scale can help in assessing pediatric sleep health by educators and clinicians.
- The weak evidence base underlying circadian plasticity hints that it is likely a red herring. Future studies should focus on other hypotheses that do not require the circadian plasticity to underie the expected effects. It is the author's opinion that if the domain of circadian rhythm science and circadian rhythm disorders research could reach a consensual refutation of circadian plasticity, this would be a huge step forward to the development of effective treatments and elucidation of fundamental sleep mechanisms.
- Adopt the circadian disruption nomenclatura of this review to improve reproducibility.
- In-hospital settings need to account and respect the patient's circadian rhythm to increase the effectiveness of drugs and reduce their adverse effects, through the reduction of disturbances during the circadian night and proper management of artificial light (circadian lighting).
- A genetic study shown that the circadian rhythm rely on a network of multiple clocks, providing redundancy and hence resilience in face of circadian disruptions. We can hypothesize that disruptions of this network may underlie some of the circadian rhythm disorders.
- Adults should be systematically screened for sleep disorders independently from co-morbid (psychological) conditions, as this may significantly improve the detection and management of psychological health outcomes.
- Up to this day, insomniacs with a co-morbid psychiatric disorder are excluded from most studies. They should instead be included as the co-morbid psychiatric disorder as insomnia is independent or even preceding the onset of psychiatric disorders, and so there is no ground to reject these subjects and doing so leaves a huge gap in the literature as most cases of severe insomnia also have psychiatric co-morbidities.
- Sleep diaries over at least 2 weeks should always be used as part of the diagnostic procedure and with a recommendation to the patient to continue to maintain it whenever there is a relapse. Although this is currently considered a standard diagnostic tool for insomnia (including psychiatrists) and circadian rhythm disorders by the AASM since 2008, sleep clinicians rarely use it. Actigraphy can also be used.
- Awareness about sleep health should be more spread among the general public, see Buysse questionnaire and definition of sleep health (see also here): sleep health was defined as “a multidimensional pattern of sleep-wakefulness, adapted to individual, social, and environmental demands, that promotes physical and mental wellbeing." Buysse proposed the acronym SATED for the items that allows to evaluate sleep health: Satisfaction with sleep, Alertness during waking hours, Timing of sleep, sleep Efficiency, and sleep Duration. An adaptation for children and teenagers named B-SATED is also available.
Leads to investigate
- Investigate and quantify whether pupil dilation to light/darkness can predict circadian rhythm shifting (and hence indicate whether dark therapy is adequately done or not, using eg shaded blue blocker sunglasses).
- Investigate whether using more intense light therapy allows to avoid or reduce the need for dark therapy (since prior exposure to bright light reduces sensitivity to light).
- Systematization of treatments timing using continuous temperature monitoring to estimate the circadian rhythm via wearables such as Thermocron iButtons.
- New drugs classes: Adenosine analogs such as cordycepin should be further investigated as potential type-0 resetters (ie, strongest type of circadian phase shifting). See this study, this journalistic vulgarization article and my informal review on dosage and safety (archive here). Caffeine was also shown to phase delay the circadian rhythm, hence it seems any molecule affecting either adenosine or melatonin pathways can potentially be used as zeitgebers.
- Investigate other drug candidates to reset the circadian rhythm such as nobiletin apparently affecting muscle clocks (could be an alternative to exercising).
- Iron supplementation (such as in reinforced nutritional yeast) can worsen circadian rhythm issues?
- Sleep deprivation builds up dopamine which then over stimulates the brain (wake maintenance zones), so something like Methylphenidat makes sense to use to ease sleep or clear up brain fog. Or melatonin too since it interacts with the dopaminergic system.
- Low doses of aripiprazole were found in two trials (here and here) to increase the entrainment of DSPD individuals to bright light. Hence, a combined therapy of low doses of aripiprazole and light therapy (especially with light therapy glasses) should be further investigated for both DSPD and non-24, especially for treatment resistant cases of circadian rhythm disorders. Other photosensitizing drugs, such as those commonly used for ADHD, could also prove similarly useful, since the ipRGC cells are the ones mediating both the pupil contraction reflex and circadian rhythm shifting. But side effects and fast tolerance build up may reduce the use case for this class of drugs.
- Adenosine agonists and potential adenosine dysregulation: The current document's author strongly suspect that circadian rhythm disorders also include sleep homeostat dysregulations, or mismatches with the circadian rhythm, as can be experimentally produced with healthy sleepers (eg, this study). More studies are needed on the adenosine regulation in circadian rhythm disorders and the potential use of adenosine agonists.
- Dopaminergic agents (aripiprazole, brexpiprazole and pramipexole) to increase the effectiveness of light therapy and melatonin in people who are less responsive. This may be a key class of agent to make these therapies more widely effective. The issue is whether they are sustainable, as dopaminergic agents build up tolerance and addiction, so it's necessary in practice to regularly stop taking them and restart after the tolerance has lowered, and hence long-term clinical trials are needed to assess their sustainability (in addition to their effectiveness to potentiate zeitgebers magnitude).
- Sudden wake up variability does NOT mean that your circadian rhythm suddenly shifted, I strongly suspect the circadian rhythm is much slower/has much more inertia to shift. I will confirm this with body temperature monitoring in the upcoming months.
- New objective measure of sleep and circadian rhythm: ocular: «infrared reflectance oculography was used to collect ocular measures of sleepiness: positive and negative amplitude/velocity ratio (PosAVR, NegAVR), mean blink total duration (BTD), the percentage of eye closure (%TEC), and a composite score of sleepiness levels (Johns Drowsiness Scale; JDS).», «The study demonstrated that objective ocular measures of sleepiness are sensitive to circadian rhythm misalignment in shift workers.» https://www.ncbi.nlm.nih.gov/pubmed/26094925
- Explore the concept of revenge bedtime procrastination and its potential relationship to the mice utopia experiment and behavioral sink by Calhoun.
- Visualization tools can help: https://www.reddit.com/r/DSPD/comments/hj4utz/a_weird_way_im_coping_with_dspd/
- Circadian syndrome links circadian rhythm disorders with metabolic syndromes, and here circadian rhythm disorders are suggested to be linked to Alzheimer, and Alzheimer is considered by some scientists as a type-3 diabetes (ie, dysregulation of insulin and glucose in the brain through brain insulin resistance, rather than in the body).
- The wide genetic diversity of ipRGC cells suggests there are complex effects of light therapy that may not be elucidated yet (eg, photic history).
- Genetics: investigate the GG mutation in MTNR1B rs10830963 and rs10830962, suspect it's common in non-24 as it was incidentally observed as a side result in this study.
- Devise a new behavioral scale to specifically diagnose circadian rhythm disorders, to complement diagnosis by sleep diary and temperature monitoring, which would help in addressing the issues of current diagnosis guidelines based on sleep diary only (too inclusive and too restrictive at the same time).
- Test ostrichpillow light or ostrichpillow loop for an on-the-go solution to replace the eye mask to complement dark therapy blue blocker glasses. Or worse, the chicken pillow, a real item for a marketing stunt...
- Test split-dosing melatonin? Low dosage before DLMO (1mg) for circadian shifting + higher-dosage before bedtime to sleep faster (3mg)? But they used here the same kind of melatonin (prolonged or instant release?). This could be improved theoretically: use instant release 0.3mg melatonin before DLMO for circadian shifting and prolonged-release 0.5 to 2mg melatonin (higher dosages causing hypothermia and maybe facilitating sleep) 1h before bedtime to induce drowsiness and consolidate sleep?
- Are zeitgebers effects relative to only one's biological night (circadian rhythm), or is time since awake (homeostatic process S) also playing a role?
- The results of the self-experiment for the presently proposed entrainment protocol should be reproduced in a clinical trial with a bigger sample, which is difficult to do since sighted non-24 is rare and underdiagnosed. Nevertheless, there is some glint of hope with some institutions currently seeking to build a database of such patients, such as the Centre for Chronobiology, University of Basel, Switzerland and the Northwestern University, United Kingdom. Another database for non24, which is crowdsourced from feedbacks by patients about their symptoms and treatments they had and then anonymized and analyzed by AI algorithms, is being built on StuffThatWorks as of September 2021. They also have a database for DSPD (thanks to u/elorenn for the tip). Members can also submit their own questions.
- Given the very strong links between the circadian rhythm and the immunological system, and after some informal feedbacks of individuals with non24 reporting a potentially high prevalence of comorbid allergies or impaired immunology, it would be worthwhile to study the prevalence of immunological disorders in people with a circadian rhythm disorder.
- Informal results from peer groups suggest that responsiveness to light therapy is very widespread among individuals with sighted non24, and maybe even to a large subpopulation (2/3rd) of blind non24.
- Higher, extracellular doses and modes of administration for such high doses of melatonin for humans, to reduce or even circumvent the cognitive and physiological damages of sleep deprivation induced by circadian rhythm disorders, other sleep disorders and even neurodegenerative disorders such as diabetes.
- "Chronic daily, awakening, and morning headache patterns are particularly suggestive of sleep disorders, including sleep-related breathing disorders, insomnia, circadian rhythm disorders, and parasomnias. [...] Evidence strongly supports screening for sleep disorders by headache practitioners. Headache management should identify and treat sleep disorders that may improve or resolve headache." https://doi.org/10.1007/s11910-008-0027-9
- Snoring is not indicative of sleep apnea and can have sleep apnea with minimal snoring: "Snoring on its own is probably of limited usefulness in assessing sleep apnea presence and severity, because of its weak relationship with AHI. Thus, the complaint of snoring should be interpreted with caution to avoid unnecessary referrals for sleep apnea testing. Conversely, clinicians should be aware of the possibility of missing diagnosis of patients with sleep apnea who have minimal snoring." https://doi.org/10.5664/jcsm.7676
- Matching employees chronotypes to shift work positions as some scientists suggest. This could lead to significantly reduced work accidents and better performance, especially in critical areas such as urgent healthcare at night.
- "Regardless, when developing lighting schemes for adolescents, it is important that both daytime and evening light be specified. When designing lighting for schools, moreover, educational materials offering guidelines for evening light exposures should be made available to students and parents." https://doi.org/10.1177/1477153517721598
- New wearables such as actigraphy and core body temperature monitoring can allow to study sleep in preindustrial and developing societies and tribes that were previously inaccessible, with potentially very interesting insights as to the natural origin and repartition of chronotypes and circadian rhythm disorders.
- Given the recent findings of how much alcohol disrupt the circadian rhythm, and how most if not all effective antidepressants also shift the circadian rhythm, it would be interesting to investigate whether children of individuals abusing alcohol chronically may be more likely to have a circadian rhythm disorder than children of non alcohol abusing parents. If that's the case, it would be interesting for fundamental biological studies to elucidate whether this is the case because parents with circadian rhythm disorders are more likely to abuse alcohol, or whether the circadian rhythm disturbances caused by alcohol can be inherited genetically by children. See this post for an extended discussion.
- There needs to be specific studies on how to treat sleep and especially circadian rhythm disorders in individuals with comorbid neuromotor disorders such as RLS and PLMD, as they contra-indicate the only few therapies that are effective to treat circadian rhythm disorders: melatonin and light therapy (likely because of the indirect increase in melatonin due to photic history). Indeed, melatonin triggers or worsen significantly the neuromotor symptoms. Hence, these individuals' circadian rhythm disorders are currently left untreated.
- There is a crucial need for more demographics studies, especially on employment status and livinghood of people with circadian rhythm disorders, especially sighted non-24 (not blinded, as the results are then confounded by the effect of blindness). The Circadian Sleep Disorders Network conducted such a survey in 2020, with a list of questions published here, but only partial results were ever published.
- Interindividual circadian flexibility/malleability should be more investigated. Freerunning caused by non24 does not appear to be related to circadian flexibility, but people, eg night shift workers, seem to have a flexible circadian phase can freerun to accommodate their new sleep window opportunity under their socioprofessional constraints. The factors defining circadian flexibility could help elucidate the difficulties of treatments of circadian rhythm disorders.
Apps ideas
Smartphone applications have the advantage to be readily available to a lot of users. Hence, this is a great avenue to positively impact the lives of a lot of users at a virtually null cost for them. Apps can be used both from a patient-centric way by designing apps to accompany them in their treatment, increase their compliance and self-measure the impact of various factors on their sleep, as well as a researcher-centric approach to collect large-scale data on sleep and sleep-related variables such as light exposure.
- blue light booster in the morning and blue light filter (gamma curves) + dimmer in the evening. Similar idea to flux but with 3 modes: blue boosting, no change and evening filtering. Would allow to do a kind of light therapy using only screen computers (only for screens without PMW).
- smartphone apps to record light sensor + gps position automatically. Anonymize gps position by allowing user to set named areas, and only the named area will be recorded (eg, at work, commuting, home, shopping, hanging out, etc). Would be a cheap way to record large scale data for spectral diet of humans.
Methods
This document can be considered to be a 3-parts work, including:
- A proposition of a new therapeutic protocol for circadian rhythm disorders, especially the sighted non-24 disorder, called VLiDACMel.
- A narrative review of the sleep and circadian rhythm scientific and medical literature, both to justify the design choices of VLiDACMel, and at other times to inform other aspects of these sleep disorders.
- Preliminary results of an N-of-1 trial (ie, a self-experiment) with a repeated ABA design.
We will discuss the design choices for each of these parts.
About the therapeutic protocol proposition
The reason for the existence of this document is to propose and iterate over an innovative therapeutic protocol. The protocol was designed with a combination of trial-and-error, author's own ideas by thought experiments, and later revised iteratively especially with insights from the scientific and medical literature.
The protocol has been extensively rewritten several times to attain a state where the choice for each item is clarified and supported by previous evidence as much as possible.
Note: a protocol is not a study: a protocol is a proposition of a set of clearly defined procedures that aims to be reproducible or even generalizable.
About the narrative review
Most of this work relies on academic sources selected according to commonly established hierarchical gradation of quality (hierarchy of evidence, see also Christopher J Blunt's 2015 PhD Thesis for a critical appraisal), eg, guidelines > systematic reviews and meta-analyses of human studies and clinical trials > narrative reviews > human studies > animal reviews > animal studies. Predatory journals are avoided, and permalinks such as DOI or PubMed are preferred. When a permalink is not available, the resource was mirrored on Web Archive or Archive.is . However, since this is an unsystematic review, there is an inherent risk of bias, in that the given overview may represent the ground truth.
Reproducibility is a crucial criterion that was systematically used to evaluate the reliability of results. Hence, systematic reviews were preferred over results from a single trial, but when unavailable, the concordance of the results of multiple studies conducted by different teams increased the confidence in the results' reliability (eg, increasing duration of bright light exposure being more effective than increasing light intensity).
However, due to the lack of data and studies on rare disorders such as sighted non-24, other circadian rhythm disorders and more generally the academic literature on circadian rhythm and sleep research were considered and put into perspective while accounting for the potential differences, as it is assumed that all circadian rhythm disorders share common pathways of causation and treatment since the circadian rhythm and sleep are highly conserved biological processes throughout evolution, not just in humans.
Where there are controversies or uncertainties, the debates are presented and the most reasonable conclusion is emphasized using bayesian reasoning and falsifiability (see also here, here and here). For example, procedures are considered ineffective to treat circadian rhythm disorders unless they demonstrate a capability to modulate the core body temperature. There is always at least one clear condition given to falsify any potentially controversial claim stated in this document.
Since patient's personal knowledge is part of medical science, this document also includes several author's anecdotal observations wherever pertinent, in the hopes this may be useful as supplementary data for other researchers to look into. Indeed, it is the author's conviction that data should never be scraped, whether the results are positive or negative.
About the preliminary self-study results
Given the author of the current document is afflicted by the target disorder, it was deemed an excellent opportunity to test and improve iteratively the protocol through trial-and-error. Along the way, data collection became more and more systematic and objective, with the use of external sensors such as non-invasive core body temperature monitoring via dual heat flux technology.
In a perspective of reproducibility and open science documentation, all the results and protocols for these side projects (such as Wearadian for external sensors) are documented as extensively but consisely as possible to help with future reproducibility experiments (if any). There is absolutely no claim of generalizability or even efficacy beyond the author's personal experience, as clearly stated in various disclaimers throughout the document.
Being afflicted with the studied disorder confers some advantages: pilot experimentations can be iterated faster, but the disadvantage is the risk of bias. Hence, objective measures were preferred whenever possible to reduce the risk of subjective bias.
It can be argued that the naturalistic condition impairs the quality of the study due to confounding and masking factors. But this is also an advantage and a purposeful requirement. Indeed, therapies efficacy need to be assessed in practice. If an intervention is only effective in an unrealistic condition that can only be done in labs, it will be of little usefulness to the clinical population. One major factor that is usually eliminated in lab settings but unavoidable in practice by patients is exposure to sunlight. Another is social duties. Hence, to be clinically significant, the effectiveness of an intervention needs to be clearly significant even in this naturalistic setting. Furthermore, masking factors can be detected with phase jumping on actograms or by monitoring the core body temperature, which should not be affected by any masking factor, only by zeitgebers. The same reasoning also applies to circadian rhythm sensors, as their measures need to robustly track the circadian rhythm in the naturalistic setting to be pertinent to improve the management of circadian rhythm disorders.
Indeed, it is the author's opinion that one of the greatest advantages of self-experimentation is that it allows to directly test for practical effectiveness, which allows for discrimination of sometimes conflicting informations between statistically significant effects but ultimately clinically insignificant.
Of course self experimenting can only count as preliminary findings with a high uncertainty of correctness (due to noise and unaccounted confusion factors) and of generalizability, that needs at least further reproduction with group studies. However, a sample size of n=1 is always better than a sample size of n=0, as there are lots of interesting experiments in need of being conducted but will not be due to the difficulty of finding adequate and willing participants with enough time to run the experiment under usually stringent experimental constraints, on top of the time-consuming administrative burden researchers face when designing even the smallest experiments. In these cases, preliminary evidence even from a single subject can allow to tremendously trim down between the realm of possible - sometimes promising - observations and the unreal or unpromising approaches.
Hence, despite the limitations, this self-study should not be considered subjective but a truly objective scientific experiment, or more accurately an explicit particular experiment according to Polanyi's epistemological framework. It is worth remembering that several pioneers in circadian rhythm science extensively used self-experimentation at the time as referenced in the "Brief history of bright light therapy" section. Self-experimentation is also not limited to this field of science, but is common across science domains and especially in medicine (see also this vulgarization article). This method of research is still being actively used and is leading to innovative insights, such as with Poldrack's MyConnectome project.
In addition, pertaining data and source codes are released per the FAIR protocol. Data analysis will be later conducted, and the self-experiments are aimed to be conducted in a N-of-1 trial methodology (aka single-patient trial method), as this approach is more reliable than single-case studies especially by allowing statistical significance assessments on objective metrics, although some practices are obviously impossible in this context, such as blinding.
Ethics
This document is the result of an extensive literature review and a self-experiment.
The self-experiment was not pre-approved by an ethical committee, but it should be considered ethical per se (see also here). Although more prevalent historically, this kind of approach has seen a resurgence lately due to its advantages, with for example the MyConnectome dataset, which allowed to further advance our knowledge of the functional brain connectivity longitudinal changes. The American Diabetes Association since 2018 is another example, with the then newly appointed CEO of the organization self-experimenting with low-carb diets and self-monitoring biomarkers, which led her to assess first-hand the effectiveness of low-carb diets in the management of metabolic disorders and hence ultimately to pivoting a century old misconception about the involvement of lipids in the pathogenesis of metabolic disorders. Self-experimenting is also one of the methods that was extensively used by sleep research pioneers such as Aschoff, or also Nathaniel Kleitman, the father of sleep research.
Conflicts of interest
The author, Stephen Karl Larroque, declares no conflict of interest. All his present and past fundings are publicly detailed on ORCID. No funding were provided for the redaction of this document nor for the development of the VLiDACMel protocol. Although several products are mentioned, the author declares no conflict of interest towards these companies and products, as they are mentioned only because they are pertinent, and all tests with these products were done with the author's own funds and from the author's own volition with no external incentives whatsoever.
Acknowledgements
The author is thankful to u/Dialectical_Warhead on Reddit for his extremely helpful critical feedbacks and sharing of bibliography, which contributed notably in clarifying some of the optimal parameters and theoretical background for light therapy, as well as critical discussions on sociological and psychological aspects of circadian rhythm disorders.
The author is indebted to Mr. Bernard Dengis MD for his wide knowledge of even rare pathologies, his wise and human approach to medical care and his support all along.
The author is very grateful to the Pr. Robert Poirrier for not only sharing bits of his in-depth knowledge, such as his crucial clues about ipRGC cells location in the retina, his critical thinking and human understanding of social issues of a disorder. Essential skills that are unfortunately rarer in the medical and scientific fields nowadays.
The author is grateful to the members of the subreddit r/N24 and the members of its relative discord for their support, questions and sharing their experience with this disorder. User Maverick on Discord provided critical feedbacks on the pharmacology and pharmacodynamics of some compounds. Thank you to Tanka, Psyz0me, and V for their continued support since the early phases of the experiment, and for their suggestions about eye physiology. Thank you to Alec Gray for their kind permission to reproduce their poetic illustration of non-24. Thank you to editoreal for reminding that Vitamin A is necessary for the synthesis of all opsins in the eyes including melanopsin, the essential pigment of ipRGC cells for bright light entrainment, and for referencing how vitamin D over supplementation can inhibit melatonin. Thank you to mmortal03 for sharing works on quantifying circadian misalignment using actigraphy. Several redditors were also of great help by sharing personal experiences, thoughts and some references. Thank you to fanfan64 for sharing details on eugeroics, glycine and epitalon. Warm thanks to u/eeror for sharing the works on circadian light subadditivity, the clarifications on the IEC/EN 62471 photohazard regulation, and for a lot of other very enlightening discussions. Thank you to u/ILikeBirdsQuiteALot for the figures on the duration of hyperphotosensitization with ADHD stimulants. Thank you to u/SelfAwareMachines for the interesting discussions and links to research on some innovative neurological pathogenesis hypotheses for circadian rhythm disorders, such as astrocytes. Thank you to u/carvo08 for kindly sharing several references, including the adenosine buildup underlying the inhibitory effect of sleep deprivation on bright light therapy. And thank you to all the others who kindly shared information that the author forgot to cite in this non exhaustive list.
The author is admirable of the giants on which he relied to design this protocol, such as Charles A. Czeisler and Josephine Arendt. Since the scientific method proceeds under a longer timeframe than pragmatic considerations, researchers sometimes regretfully feel like their work is remote from reality. This protocol demonstrates an instance of a pragmatic application that would have been impossible without the various and varied work of numerous researchers.
Finally, the author would like to especially thank his significant other: thank you for your support all along, your wise and clever advices, and your continuous help which made these experiments easier with minimal external disturbances. You made this all possible. And to my future children, who were my strongest motivation, I hope you will never have to suffer through what I had to, and will have more opportunities than I and your ancestors had.